Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the EOs
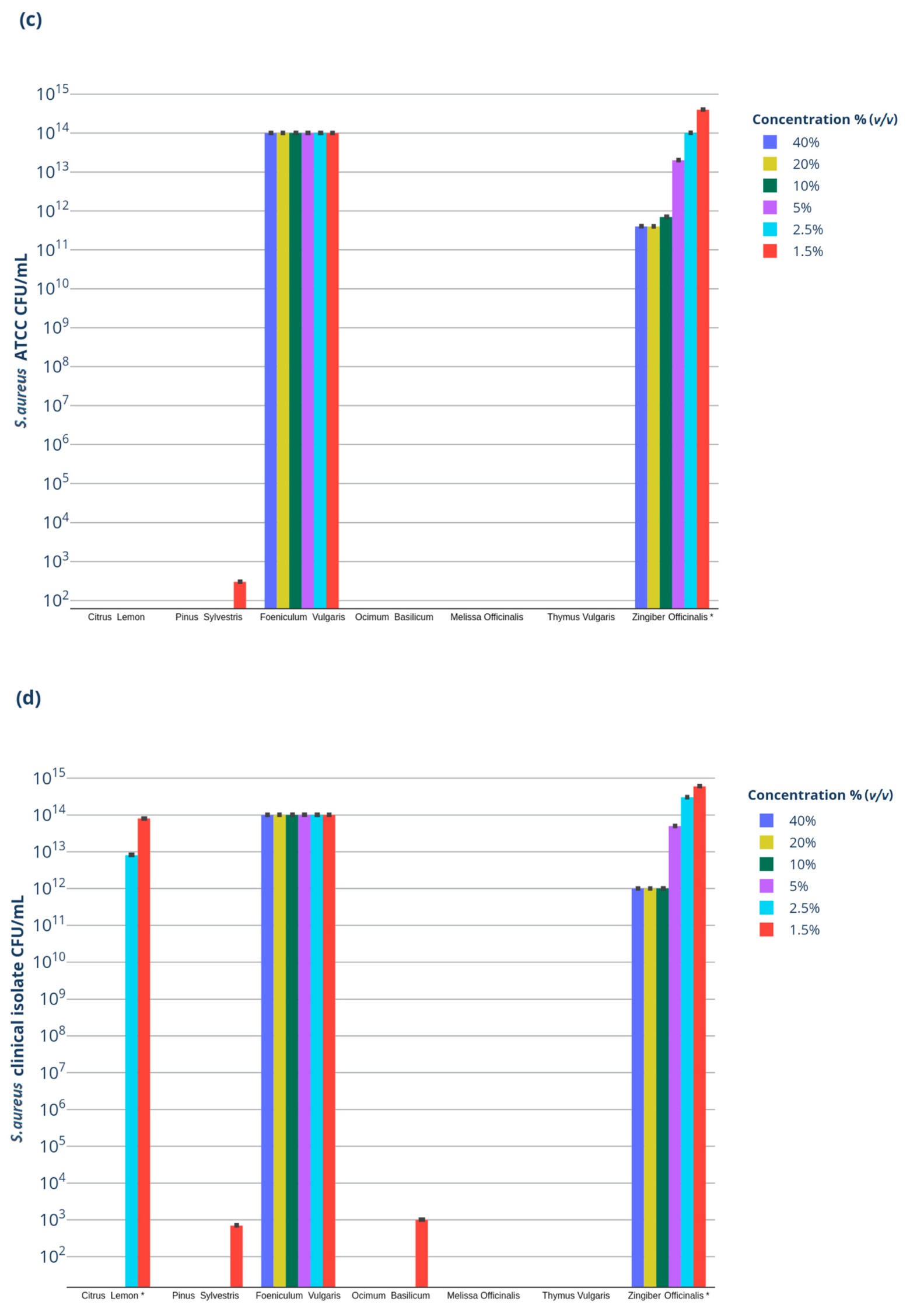
2.2. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Essential Oil
4.2. Gas Chromatography/Mass Spectrophotometry (GC/MS)
4.3. Compound Identification
4.4. Bacteria Strains
4.5. Screening for MDR Activity
4.6. Screening for Antibacterial Activity of EOs
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Poolman, J.T.; Anderson, A.S. Escherichia coli and Staphylococcus aureus: Leading bacterial pathogens of healthcare associated infections and bacteremia in older-age populations. Expert Rev. Vaccines 2018, 17, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [Green Version]
- Bradley, S.F. Staphylococcus aureusInfections and Antibiotic Resistance in Older Adults. Clin. Infect. Dis. 2002, 34, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef]
- Loayza, F.; Graham, J.P.; Trueba, G. Factors Obscuring the Role of E. coli from Domestic Animals in the Global Antimicrobial Resistance Crisis: An Evidence-Based Review. Int. J. Environ. Res. Public Health 2020, 17, 3061. [Google Scholar] [CrossRef]
- Cain, C.L. Antimicrobial Resistance in Staphylococci in Small Animals. Vet.-Clin. N. Am. Small Anim. Pract. 2013, 43, 19–40. [Google Scholar] [CrossRef]
- Wieler, L.H.; Ewers, C.; Guenther, S.; Walther, B.; Lübke-Becker, A. Methicillin-resistant staphylococci (MRS) and extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae in companion animals: Nosocomial infections as one reason for the rising prevalence of these potential zoonotic pathogens in clinical samples. Int. J. Med Microbiol. 2011, 301, 635–641. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Camero, M.; Lanave, G.; Catella, C.; Capozza, P.; Gentile, A.; Fracchiolla, G.; Britti, D.; Martella, V.; Buonavoglia, C.; Tempesta, M. Virucidal activity of ginger essential oil against caprine alphaherpesvirus-1. Vet.-Microbiol. 2019, 230, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cui, P.; Shi, W.; Zhang, Y. Identification of essential oils with activity against stationary phase Staphylococcus aureus. BMC Complement. Med. Ther. 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.S.; Sumita, T.C.; Furlan, M.R.; Jorge, A.O.; Ueno, M. Antibacterial activity of essential oils on microorganisms isolated from urinary tract infection. Rev. Saude Publica 2004, 38, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in Nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Perrino, E.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and Plant Community Implication on Essential Oils Composition in Useful Wild Officinal Species: A Pilot Case Study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007; 804p. [Google Scholar]
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and antiviral effects of Thymus vulgaris essential oil on feline coronavirus. Res. Vet.-Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Cronan, J.E., Jr. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 1999, 33, 249–259. [Google Scholar] [CrossRef]
