Abstract
Background: Fixed-dose combination (FDC) antibiotics can be clinically inappropriate and are concerning with regards to antimicrobial resistance, with little usage data available in low- and middle-income countries. Methods: Based on retrospective data from the Center for Antibacterial Surveillance, we investigated the consumption of FDC antibiotics in hospital inpatient settings in China from 1 January 2013 to 31 December 2019. The metric for assessing antibiotic consumption was the number of daily defined doses per 100 bed days (DDD/100BDs). FDC antibiotics were classified according to their composition and the Access, Watch, Reserve (AWaRe) classification of the World Health Organization. Results: A total of 24 FDC antibiotics were identified, the consumption of which increased sharply from 8.5 DDD/100BDs in 2013 to 10.2 DDD/100BDs in 2019 (p < 0.05) despite the reduction in the total antibiotic consumption in these hospitals. The increase was mainly driven by FDC antibiotics in the Not Recommended group of the AWaRe classification, whose consumption accounted for 63.0% (6.4 DDD/100BDs) of the overall FDC antibiotic consumption in 2019, while the consumption of FDC antibiotics in the Access group only accounted for 13.5% (1.4 DDD/100BDs). Conclusion: FDC antibiotic consumption significantly increased during the study period and accounted for a substantial proportion of all systemic antibiotic usage in hospitals in China. FDC antibiotics in the Not Recommended group were most frequently consumed, which raises concerns about the appropriateness of FDC antibiotic use.
1. Introduction
Rising antimicrobial resistance (AMR) is a global health crisis, with serious consequences for morbidity, mortality, and healthcare costs [1,2,3,4]. However, the development of new antibiotics, which can take a decade or longer, can hardly keep pace with the emergence and spread of AMR, making the economic use of currently available antibiotics necessary [5,6,7,8]. Fixed-dose combination (FDC) medications, which contain two or more active substances within a single dosage form, represent a strategy to circumvent much of the early phases of drug development and reduce the lengthy timeline [7,9]. FDCs can improve treatment response compared to monotherapy through synergistic mechanisms of action (such as sulfamethoxazole/trimethoprim) or by improving the medication adherence of patients [10]. FDCs are well-established in treating conditions such as tuberculosis, malaria, and HIV [11,12]. Recent research has indicated that the sensible use of pharmacodynamically synergistic antibiotics through FDC may delay or even prevent the emergence of AMR [13]. One of the prominent groups of these FDC antibiotics with broad-spectrum activity is the combination of β-lactam and β-lactamase inhibitor (BL-BLI), which is regarded as a potentially effective strategy for infections caused by drug-resistant Gram-negative bacilli [14,15].
However, FDC antibiotics can be clinically inappropriate, as reported in some countries [16,17,18]. Concerns have been raised due to a lack of efficacy, increasing toxicity, and their potential impact on resistance [19]. High consumption of FDC antibiotics was observed in some low- and middle-income countries (LMICs) where FDCs accounted for a substantial proportion of the total antibiotic consumption [11]. In the past two decades, China is among the countries consuming the most antibiotics in the world [20]. Hence, China has committed to confining AMR through a series of policies and made considerable progress in limiting its total antibiotic consumption [21,22]. Nonetheless, a recent study showed that the country remained the second largest consumer of FDC antibiotics among 75 countries in 2015 as measured by sales data [11], but the data on the trends and patterns of FDC consumption were limited. In this study, we aim to describe the trends and patterns of FDC antibiotic consumption in hospitals in China over seven years. The rest of this paper is organized as follows. The methodology of the study is presented in Section 2. Then, the detailed results of FDC antibiotic consumption are proposed in Section 3. In Section 4, we discuss the findings of the study. The conclusion of the study is presented in Section 5.
2. Materials and Methods
2.1. Study Design
This is an observational study investigating the consumption of FDC antibiotics in hospital inpatient settings in China from 1 January 2013 to 31 December 2019.
2.2. Data Source
Data were retrieved from the Center for Antibacterial Surveillance (CAS), the largest nationwide surveillance database collecting data on antibacterial use from 31 (out of 34) provinces in China (excluding Hong Kong, Macao, and Taiwan). All secondary and tertiary hospitals that reported data on antibacterial use to the CAS database during the study period were included in the analyses. The specific numbers of hospitals included each year varied between 1630 in 2013 and 2486 in 2019. Hospital characteristics are shown in Table A1. The database collected quarterly data on inpatient antibacterial use. The CAS Quality Control Committee audited the data quality to ensure the data met the relevant requirements.
2.3. Data Collection
We extracted data on the annualized consumption of antibiotics and aggregated data at the level of active substances. The CAS database contained information about the following variables: region, hospital name, year, drug specification, unit, active substance designation, the quantity of consumption, and bed days.
We defined FDC antibiotics as medications consisting of two active substances, in which at least one was an antibiotic under the Anatomical Therapeutic Chemical (ATC) classification J01, as recommended by the World Health Organization (WHO) Collaborating Centre for Drug Statistic Methodology [23]. FDC antibiotics were then classified into antibiotic plus antibiotic adjuvant (A and A) or dual antibiotics (DA) according to the composition for further analysis [7,11,16].
2.4. Measurement
2.4.1. Indicators
Antibiotic consumption was expressed as the number of defined daily doses per 100 bed days (DDD/100BDs) based on the WHO Collaborating Center for Drug Statistic Methodology [24,25]. For drugs that could not be coded according to the ATC system, the dosage regimen recommended in the manufacturers’ instructions was used as an alternative to gauge their DDD, as approved by the Chinese Food and Drug Administration [26].
We calculated the compound annual growth rate (CAGR) [27] of the consumption of FDC antibiotics to calculate a comparable metric across time.
C2019: Total FDC antibiotic consumption for the year 2019 (expressed as DDD/100BDs).
C2013: Total FDC antibiotic consumption for the year 2013 (expressed as DDD/100BDs).
