Profiling Antibiotic Resistance in Acinetobacter calcoaceticus
Abstract
1. Introduction
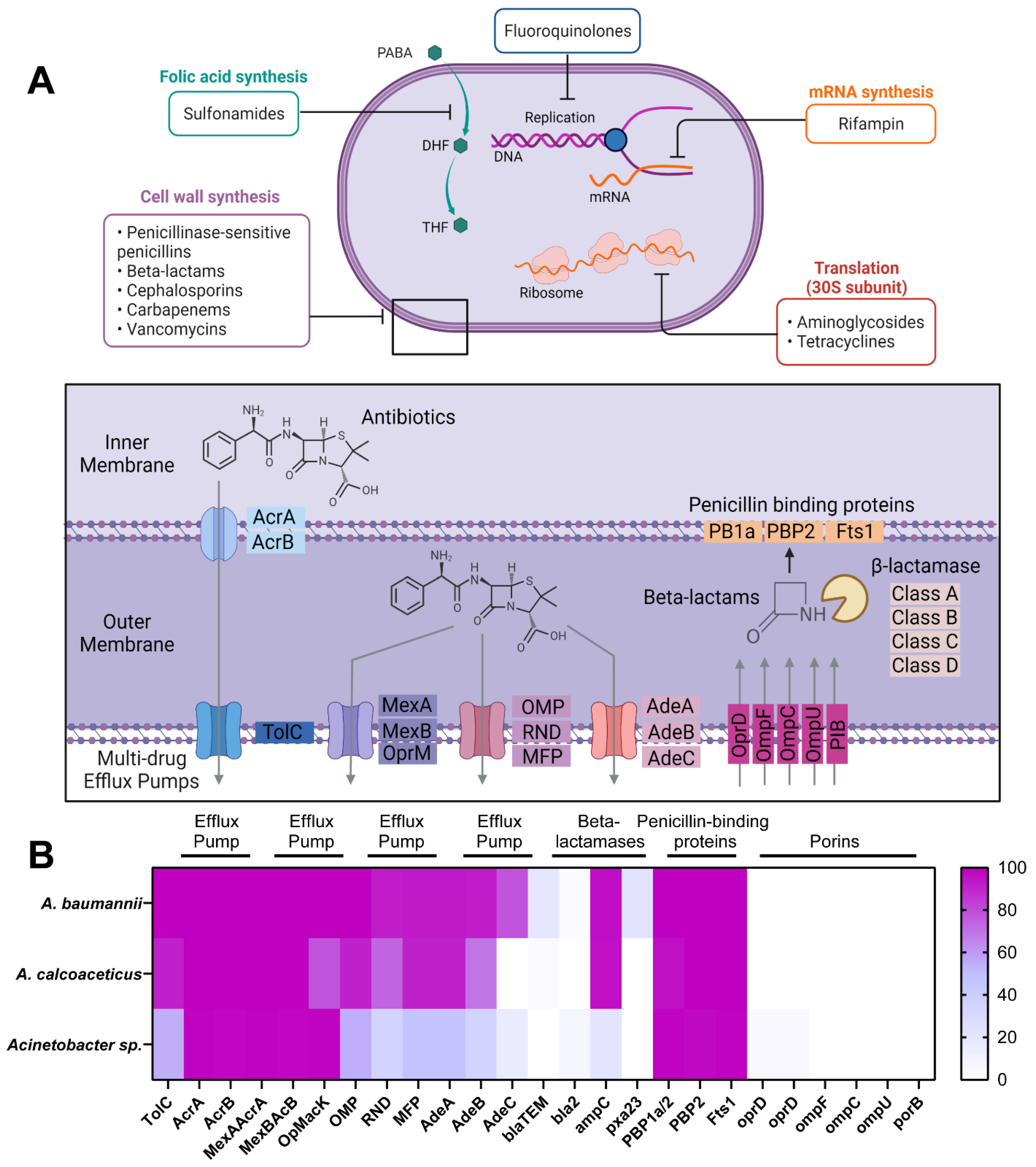
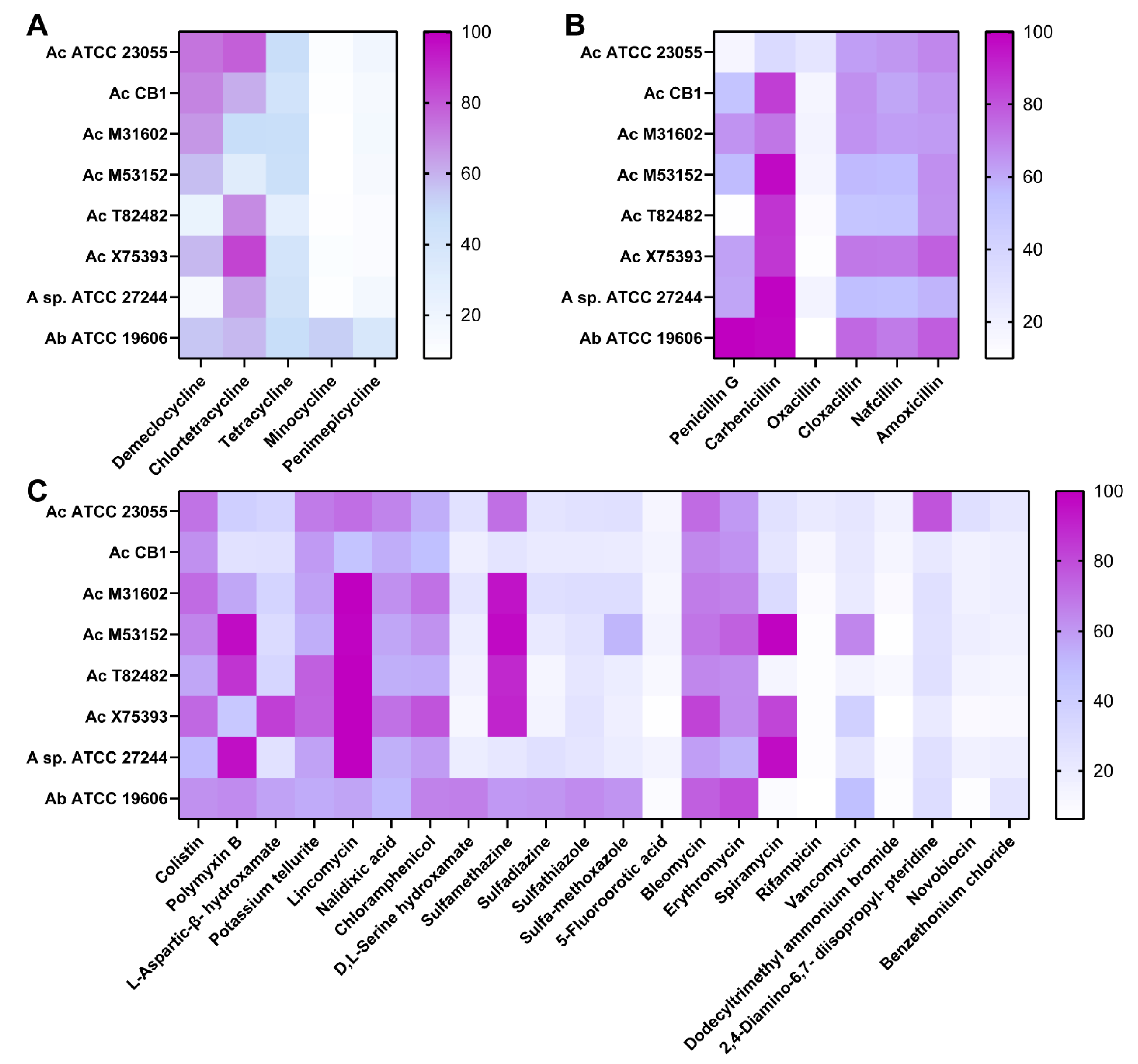
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Culture Conditions
5.2. Antibiotic Treatment
5.3. Computational Analysis
5.4. Statistics
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mancilla-Rojano, J.; Ochoa, S.A.; Reyes-Grajeda, J.P.; Flores, V.; Medina-Contreras, O.; Espinosa-Mazariego, K.; Parra-Ortega, I.; Rosa-Zamboni, D.D.L.; Castellanos-Cruz, M.C.; Arellano-Galindo, J.; et al. Molecular Epidemiology of Acinetobacter calcoaceticus-Acinetobacter baumannii Complex Isolated From Children at the Hospital Infantil de México Federico Gómez. Front. Microbiol. 2020, 11, 576673. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Vignon-Whaley, J.J.J.; Vaamonde, J.A.A.; Alonzo, L.A.P.; Reséndiz, A.R.; Álvarez, M.M.; López, E.N.V.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, R.; Edwards, J.R.; National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005, 41, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Blossom, D.B.; Srinivasan, A. Drug-Resistant Acinetobacter baumannii-calcoaceticus Complex: An Emerging Nosocomial Pathogen With Few Treatment Options. Infect. Dis. Clin. Pract. 2008, 16, e1–e94. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1334. [Google Scholar] [CrossRef]
- Visca, P.; Seifert, H.; Towner, K.J. Acinetobacter infection—An emerging threat to human health. IUBMB Life 2011, 63, 1048–1054. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, J.; Xu, Y.; Zhang, F.; Chen, Z. Comparison of clinical manifestations and antibiotic resistances among three genospecies of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. PLoS ONE 2018, 13, e0191748. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef]
- Amatya, R.; Acharya, D. Prevalence of Tigecycline Resistant Multidrug Resistant Acinetobacter calcoaceticus-Acinetobacter baumannii Complex from a Tertiary Care Hospital in Nepal. Nepal Med. Coll. J. 2015, 17, 83–86. [Google Scholar]