- Mohsenipour, Z.; Hassanshahian, M. The inhibitory effect of Thymus vulgaris extracts on the planktonic form and biofilm structures of six human pathogenic bacteria. Avicenna J. Phytomed. 2015, 5, 309–318. [Google Scholar] [CrossRef]
- Gömöri, C.; Vidács, A.; Kerekes, E.B.; Nacsa-Farkas, E.; Böszörményi, A.; Vágvölgyi, C.; Krisch, J. Altered Antimicrobial and Anti-biofilm Forming Effect of Thyme Essential Oil due to Changes in Composition. Nat. Prod. Commun. 2018, 13, 1934578X1801300426. [Google Scholar] [CrossRef] [Green Version]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial Activity of Five Essential Oils against Bacteria and Fungi Responsible for Urinary Tract Infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.M.; Sokeng, A.J.T.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- López, E.I.C.; Balcázar, M.F.H.; Mendoza, J.M.R.; Ortiz, A.D.R.; Melo, M.T.O.; Parrales, R.S.; Delgado, T.H. Antimicrobial Activity of Essential Oil of Zingiber officinale Roscoe (Zingiberaceae). Am. J. Plant Sci. 2017, 8, 1511–1524. [Google Scholar] [CrossRef] [Green Version]
- Al-Jabri, N.N.; Hossain, M.A. Comparative chemical composition and antimicrobial activity study of essential oils from two imported lemon fruits samples against pathogenic bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, P.; Pruss, A.; Masiuk, H.; Mnichowska-Polanowska, M.; Kaczmarek, M.; Giedrys-Kalemba, S.; Dołęgowska, B.; Zielińska-Bliźniewska, H.; Olszewski, J.; Sienkiewicz, M. The effect of fennel essential oil and trans-anethole on antibacterial activity of mupirocin against Staphylococcus aureus isolated from asymptomatic carriers. Adv. Dermatol. Allergol. 2019, 36, 308–314. [Google Scholar] [CrossRef]
- Krüger, H.; Hammer, K. Chemotypes of Fennel (Foeniculum vulgare Mill.). J. Essent. Oil Res. 1999, 11, 79–82. [Google Scholar] [CrossRef]
- Badoc, A.; Deffieux, G.; Lamarti, A.; Bourgeois, G.; Carde, J.-P. Essential Oil of Foeniculum vulgare Mill. (Fennel) subsp. piperitum (Ucria) Cout. Fruit. J. Essent. Oil Res. 1994, 6, 333–336. [Google Scholar] [CrossRef]
- Mahboubi, M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin. Phytoscience 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Ebani, V.V.; Mancianti, F. Use of Essential Oils in Veterinary Medicine to Combat Bacterial and Fungal Infections. Vet.-Sci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Mirskaya, E.; Agranovski, I.E. Control of Airborne Microorganisms by Essential Oils Released by VaxiPod. Atmosphere 2021, 12, 1418. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A review on recent research results (2008-2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Koo, I.; Kim, S.; Zhang, X. Comparative analysis of mass spectral matching-based compound identification in gas chromatography–mass spectrometry. J. Chromatogr. A 2013, 1298, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingledew, W.M.; Sivaswamy, G.; Burton, J.D. The API 20E microtube system for rapid identification of gram negative brewery bacteria. J. Inst. Brew. 1980, 86, 165–168. [Google Scholar] [CrossRef]