2.4.2. WHO AWaRe Classification
FDC antibiotics were assessed based on the 2021 revision of the WHO Access, Watch, Reserve (AWaRe) classification [28]. The AWaRe classification was established based on the strength of the antibiotics and the potential impacts on antimicrobial resistance. Antibiotics in the Access group were the first- or second-line treatments for common infections and should be widely accessible. Antibiotics in the Watch group should only be used for a limited group of well-defined syndromes and under close surveillance. Antibiotics in the Reserve group should be primarily referred to as the last resort to treat infections caused by multi- or extensively drug-resistant bacteria. The fourth group, “Not Recommended”, consisted of antibiotic combinations whose use may negatively impact AMR and patient safety. In our study, we added a fifth group, “Not Included, to refer to antibiotics not included in the WHO AWaRe classification, but which were used in China.
2.5. Data Analysis
We assessed the consumption of FDC antibiotics in hospitals at a national level and comparatively analyzed the total antibiotic consumption to better understand the trend change. The consumption of FDC antibiotics at a provincial level was also analyzed to identify potential regional differences. Measures of relative consumption, expressed as a proportion of the total consumption of FDC antibiotics, were calculated based on FDC composition as well as the AWaRe classification for further analysis. Trends and ranking of the most frequently used FDC antibiotics were also assessed.
We computed linear regressions to assess the trends in the consumption of FDC antibiotics. The dependent variable was the consumption of FDC antibiotics, and the independent variable was time. Data were managed and analyzed in Microsoft Excel 2019 (Microsoft, Washington, DC, USA) and STATA 15.1 (StataCorp LLC, College Station, TX, USA). Figures were plotted using Origin (Pro), Version 2020b (OriginLab Corporation, Northampton, MA, USA). A difference of p < 0.05 was considered indicative of statistical significance.
3. Results
A total of 24 FDC antibiotic agents were identified in the CAS database from 1 January 2013 to 31 December 2019, including 7 DA agents and 17 A and A agents. Among these, four were not included in the AWaRe classification, fourteen contained agents categorized as Not Recommended, three contained agents in the Access group, two contained agents in the Watch group, and one contained agents in the Reserve group (Table A2).
3.1. Total Consumption of FDC Antibiotics
From 2013 to 2019, the consumption of FDC antibiotics in hospital inpatient settings in China significantly increased from 8.5 DDD/100BDs recorded in 1630 hospitals in 2013 to 10.2 DDD/100BDs recorded in 2486 hospitals in 2019 (p < 0.05), with a CAGR of 3.0%. Meanwhile, the contribution of FDC antibiotics to the total consumption of antibiotics in these hospitals increased by 6.2% (p < 0.05). However, a contrasting trend was found in the total consumption of antibiotics in hospitals, which showed a significant decrease from 48.8 DDD/100BDs in 2013 to 43.0 DDD/100BDs in 2019 (Figure 1, Table A2).
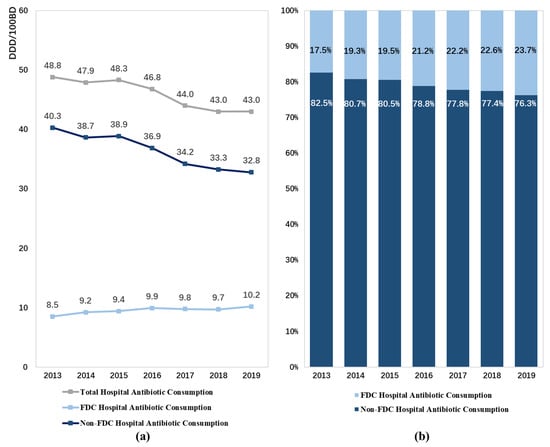
Figure 1.
Patterns of fixed-dose combination antibiotic consumption in hospitals in China from 2013 to 2019: (a) expressed as the number of DDD/100 bed days, (b) expressed as percentage proportions. Abbreviation: FDC, fixed-dose combination.
3.2. Regional Distribution of FDC Antibiotic Consumption
Figure 2a presents the consumption of FDC antibiotics, stratified by region, in China in 2019. Xizang, Guangxi, and Jiangxi were the top three largest consumers of FDC antibiotics in 2019, with a consumption of 14.4 DDD/100BDs, 13.1 DDD/100BDs, and 12.8 DDD/100BDs, respectively. The consumption of FDC antibiotics substantially increased in most regions (28 provinces) across China, while only three provinces (Shanghai, Zhejiang, and Hunan) demonstrated a moderately decreasing trend between 2013 and 2019 (Figure 2b).
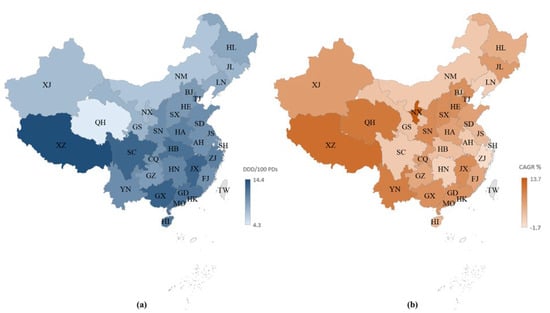
Figure 2.
Fixed-dose combination antibiotic consumption in hospitals in China: (a) expressed as DDD/100 bed days in 2019, (b) expressed as compound annual growth rates between 2013 and 2019. Abbreviations: BJ, Beijing; SH, Shanghai; TJ, Tianjin; CQ, Chongqing; HE, Hebi; SX, Shanxi; NM, Inner Mongolia; LN, Liaoning; JL, Jilin; HL, Heilongjiang; JS, Jiangsu; ZJ, Zhejiang; AH, Anhui; FJ, Fujian; JX, Jiangxi; SD, Shandong; HA, Henan; HN, Hunan; GD, Guangdong; GX, Guangxi; HI, Hainan; SC, Sichuan; GZ, Guizhou; YN, Yunnan; XZ, Tibet; SN, Shaanxi; GS, Gansu; QH, Qinghai; NX, Ningxia; XJ, Xinjiang.