- Koczura, R.; Przyszlakowska, B.; Mokracka, J.; Kaznowski, A. Class 1 integrons and antibiotic resistance of clinical Acinetobacter calcoaceticus-baumannii complex in Poznań, Poland. Curr. Microbiol. 2014, 69, 258–262. [Google Scholar] [CrossRef][Green Version]
- Cerqueira, G.M.; Peleg, A.Y. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 2011, 63, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Gidabayda, J.G.; Philemon, R.; Abdallah, M.S.; Saajan, A.M.; Temu, T.; Kunjumu, I.; Mmbaga, B.T.; Msuya, L.J. Prevalence, Aetiology, and Antimicrobial Susceptibility Patterns of Urinary Tract Infection amongst Children Admitted at Kilimanjaro Christian Medical Centre, Moshi, Tanzania. East Afr. Health Res. J. 2017, 1, 53–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jara, L.M.; Pérez-Varela, M.; Corral, J.; Arch, M.; Cortés, P.; Bou, G.; Aranda, J.; Barbé, J. Novobiocin Inhibits the Antimicrobial Resistance Acquired through DNA Damage-Induced Mutagenesis in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 60, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Gwin, C.M.; Prakash, N.; Rigel, N.W. Identification of factors needed by a clinical isolate of Acinetobacter baumannii to resist antibacterial compounds. Bios 2019, 90, 149–157. [Google Scholar] [CrossRef]
- Ramirez, D.; Berry, L.; Domalaon, R.; Brizuela, M.; Schweizer, F. Dilipid Ultrashort Tetrabasic Peptidomimetics Potentiate Novobiocin and Rifampicin Against Multidrug-Resistant Gram-Negative Bacteria. ACS Infect. Dis. 2020, 6, 1413–1426. [Google Scholar] [CrossRef]
- Redder, P.; Linder, P. New range of vectors with a stringent 5-fluoroorotic acid-based counterselection system for generating mutants by allelic replacement in Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 3846–3854. [Google Scholar] [CrossRef]
- Bharathi, S.V.; Venkataramaiah, M.; Rajamohan, G. Genotypic and Phenotypic Characterization of Novel Sequence Types of Carbapenem-Resistant Acinetobacter baumannii, with Heterogeneous Resistance Determinants and Targeted Variations in Efflux Operons. Front. Microbiol. 2021, 12, 738371. [Google Scholar] [CrossRef]
- Rathod, P.K.; Khatri, A.; Hubbert, T.; Milhous, W.K. Selective activity of 5-fluoroorotic acid against Plasmodium falciparum In Vitro. Antimicrob. Agents Chemother. 1989, 33, 1090–1094. [Google Scholar] [CrossRef]