- Devenish, J.A.; Barnum, D.A. Evaluation of the API 20E system for the identification of gram-negative nonfermenters from animal origin. Can. J. Comp. Med. Rev. Can. Med. Comp. 1982, 46, 80–84. [Google Scholar]
- Kloos, W.E.; Wolfshohl, J.F. Identification of Staphylococcus species with the API STAPH-IDENT system. J. Clin. Microbiol. 1982, 16, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlyn, C. Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J. Clin. Microbiol. 2000, 38, 3341–3348. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, M.P.; Lewis, J.S. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions, and Processes. J. Clin. Microbiol. 2020, 58, e01864-19. [Google Scholar] [CrossRef]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| N | Components | AI | LEO | PEO | FEO | BEO | MEO | TEO | GEO |
|---|---|---|---|---|---|---|---|---|---|
| % ± SEM | % ± SEM | % ± SEM | % ± SEM | % ± SEM | % ± SEM | % ± SEM | |||
| 1 | α-pinene a | 931 | 2.4 ± 0.5 | 29 ± 3 | 6.4 ± 0.9 | 0.34 ± 0.04 | 1.81 ± 0.10 | 1.73 ± 0.12 | |
| 2 | camphene a | 952 | 1.6 ± 0.3 | 0.12 ± 0.01 | 0.52 ± 0.05 | 1.89 ± 0.11 | 4.74 ± 0.27 | ||
| 3 | β-thujene | 968 | 1.94 ± 0.20 | 1.24 ± 0.23 | 0.20 ± 0.01 | 0.71 ± 0.06 | |||
| 4 | β-pinene a | 980 | 14.5 ± 1.0 | 17.2 ± 1.2 | 0.65 ± 0.07 | 0.65 ± 0.05 | 0.56 ± 0.03 | ||
| 5 | α-phellandrene a | 1003 | 5.2 ± 0.6 | 0.15 ± 0.01 | 4.4 ± 0.6 | ||||
| 6 | 3-carene a | 1016 | 13.1 ± 2.5 | ||||||
| 7 | o-cymene | 1021 | 1.1 ± 0.8 | 1 ± 0.1 | 19.6 ± 1.5 | ||||
| 8 | eucalyptol a | 1023 | 0.29 ± 0.02 | 1.2 ± 0.5 | 0.89 ± 0.05 | 1.9 ± 0.4 | |||
| 9 | limonene a | 1032 | 53 ± 5 | 9.8 ± 1.2 | 5.1 ± 1 | 4.3 ± 1 | 0.60 ± 0.04 | ||
| 10 | γ-terpinene a | 1064 | 5.9 ± 1.0 | 0.15 ± 0.02 | 9 ± 1 | ||||
| 11 | β-linalool a | 1101 | 0.21 ± 0.02 | 17 ± 2.6 | 0.96 ± 0.25 | 4 ± 1 | |||
| 12 | endo-borneol a | 1167 | 1.8 ± 0.7 | 1.01 ± 0.12 | |||||
| 13 | estragole a | 1198 | 1.5 ± 0.1 | 73 ± 6 | |||||
| 14 | citral a | 1240 | 3.8 ± 0.9 | 1.1 ± 0.08 | 43 ± 3 | ||||
| 15 | geraniol | 1254 | 2 ± 1 | ||||||
| 16 | anethole a | 1284 | 58.7 ± 3.9 | ||||||
| 17 | bornylacetate a | 1289 | 5.7 ± 1.3 | ||||||
| 18 | thymol a | 1290 | 47 ± 3 | ||||||
| 19 | geranyl aceate | 1385 | 0.87 ± 0.06 | 1.95 ± 0.10 | |||||
| 20 | caryophyllene a | 1415 | 0.136 ± 0.012 | 4.9 ± 0.9 | 0.43 ± 0.02 | 25 ± 1 | 2.2 ± 0.9 | ||
| 21 | α-bergamotene | 1430 | 0.212 ± 0.020 | 0.105 ± 0.012 | 3.2 ± 0.4 | 0.14 ± 0.01 | |||
| 22 | humulene | 1451 | 0.47 ± 0.02 | 0.237 ± 0.023 | 4.4 ± 0.9 | ||||
| 23 | α-curcumene a | 1481 | 15 ± 1 | ||||||
| 24 | zingiberene a | 1493 | 32.1 ± 1.8 | ||||||
| 25 | β-sesquiphellandrene a | 1521 | 11 ± 1 | ||||||