3.3. The Consumption of FDC Antibiotics in Different Compositions and AWaRe Classification
Of the two types of FDC antibiotics, A and A agents were more frequently consumed, contributing 10.0 DDD/100BDs (98.4%) to the total consumption of FDC antibiotics in 2019. Both DA and A and A agents demonstrated increasing trends in consumption over time (Figure 3a).
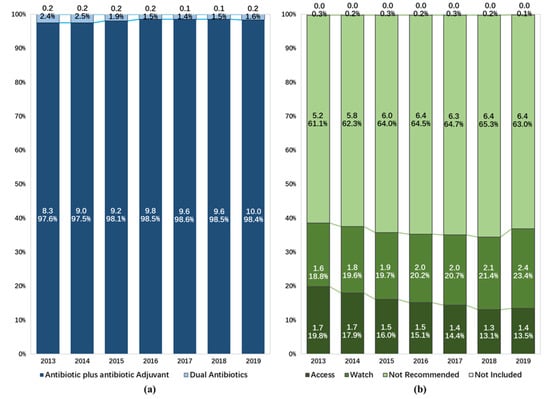
Figure 3.
Composition of fixed-dose combination antibiotic consumption in hospitals in China from 2013 to 2019: (a) expressed as different types of chemical composition, (b) expressed as the Access, Watch, Reserve classification of the World Health Organization.
The proportion of the consumption of FDC antibiotics not included in the 2021 WHO AWaRe classification dropped from 0.3% to 0.1% during the study period. When assessing FDC antibiotics included in the AWaRe classification, agents in the Not Recommended group (6.4 DDD/100BDs) accounted for 63.0% of the consumption of all FDC antibiotics in 2019. The consumption of FDC agents in the Access group and the Watch group were 1.4 and 2.4 DDD/100BDs, accounting for 13.5% and 23.4%, respectively. There was a reduction in the proportion of the consumption of FDC antibiotics in the Access group (from 19.8% to 13.5%), while increasing trends were observed in the consumption of FDC antibiotics in the Not Recommended group (from 61.1% to 63.0%) and the Watch group (from 18.8% to 23.4%) (Figure 3b, Table A2).
3.4. The Consumption of Most Frequently Used FDC Antibiotics
Among the 24 FDC antibiotics identified, the consumption of only four FDCs (cefoperazone/sulbactam, piperacillin/tazobactam, mezlocillin/sulbactam, and cefotaxime/sulbactam) significantly increased from 5.2 DDD/100BDs in 2013 to 6.5 DDD/100BDs in 2019 and contributed to 63.4% of the total consumption of FDC antibiotics in hospital inpatient settings in China in 2019 (Table A2).
The seven most frequently consumed FDC antibiotics were cefoperazone/sulbactam, piperacillin/tazobactam, amoxicillin/clavulanic acid, mezlocillin/sulbactam, cefoperazone/tazobactam, piperacillin/sulbactam, and imipenem/cilastatin in both 2013 and 2019. These FDC antibiotics accounted for 90.6% of the total consumption of FDC antibiotics in China in 2019. Among these frequently consumed FDC antibiotics, significant increasing trends were observed in cefoperazone/sulbactam (p < 0.05), piperacillin/tazobactam (p < 0.05), and mezlocillin/sulbactam (p < 0.05), while amoxicillin/clavulanic acid was the only antibiotic that experienced a significant decrease during the study period (p < 0.05) (Table A2).
4. Discussion
To our knowledge, this study is the first to estimate the consumption of FDC antibiotics over time at a national level using surveillance data in China. In contrast to the reduction in the total consumption of antibiotics in hospital inpatient settings in China, the consumption of FDC antibiotics significantly increased between 1 January 2013 and 31 December 2019.
Overall, the consumption of FDC antibiotics accounted for a notable proportion (23.7% in 2019) of all systemic antibiotics in hospitals in China. Several studies estimated the FDC antibiotic consumption using sales data and showed varying findings on the FDC proportion. Our finding is lower than the proportion in India (33.8%) [16] but slightly higher than the proportion shown in a study conducted in eight Latin American countries (21.0%) [18] and a multinational study involving 75 countries (22.5%) [11]. Although these results cannot be directly compared due to the discrepancy between sales data and usage data, they, in some way, provided a reference for how frequently FDC antibiotics are used in China. The considerable proportion of FDC antibiotics shown in our study is mainly attributable to A and A agents, most of which were comprised of β-lactam and β-lactamase inhibitor (BL-BLI) (16/17). The potential reason for the extensive use of BL-BLI may be driven by increasing incidences of extended-spectrum β-lactamases (ESBLs) producing bacteria in hospital settings in China [29,30,31]. To confine the resistance of β-lactamase, medical institutions prefer the FDC of β-lactam antibiotics and β-lactamase inhibitors to improve their antimicrobial activity [32,33]. However, indiscriminate and widespread use of such combinations may significantly compromise patient care and trigger large-scale resistance development [34,35]. Therefore, further research is needed to investigate the safety and efficacy profiles of these BL-BLI combinations.
Previous studies raised concerns about the widespread use of unorthodox BL-BLI in India and about China flouting antimicrobial stewardship (ASP) and compromising patient care [34]. Unorthodox combinations may compromise clinical outcomes and potentially contribute to resistance development [35]. These FDC antibiotics have been introduced into clinical practice without mandatory drug development studies involving pharmacokinetic/pharmacodynamic, safety, and efficacy assessments being undertaken [36]. The Indian Government has already taken a series of initiatives to deal with this problem [37]. Despite the increasing concern of AMR, the usage of FDC antibiotics in China is still largely overlooked. Although in vitro studies were conducted [38,39,40], evidence on the safety and/or efficacy of FDC antibiotics is far from adequate [41,42] to support these unorthodox combinations that are categorized as Not Recommended by the AWaRe classification. Rather than imitating the composition of BL-BLIs through rigorous drug development, sulbactam or tazobactam has been arbitrarily combined with cephalosporins by indigenous drug manufacturers in China [34]. These unorthodox BL-BLIs may cause uncertain clinical outcomes or even clinical failure, resistance development, and drug toxicity [11,43]. Thus, more studies are required to evaluate their safety, efficacy, and effectiveness to promote the appropriate use of antibiotics.