- Horvath, T.D.; Ihekweazu, F.D.; Haidacher, S.J.; Ruan, W.; Engevik, K.A.; Fultz, R.; Hoch, K.M.; Luna, R.A.; Oezguen, N.; Spinler, J.K.; et al. Bacteroides ovatus colonization influences the abundance of intestinal short chain fatty acids and neurotransmitters. iScience 2022, 25, 104158. [Google Scholar] [CrossRef]
- Glover, J.S.; Ticer, T.D.; Engevik, M.A. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci. Rep. 2022, 12, 8456. [Google Scholar] [CrossRef]
- Engevik, M.A.; Stripe, L.K.; Baatz, J.E.; Wagner, C.L.; Chetta, K.E. Identifying single-strain growth patterns of human gut microbes in response to preterm human milk and formula. Food Funct. 2022, 13, 5571–5589. [Google Scholar] [CrossRef] [PubMed]
- Fultz, R.; Ticer, T.; Glover, J.; Stripe, L.; Engevik, M.A. Select Streptococci Can Degrade Candida Mannan to Facilitate Growth. Appl. Environ. Microbiol. 2022, 88, e0223721. [Google Scholar] [CrossRef]
- Luck, B.; Horvath, T.D.; Engevik, K.A.; Ruan, W.; Haidacher, S.J.; Hoch, K.M.; Oezguen, N.; Spinler, J.K.; Haag, A.M.; Versalovic, J.; et al. Neurotransmitter Profiles Are Altered in the Gut and Brain of Mice Mono-Associated with Bifidobacterium dentium. Biomolecules 2021, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.S.; Browning, B.D.; Ticer, T.D.; Engevik, A.C.; Engevik, M.A. Acinetobacter calcoaceticus is Well Adapted to Withstand Intestinal Stressors and Modulate the Gut Epithelium. Front. Physiol. 2022, 13, 880024. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.; Hawkey, P.M.; Heritage, J.; Snelling, A.M. Description of Leeds Acinetobacter Medium, a new selective and differential medium for isolation of clinically important Acinetobacter spp., and comparison with Herellea agar and Holton’s agar. J. Clin. Microbiol. 1994, 32, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Zhang, G.; Mills, D.A.; Block, D.E. Development of chemically defined media supporting high-cell-density growth of lactococci, enterococci, and streptococci. Appl. Environ. Microbiol. 2009, 75, 1080–1087. [Google Scholar] [CrossRef]