| 26 | caryophylleneoxyde | 1592 | 0.31 ± 0.05 | 1.68 ± 0.29 | 0.223 ± 0.012 | 2.2 ± 0.9 | 0.58 ± 0.03 |
| (a) | |||||||
|---|---|---|---|---|---|---|---|
| EO Concentration % (v/v) | LEO * | PEO * | FEO * | BEO * | MEO * | TEO * | GEO * |
| 40% | 1011 | 1011 | 107 | 1012 | 1011 | n.g. | 108 |
| 20% | 108 | 1011 | 107 | 1012 | 1011 | n.g. | 108 |
| 10% | 104 | 109 | 107 | 1011 | 1011 | n.g. | 108 |
| 5% | 104 | 108 | 107 | 108 | 108 | n.g. | 108 |
| 2.50% | 103 | 108 | 104 | 108 | 109 | n.g. | 107 |
| 1.25% | 101 | 104 | 104 | 108 | 109 | n.g. | 106 |
| (b) | |||||||
| EO Concentration % (v/v) | LEO* | PEO* | FEO* | BEO* | MEO* | TEO* | GEO* |
| 40% | 108 | 1010 | 109 | 107 | 109 | n.g. | 106 |
| 20% | 108 | 1010 | 107 | 107 | 107 | n.g. | 105 |
| 10% | 105 | 109 | 105 | 105 | 107 | n.g. | 105 |
| 5% | 105 | 108 | 105 | 105 | 107 | n.g. | 105 |
| 2.50% | 103 | 107 | 105 | 102 | 104 | n.g. | 104 |
| 1.25% | 101 | 106 | 104 | n.i. | 103 | n.g. | 101 |
| (c) | |||||||
| EO Concentration % (v/v) | LEO* | PEO* | FEO* | BEO* | MEO* | TEO* | GEO* |
| 40% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1011 |
| 20% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1011 |
| 10% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1011 |
| 5% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1013 |
| 2.50% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1014 |
| 1.25% | n.g. | 102 | n.i. | n.g. | n.g. | n.g. | 1014 |
| (d) | |||||||
| EO Concentration % (v/v) | LEO* | PEO* | FEO* | BEO* | MEO* | TEO* | GEO* |
| 40% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1012 |
| 20% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1012 |
| 10% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1012 |
| 5% | n.g. | n.g. | n.i. | n.g. | n.g. | n.g. | 1013 |
| 2.50% | 1012 | n.g. | n.i. | n.g. | n.g. | n.g. | 1014 |
| 1.25% | 1013 | 102 | n.i. | n.g. | n.g. | n.g. | 1014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus. Antibiotics 2022, 11, 979. https://doi.org/10.3390/antibiotics11070979
Galgano M, Capozza P, Pellegrini F, Cordisco M, Sposato A, Sblano S, Camero M, Lanave G, Fracchiolla G, Corrente M, et al. Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus. Antibiotics. 2022; 11(7):979. https://doi.org/10.3390/antibiotics11070979
Chicago/Turabian StyleGalgano, Michela, Paolo Capozza, Francesco Pellegrini, Marco Cordisco, Alessio Sposato, Sabina Sblano, Michele Camero, Gianvito Lanave, Giuseppe Fracchiolla, Marialaura Corrente, and et al. 2022. "Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus" Antibiotics 11, no. 7: 979. https://doi.org/10.3390/antibiotics11070979
APA StyleGalgano, M., Capozza, P., Pellegrini, F., Cordisco, M., Sposato, A., Sblano, S., Camero, M., Lanave, G., Fracchiolla, G., Corrente, M., Cirone, F., Trotta, A., Tempesta, M., Buonavoglia, D., & Pratelli, A. (2022). Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus. Antibiotics, 11(7), 979. https://doi.org/10.3390/antibiotics11070979