In the past two decades, the Chinese Government has made various attempts to confine AMR through policies and measures, including limiting the usage of antibiotics [44]. However, the implementation of ASP overlooked the inappropriate use of FDC antibiotics [34]. A core element of the ASP, the Antibiotic Formulary Restriction (AFR), categorized antimicrobials into three classes (Non-Restricted, Restricted, and Highly Restricted) [21]. It was developed at a provincial level instead of a national level to accommodate for regional differences in the prevalence of bacteria resistance [45]. Our study finds that among the seven most frequently consumed FDC antibiotics that contributed 90% to the total consumption of FDC antibiotics in China, cefoperazone/sulbactam, mezlocillin/sulbactam, cefoperazone/tazobactam, and piperacillin/sulbactam were classified as Restricted in most provinces, though they were classified as Not Recommended according to the AWaRe classification. This discrepancy implies that these medications, despite having limited evidence on safety and efficacy profiles and thus, need to be used with caution, can be easily accessed in China. In addition, these FDC antibiotics were classified into levels of higher restriction in provinces such as Shanghai and Zhejiang, where the consumption of FDC antibiotics showed a negative CAGR during the study period. This indicates that in regions with a higher level of development, more medical resources, and a higher level of management, FDC antibiotics were used more prudently compared to less developed regions.
As globalization allows resistant micro-organisms to spread rapidly to distant countries and continents, the regulation of FDC antimicrobials in China has implications beyond the country itself [37]. A multisectoral approach involving all stakeholders is needed to curb the potentially inappropriate use of FDC antibiotics, and regulators should strengthen the enforcement of relevant regulations. The industry should also act responsibly, ensuring that the development of FDC antibiotics adheres to scientific standards and generates robust efficacy and safety data. Moreover, physicians should be better educated through formal education or training sessions about the public health implications of the inappropriate use of FDC antibiotics. Pharmacists can also help to address the inappropriate use of FDC antibiotics through their professional knowledge of drugs.
Limitation
This study has several limitations. Firstly, the reporting of hospitals to the CAS database is on a voluntary basis, and each year the number of hospitals included in the CAS database varies, which might have introduced selection bias. However, since these hospitals included mostly tertiary hospitals and spanned most provinces in China, our study sample remained representative to a certain extent. Secondly, the CAS database mainly covered secondary and tertiary hospitals across China with a paucity of data from primary healthcare settings. As primary healthcare facilities generally had very limited inpatient capacity, our results could still reflect patterns in the consumption of FDC antibiotics in inpatient settings in China. Thirdly, only aggregated data, instead of hospital-level data, were available, and details regarding quality control were inaccessible. However, the CAS database conducts internal quality control measures. The National Health Commission also carried out training programs to improve data collection, which could ensure the quality of the data. Fourthly, due to limited data availability, we only analyzed the use of FDC antibiotics in inpatient settings. This might lead to an underestimation of the consumption of some antibiotics, such as amoxicillin–clavulanic acid and sulfamethoxazole–trimethoprim. However, as most FDC antibiotics are restricted in outpatient settings according to antibiotic formulary restriction management in China [46], our results therefore remain considerably representative of the trends and patterns of FDC antibiotics used in China.
5. Conclusions
Our study finds that the consumption of FDC antibiotics represented a substantial proportion of all systemic antibiotics in hospital inpatient settings in China, with a significant increasing trend despite the reduction in the total consumption of antibiotics. FDC antibiotics in the Not Recommended group were most frequently consumed, which might indicate potentially inappropriate use of these medications. More attention should be paid to establishing specialized management approaches promoting the appropriate use of FDC antibiotics to better confine AMR nationally and internationally.
Author Contributions
Conceptualization, H.W., L.H. and X.G.; Data curation, L.H., Y.Z., Y.Y., K.D., Q.Y., X.Y. and Z.C.; Formal analysis, L.H.; Funding acquisition, L.S.; Investigation, H.W., Y.Z., Y.D. and B.Z.; Methodology, L.H. and Y.Z.; Project administration, B.Z., Y.D., X.G. and L.S.; Resources, Y.D., Q.Y., X.Y., Z.C. and B.Z.; Software, L.H.; Supervision, X.G. and L.S.; Validation, X.G.; Visualization, L.H. and Y.Z.; Writing—original draft, H.W. and L.H.; Writing—review and editing, H.W. and X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81861138048 and No. 71774005) (http://www.nsfc.gov.cn/ [Accessed 15 July 2022]). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study used secondary aggregated data on antibiotic consumption. As such, ethical approval was not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
All authors declare that they have no conflict of interest.
Appendix A

Table A1.
A Characteristics of the sample hospitals from China Center for Antibacterial Surveillance, 2013-2019.
Table A1.
A Characteristics of the sample hospitals from China Center for Antibacterial Surveillance, 2013-2019.