- Chen, I.A.; Chu, K.; Palaniappan, K.; Ratner, A.; Huang, J.; Huntemann, M.; Hajek, P.; Ritter, S.; Varghese, N.; Seshadri, R.; et al. The IMG/M data management and analysis system v.6.0: New tools and advanced capabilities. Nucl. Acids Res. 2021, 49, D751–D763. [Google Scholar] [CrossRef] [PubMed]

| A. calcoaceticus | A. baumannii | Acinetobacter spp. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Antibiotics | Concentration | 23055 | CB1 | M31602 | M53152 | T82482 | X75393 | Category | 19606 | Category | 27244 | Category |
| Cephalosporins | Ceftazidime | 30 µg | 19 | 20 | 19 | 19 | 19 | 19 | I | 19 | I | 19 | I |
| Carbapenem | Imipenem | 10 µg | 28 | 30 | 29 | 29 | 29 | 29 | S | 29 | S | 29 | S |
| Carbapenem | Meropenem | 10 µg | 25 | 24 | 24 | 24 | 24 | 24 | I | 24 | I | 24 | I |
| Aminoglycoside | Amikacin | 30 µg | 20 | 21 | 20 | 20 | 20 | 20 | S | 20 | S | 20 | S |
| Aminoglycoside | Gentamicin | 10 µg | 24 | 22 | 23 | 22 | 23 | 22 | S | 23 | S | 22 | S |
| Aminoglycoside | Tobramycin | 10 µg | 21 | 21 | 21 | 21 | 21 | 21 | S | 21 | S | 21 | S |
| Tetracyclines | Tetracycline | 30 µg | 15 | 16 | 15 | 15 | 15 | 15 | I | 15 | I | 15 | I |
| Tetracyclines | Tigecycline | 15 µg | 23 | 24 | 23 | 23 | 23 | 23 | S | 23 | S | 23 | S |
| Macrolides | Erythromycin | 15 µg | 15 | 11 | 13 | 12 | 13 | 12 | R | 12 | R | 12 | R |
| Miscellaneous | Colistin Sulphate | 10 µg | 15 | 14 | 14 | 14 | 14 | 14 | R | 14 | R | 14 | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glover, J.S.; Ticer, T.D.; Engevik, M.A. Profiling Antibiotic Resistance in Acinetobacter calcoaceticus. Antibiotics 2022, 11, 978. https://doi.org/10.3390/antibiotics11070978
Glover JS, Ticer TD, Engevik MA. Profiling Antibiotic Resistance in Acinetobacter calcoaceticus. Antibiotics. 2022; 11(7):978. https://doi.org/10.3390/antibiotics11070978
Chicago/Turabian StyleGlover, Janiece S., Taylor D. Ticer, and Melinda A. Engevik. 2022. "Profiling Antibiotic Resistance in Acinetobacter calcoaceticus" Antibiotics 11, no. 7: 978. https://doi.org/10.3390/antibiotics11070978
APA StyleGlover, J. S., Ticer, T. D., & Engevik, M. A. (2022). Profiling Antibiotic Resistance in Acinetobacter calcoaceticus. Antibiotics, 11(7), 978. https://doi.org/10.3390/antibiotics11070978