| Provinces | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | S | A | T | S | A | T | S | A | T | S | A | T | S | A | T | S | A | T | S | A | |
| C | 237 | 95 | 332 | 253 | 104 | 357 | 272 | 113 | 385 | 270 | 112 | 382 | 274 | 113 | 387 | 284 | 123 | 407 | 286 | 135 | 421 |
| AH | 40 | 7 | 47 | 42 | 9 | 51 | 43 | 11 | 54 | 44 | 11 | 55 | 48 | 14 | 62 | 52 | 18 | 70 | 56 | 22 | 78 |
| HA | 64 | 44 | 108 | 66 | 48 | 114 | 67 | 54 | 121 | 65 | 53 | 118 | 66 | 50 | 116 | 68 | 52 | 120 | 67 | 54 | 121 |
| HB | 40 | 4 | 44 | 43 | 4 | 47 | 49 | 3 | 52 | 50 | 5 | 55 | 48 | 5 | 53 | 49 | 3 | 52 | 48 | 4 | 52 |
| HN | 32 | 19 | 51 | 34 | 19 | 53 | 34 | 19 | 53 | 34 | 17 | 51 | 33 | 17 | 50 | 32 | 13 | 45 | 30 | 14 | 44 |
| JX | 25 | 7 | 32 | 33 | 10 | 43 | 42 | 12 | 54 | 40 | 11 | 51 | 41 | 13 | 54 | 48 | 22 | 70 | 49 | 25 | 74 |
| SX | 36 | 14 | 50 | 35 | 14 | 49 | 37 | 14 | 51 | 37 | 15 | 52 | 38 | 14 | 52 | 35 | 15 | 50 | 36 | 16 | 52 |
| E | 449 | 132 | 581 | 481 | 137 | 618 | 505 | 150 | 655 | 533 | 182 | 715 | 545 | 183 | 728 | 591 | 245 | 836 | 612 | 289 | 901 |
| BJ | 45 | 9 | 54 | 47 | 8 | 55 | 46 | 8 | 54 | 45 | 8 | 53 | 39 | 8 | 47 | 38 | 7 | 45 | 36 | 7 | 43 |
| FJ | 39 | 15 | 54 | 38 | 14 | 52 | 41 | 16 | 57 | 42 | 18 | 60 | 45 | 18 | 63 | 53 | 27 | 80 | 54 | 28 | 82 |
| GD | 70 | 14 | 84 | 77 | 15 | 92 | 80 | 16 | 96 | 92 | 16 | 108 | 97 | 23 | 120 | 126 | 70 | 196 | 148 | 114 | 262 |
| HI | 14 | 16 | 30 | 15 | 16 | 31 | 16 | 17 | 33 | 16 | 18 | 34 | 17 | 17 | 34 | 19 | 17 | 36 | 19 | 17 | 36 |
| HE | 22 | 4 | 26 | 30 | 7 | 37 | 31 | 8 | 39 | 36 | 9 | 45 | 37 | 7 | 44 | 39 | 8 | 47 | 41 | 9 | 50 |
| JS | 85 | 23 | 108 | 82 | 23 | 105 | 90 | 27 | 117 | 94 | 30 | 124 | 95 | 29 | 124 | 93 | 29 | 122 | 91 | 27 | 118 |
| SD | 61 | 9 | 70 | 75 | 11 | 86 | 76 | 10 | 86 | 78 | 15 | 93 | 79 | 15 | 94 | 81 | 16 | 97 | 80 | 15 | 95 |
| SH | 41 | 25 | 66 | 39 | 25 | 64 | 45 | 30 | 75 | 46 | 48 | 94 | 47 | 46 | 93 | 46 | 48 | 94 | 46 | 46 | 92 |
| TJ | 23 | 8 | 31 | 27 | 8 | 35 | 28 | 8 | 36 | 28 | 9 | 37 | 32 | 8 | 40 | 34 | 9 | 43 | 33 | 9 | 42 |
| ZJ | 49 | 9 | 58 | 51 | 10 | 61 | 52 | 10 | 62 | 56 | 11 | 67 | 57 | 12 | 69 | 62 | 14 | 76 | 64 | 17 | 81 |
| HL | 59 | 1 | 60 | 60 | 2 | 62 | 67 | 2 | 69 | 67 | 2 | 69 | 69 | 2 | 71 | 68 | 2 | 70 | 67 | 2 | 69 |
| JL | 22 | 4 | 26 | 23 | 4 | 27 | 22 | 5 | 27 | 23 | 4 | 27 | 22 | 5 | 27 | 21 | 4 | 25 | 20 | 4 | 24 |
| LN | 42 | 14 | 56 | 42 | 13 | 55 | 48 | 16 | 64 | 67 | 32 | 99 | 72 | 41 | 113 | 88 | 70 | 158 | 94 | 71 | 165 |
| W | 327 | 248 | 575 | 338 | 268 | 606 | 367 | 303 | 670 | 373 | 324 | 697 | 389 | 371 | 760 | 429 | 431 | 860 | 445 | 461 | 906 |
| CQ | 20 | 13 | 33 | 21 | 13 | 34 | 23 | 17 | 40 | 23 | 23 | 46 | 27 | 26 | 53 | 28 | 29 | 57 | 29 | 29 | 58 |
| GS | 30 | 28 | 58 | 30 | 34 | 64 | 31 | 35 | 66 | 29 | 35 | 64 | 32 | 41 | 73 | 35 | 43 | 78 | 36 | 50 | 86 |
| GX | 43 | 16 | 59 | 43 | 17 | 60 | 43 | 17 | 60 | 44 | 18 | 62 | 48 | 27 | 75 | 50 | 27 | 77 | 52 | 29 | 81 |
| GZ | 23 | 8 | 31 | 27 | 8 | 35 | 33 | 18 | 51 | 38 | 27 | 65 | 40 | 35 | 75 | 41 | 53 | 94 | 43 | 61 | 104 |
| NM | 28 | 5 | 33 | 26 | 7 | 33 | 31 | 9 | 40 | 32 | 10 | 42 | 33 | 10 | 43 | 33 | 9 | 42 | 33 | 9 | 42 |
| NX | 15 | 42 | 57 | 15 | 40 | 55 | 15 | 39 | 54 | 15 | 39 | 54 | 15 | 39 | 54 | 15 | 39 | 54 | 14 | 38 | 52 |
| QH | 17 | 12 | 29 | 19 | 17 | 36 | 20 | 23 | 43 | 20 | 24 | 44 | 19 | 25 | 44 | 19 | 29 | 48 | 19 | 26 | 45 |
| SN | 42 | 58 | 100 | 42 | 60 | 102 | 46 | 69 | 115 | 45 | 67 | 112 | 45 | 70 | 115 | 48 | 65 | 113 | 50 | 59 | 109 |
| SC | 47 | 3 | 50 | 50 | 3 | 53 | 54 | 3 | 57 | 54 | 4 | 58 | 54 | 3 | 57 | 82 | 31 | 113 | 92 | 43 | 135 |
| XJ | 32 | 26 | 58 | 33 | 30 | 63 | 34 | 32 | 66 | 35 | 37 | 72 | 35 | 48 | 83 | 35 | 50 | 85 | 35 | 55 | 90 |
| XZ | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||
| YN | 30 | 37 | 67 | 32 | 39 | 71 | 37 | 41 | 78 | 38 | 40 | 78 | 40 | 47 | 87 | 42 | 56 | 98 | 41 | 62 | 103 |
| Total | 1136 | 494 | 1630 | 1197 | 528 | 1725 | 1281 | 589 | 1870 | 1333 | 656 | 1989 | 1371 | 715 | 2086 | 1481 | 875 | 2356 | 1524 | 962 | 2486 |
A, All; T, tertiary; S, secondary; C, Central; E, Eastern; W, Western; BJ, Beijing; SH, Shanghai; TJ, Tianjin; CQ, Chongqing; HE, Hebei; SX, Shanxi; NM, Inner Mongolia; LN, Liaoning; JL, Jilin; HL, Heilongjiang; JS, Jiangsu; ZJ, Zhejiang; AH, Anhui; FJ, Fujian; JX, Jiangxi; SD, Shandong; HA, Henan; HB, Hubei; HN, Hunan; GD, Guangdong; GX, Guangxi; HI, Hainan; SC, Sichuan; GZ, Guizhou; YN, Yunnan; XZ, Tibet; SN, Shaanxi; GS, Gansu; QH, Qinghai; NX, Ningxia; XJ, Xinjiang.

Table A2.
Individual Fixed-Dose Combination Antibiotic Consumption, 2013-2019, DDD/100 bed days.
Table A2.
Individual Fixed-Dose Combination Antibiotic Consumption, 2013-2019, DDD/100 bed days.
| FDC | ATC Code | Composition | AWaRe | Ranking in 2013 | Ranking in 2019 | Cum. in 2019, % | Trend | CAGR, % | Trends in Antibiotic Use | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All FDC Antibiotics | - | - | - | - | - | - | ↑* | 3.0 |  | 8.521 | 9.246 | 9.428 | 9.926 | 9.785 | 9.729 | 10.201 |
| Cefoperazone/Sulbactam | J01DD62 | A and A | NR | 1 | 1 | 33.0 | ↑* | 3.9 |  | 2.672 | 2.959 | 2.876 | 3.089 | 3.249 | 3.278 | 3.367 |
| Piperacillin/Tazobactam | J01CR05 | A and A | W | 3 | 2 | 50.9 | ↑* | 7.7 |  | 1.172 | 1.337 | 1.295 | 1.393 | 1.446 | 1.543 | 1.824 |
| Amoxicillin/Clavulanic Acid | J01CR02 | A and A | A | 2 | 3 | 62.9 | ↓* | −3.2 |  | 1.491 | 1.441 | 1.357 | 1.342 | 1.239 | 1.146 | 1.227 |
| Mezlocillin/Sulbactam | - | A and A | NR | 4 | 4 | 72.2 | ↑* | 5.7 |  | 0.682 | 0.781 | 0.886 | 1.052 | 0.993 | 1.014 | 0.951 |
| Piperacillin/Sulbactam | J01CR05 | A and A | NR | 5 | 5 | 78.7 | - | 2.5 |  | 0.565 | 0.648 | 0.729 | 0.693 | 0.644 | 0.640 | 0.654 |
| Cefoperazone/Tazobactam | J01DD62 | A and A | NR | 6 | 6 | 85.0 | - | 4.1 |  | 0.510 | 0.567 | 0.649 | 0.674 | 0.668 | 0.632 | 0.648 |
| Imipenem/Cilastatin | J01DH51 | A and A | W | 7 | 7 | 90.6 | - | 4.8 |  | 0.429 | 0.472 | 0.563 | 0.609 | 0.579 | 0.537 | 0.567 |
| Cefotaxime/Sulbactam | J01DD51 | A and A | NR | 11 | 8 | 93.8 | ↑* | 18.2 |  | 0.120 | 0.148 | 0.257 | 0.288 | 0.271 | 0.287 | 0.327 |
| Ceftriaxone/Tazobactam | J01DD63 | A and A | NR | 9 | 9 | 96.4 | - | 2.0 |  | 0.236 | 0.276 | 0.284 | 0.312 | 0.259 | 0.265 | 0.266 |
| Amoxicillin/Sulbactam | J01CR02 | A and A | NR | 8 | 10 | 97.8 | ↓** | −13.7 |  | 0.342 | 0.310 | 0.287 | 0.240 | 0.197 | 0.173 | 0.141 |
| Sulfamethoxazole/Trimethoprim | J01EE01 | DA | A | 10 | 11 | 98.9 | ↓* | −3.7 |  | 0.140 | 0.178 | 0.125 | 0.120 | 0.101 | 0.089 | 0.112 |
| Amoxicillin/Flucloxacillin | J01RA01 | DA | NR | 13 | 12 | 99.3 | - | 1.9 |  | 0.043 | 0.040 | 0.038 | 0.021 | 0.036 | 0.049 | 0.048 |
| Ampicillin/Sulbactam | J01CR01 | A and A | A | 12 | 13 | 99.7 | - | −8.5 |  | 0.057 | 0.039 | 0.029 | 0.040 | 0.065 | 0.037 | 0.033 |
| Ticarcillin/Clavulanic acid | J01CR03 | A and A | NI | 14 | 14 | 99.8 | ↓* | −9.3 |  | 0.023 | 0.021 | 0.021 | 0.021 | 0.022 | 0.018 | 0.013 |
| Ceftriaxone/Sulbactam | J01DD63 | A and A | NR | 15 | 15 | 99.9 | - | 2.1 |  | 0.010 | 0.012 | 0.013 | 0.023 | 0.007 | 0.013 | 0.012 |
| Ampicillin/Cloxacillin | J01CR50 | DA | NR | 16 | 16 | 99.9 | ↓* | −15.4 |  | 0.009 | 0.007 | 0.005 | 0.001 | 0.001 | 0.001 | 0.003 |
| Amoxicillin/Dicloxacillin | J01RA01 | DA | NR | 17 | 17 | 100.0 | - | −20.8 |  | 0.008 | 0.001 | 0.003 | 0.001 | 0.001 | 0.001 | 0.002 |
| Cefalexin/Trimethoprim | - | DA | NR | 20 | 18 | 100.0 | ↓* | −11.0 |  | 0.003 | 0.002 | 0.003 | 0.002 | 0.002 | 0.001 | 0.001 |
| Cefazolin/Sulbactam | - | A and A | NI | 23 | 19 | 100.0 | - | - |  | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.003 | 0.001 |
| Ceftazidime/Sulbactam | J01DD52 | A and A | NR | 22 | 20 | 100.0 | - | - |  | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 |
| Ampicillin/Probenecid | J01CA51 | A and A | NI | 19 | 21 | 100.0 | - | −23.7 |  | 0.003 | 0.001 | 0.004 | 0.002 | 0.001 | 0.002 | 0.001 |
| Fosfomycin/Trimethoprim | - | DA | NR | 21 | 22 | 100.0 | - | −6.7 |  | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Cefadroxil/Trimethoprim | - | DA | NR | 18 | 23 | 100.0 | ↓* | −47.5 |  | 0.005 | 0.003 | 0.002 | 0.001 | 0.001 | 0.001 | 0.000 |
| Ceftazidime/Avibactam | J01DD52 | A and A | R | 24 | 24 | 100.0 | - | - |  | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
DA: Dual antimicrobials; A and A: Antibiotic plus antibiotic adjuvant; A: Access; W: Watch; R: Reserve; NR: Not Recommended; NI: Not Included; CAGR, compound annual growth rate. ↑↓ indicates the upward/downward trend; - indicates no statistically significant change. * Note: * implies p < 0.05; ** implies p < 0.001.
References
- Roberts, S.C.; Zembower, T.R. Global Increases in Antibiotic Consumption: A Concerning Trend for WHO Targets. Lancet Infect. Dis. 2021, 21, 10–11. [Google Scholar] [CrossRef]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO Antibiotic Consumption and Access Targets in 76 Countries, 2000–2015: An Analysis of Pharmaceutical Sales Data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef]
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 30 March 2022).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic Resistance Breakers: Current Approaches and Future Directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the Pharmaceutical Industry: New Estimates of R&D Costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef]
- Boyd, N.K.; Teng, C.; Frei, C.R. Brief Overview of Approaches and Challenges in New Antibiotic Development: A Focus on Drug Repurposing. Front. Cell. Infect. Microbiol. 2021, 11, 684515. [Google Scholar] [CrossRef]
- Micek, S.T.; Welch, E.C.; Khan, J.; Pervez, M.; Doherty, J.A.; Reichley, R.M.; Kollef, M.H. Empiric Combination Antibiotic Therapy Is Associated with Improved Outcome against Sepsis Due to Gram-Negative Bacteria: A Retrospective Analysis. Antimicrob. Agents Chemother. 2010, 54, 1742–1748. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Fixed Combination Medicinal Products. 2009. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-development-fixed-combination-medicinal-products-revision-1_en.pdf (accessed on 30 March 2022).
- Connor, J.; Rafter, N.; Rodgers, A. Do Fixed-Dose Combination Pills or Unit-of-Use Packaging Improve Adherence? A Systematic Review. Bull. World Health Organ. 2004, 82, 935–939. [Google Scholar] [CrossRef]
- Bortone, B.; Jackson, C.; Hsia, Y.; Bielicki, J.; Magrini, N.; Sharland, M. High Global Consumption of Potentially Inappropriate Fixed Dose Combination Antibiotics: Analysis of Data from 75 Countries. PLoS ONE 2021, 16, e0241899. [Google Scholar] [CrossRef]
- Harries, A.D.; Lawn, S.D.; Suthar, A.B.; Granich, R. Benefits of Combined Preventive Therapy with Co-Trimoxazole and Isoniazid in Adults Living with HIV: Time to Consider a Fixed-Dose, Single Tablet Coformulation. Lancet Infect. Dis. 2015, 15, 1492–1496. [Google Scholar] [CrossRef]
- Innovation, Nontraditional Antibacterial Drugs, and Clinical Utility—ACS Infectious Diseases. Available online: https://pubs.acs.org/doi/10.1021/acsinfecdis.1c00227 (accessed on 22 June 2022).
- Mauri, C.; Maraolo, A.E.; Di Bella, S.; Luzzaro, F.; Principe, L. The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases. Antibiotics 2021, 10, 1012. [Google Scholar] [CrossRef]
- Heo, Y.-A. Imipenem/Cilastatin/Relebactam: A Review in Gram-Negative Bacterial Infections. Drugs 2021, 81, 377–388. [Google Scholar] [CrossRef]
- McGettigan, P.; Roderick, P.; Kadam, A.; Pollock, A. Threats to Global Antimicrobial Resistance Control: Centrally Approved and Unapproved Antibiotic Formulations Sold in India. Br. J. Clin. Pharmacol. 2019, 85, 59–70. [Google Scholar] [CrossRef]
- Poudel, A.; Mohamed Ibrahim, M.I.; Mishra, P.; Palaian, S. Assessment of the Availability and Rationality of Unregistered Fixed Dose Drug Combinations in Nepal: A Multicenter Cross-Sectional Study. Glob. Health Res. Policy 2017, 2, 14. [Google Scholar] [CrossRef][Green Version]
- Wirtz, V.J.; Mol, P.G.M.; Verdijk, J.; Vander Stichele, R.H.; Taxis, K. Use of Antibacterial Fixed-Dose Combinations in the Private Sector in Eight Latin American Countries between 1999 and 2009. Trop. Med. Int. Health 2013, 18, 416–425. [Google Scholar] [CrossRef]
- Why Are Fixed Dose Combinations of Antibiotics Generally Not a Good Idea?—2018—ReAct. Available online: https://www.reactgroup.org/news-and-views/news-and-opinions/year-2018/why-are-fixed-dose-combinations-of-antibiotics-generally-not-a-good-idea/ (accessed on 30 March 2022).
- Zhao, H.; Wei, L.; Li, H.; Zhang, M.; Cao, B.; Bian, J.; Zhan, S. Appropriateness of Antibiotic Prescriptions in Ambulatory Care in China: A Nationwide Descriptive Database Study. Lancet Infect. Dis. 2021, 21, 847–857. [Google Scholar] [CrossRef]
- Xiao, Y. Antimicrobial Stewardship in China: Systems, Actions and Future Strategies. Clin. Infect. Dis. 2018, 67, S135–S141. [Google Scholar] [CrossRef]
- Li, H.; Yan, S.; Li, D.; Gong, Y.; Lu, Z.; Yin, X. Trends and Patterns of Outpatient and Inpatient Antibiotic Use in China’s Hospitals: Data from the Center for Antibacterial Surveillance, 2012–2016. J. Antimicrob. Chemother. 2019, 74, 1731–1740. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for ATC Classification and DDD Assignment. 2020. Available online: https://www.whocc.no/filearchive/publications/2020_guidelines_web.pdf (accessed on 30 December 2021).
- World Health Organization. Introduction to DDD Indicators. Available online: https://www.who.int/tools/atc-ddd-toolkit/indicators (accessed on 24 June 2022).
- WHOCC-ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 30 March 2022).
- Wushouer, H.; Zhou, Y.; Zhang, X.; Fu, M.; Fan, D.; Shi, L.; Guan, X. Secular Trend Analysis of Antibiotic Utilisation in China’s Hospitals 2011-2018, a Retrospective Analysis of Procurement Data. Antimicrob. Resist. Infect. Control 2020, 9, 53. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global Antibiotic Consumption 2000 to 2010: An Analysis of National Pharmaceutical Sales Data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- World Health Organization. 2021 AWaRe Classification. 2021. Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 22 June 2022).
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef]
- Expert group of “Expert Consensus on clinical Application of β -lactam antibiotic β -lactam inhibitor Compound Preparation”. Expert Consensus on clinical Application of β -lactam antibiotic β -lactam inhibitor Compound Preparation (2020 Edition). Natl. Med. J. China 2020, 100, 738–747. (In Chinese) [Google Scholar] [CrossRef]
- CARSS-2020 China Antimicrobial Resistance Surveillance Report. Available online: http://www.carss.cn/Report/Details?aId=808 (accessed on 31 March 2022). (In Chinese).
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Pascual, A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31, e00079-17. [Google Scholar] [CrossRef]
- Chinese expert consensus group on emergency diagnosis and treatment of extended-spectrum β-lactamase-producing Enterobacter infection. Chinese expert consensus on emergency diagnosis and treatment of extended-spectrum β-lactamase-producing Enterobacter infection. Chin. J. Emerg. Med. 2020, 29, 1520–1526. (In Chinese) [CrossRef]
- Palwe, S.; Veeraraghavan, B.; Periasamy, H.; Khobragade, K.; Kharat, A.S. Unorthodox Parenteral β-Lactam and β-Lactamase Inhibitor Combinations: Flouting Antimicrobial Stewardship and Compromising Patient Care. Antimicrob. Agents Chemother. 2020, 64, e00168-20. [Google Scholar] [CrossRef]
- Anand, P.; Kaur, N.; Verma, V.; Shafiq, N.; Malhotra, S. Assessment of Rationality of Available Fixed Dose Combinations of Antibiotics in India. Expert Rev. Anti Infect. Ther. 2022, 20, 797–808. [Google Scholar] [CrossRef]
- Sayer, B.; Bortone, B.; Sharland, M.; Hsia, Y. Fixed-Dose Combination Antibiotics: The Search for Evidence Using the Example of Ampicillin-Cloxacillin. Br. J. Clin. Pharmacol. 2021, 87, 2996–2999. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, M.U.; Balkrishnan, R. Fixed-Dose Combination Antibiotics in India: Global Perspectives. Lancet Glob. Health 2016, 4, e521. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Han, R.; Huang, Z.; Zhang, X.; Hu, F.; Yang, F. Sulbactam Enhances In Vitro Activity of β-Lactam Antibiotics Against Acinetobacter Baumannii. Infect. Drug Resist. 2021, 14, 3971–3977. [Google Scholar] [CrossRef]
- Xiao, S.; Zhuo, C.; Zhuo, C. In Vitro Activity of Various Sulbactam Compounds and Piperacillin/Tazobactam against Clinical Isolates of Different Gram-Negative Bacteria. Comput. Math Methods Med. 2021, 2021, 1175379. [Google Scholar] [CrossRef]
- Wareham, D.W.; Momin, M.H.F.A.; Phee, L.M.; Hornsey, M.; Standing, J.F. Cefepime/Sulbactam as an Enhanced Antimicrobial Combination Therapy for the Treatment of MDR Gram-Negative Infections. J. Antimicrob. Chemother. 2020, 75, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, P.G.; Lomovskaya, O.; Griffith, D.C.; Dudley, M.N.; VanScoy, B. β-Lactamase Inhibitors: What You Really Need to Know. Curr. Opin. Pharmacol. 2017, 36, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Crass, R.L.; Pai, M.P. Pharmacokinetics and Pharmacodynamics of β-Lactamase Inhibitors. Pharmacotherapy 2019, 39, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.B. Kinetics of Sulbactam Hydrolysis by β-Lactamases, and Kinetics of β-Lactamase Inhibition by Sulbactam. Antimicrob. Agents Chemother. 2017, 61, e01612-17. [Google Scholar] [CrossRef]
- He, P.; Sun, Q.; Shi, L.; Meng, Q. Rational Use of Antibiotics in the Context of China’s Health System Reform. BMJ 2019, 365, l4016. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission of China. Questions and Answers for Administrative Measures for the Clinical Application of Antibacterial Drugs. 2012. Available online: http://www.nhc.gov.cn/wjw/zcjd/201304/43c21ad2846d4cd58785310fc1fc775a.shtml (accessed on 30 March 2022). (In Chinese)
- National Health and Family Planning Commission of China. Administrative Measures for the Clinical Application of Antibacterial Drugs. 2012. Available online: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=347e8d20a6d442ddab626312378311b4 (accessed on 30 March 2022). (In Chinese)
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).