Characterization of Pathway-Specific Regulator NigR for High Yield Production of Nigericin in Streptomyces malaysiensis F913
Abstract
1. Introduction
2. Results
2.1. NigR Encodes a Putative SARP Family Transcriptional Regulator
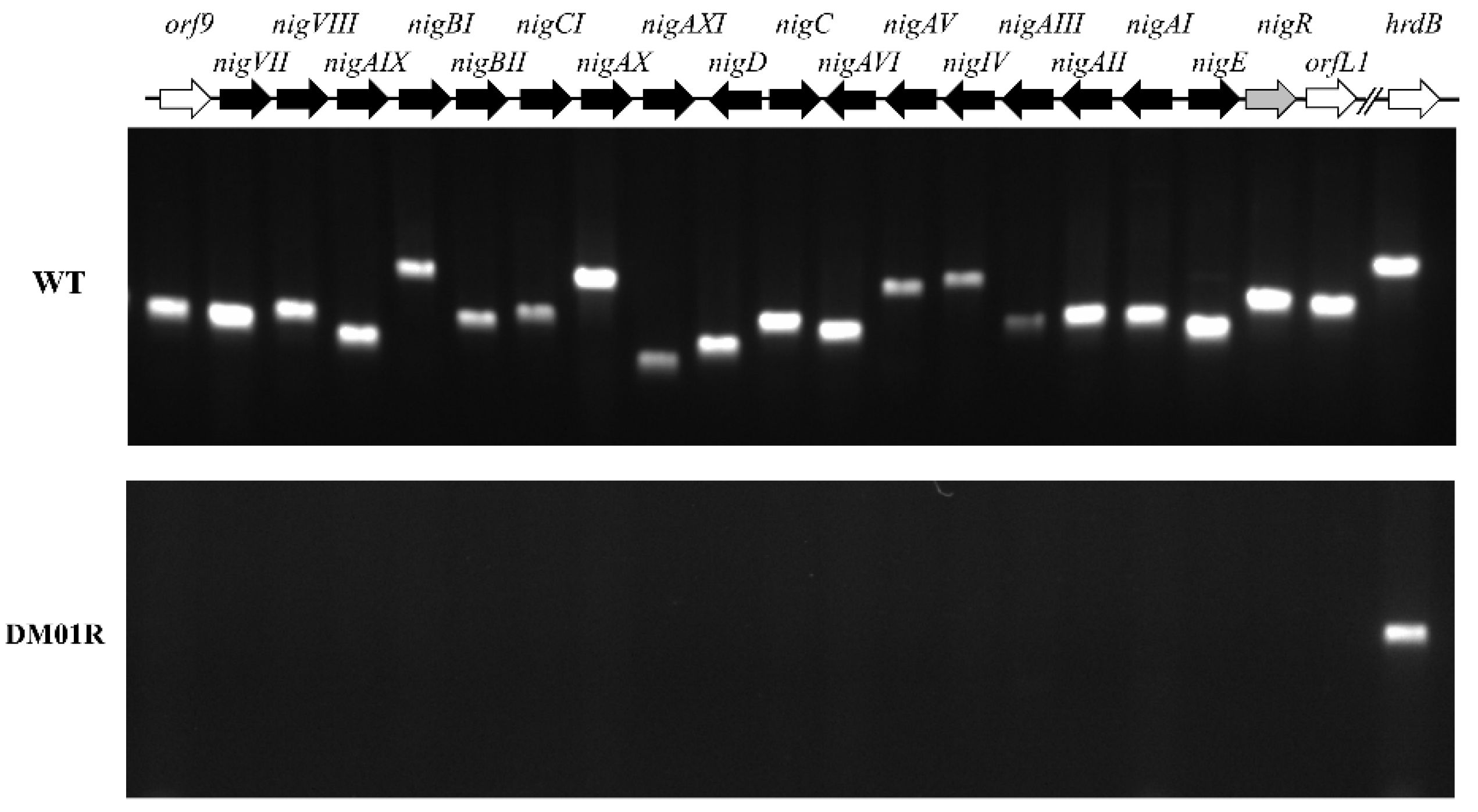
2.2. Disruption of NigR Abolished Nigericin Production
2.3. Gene Expression Analysis in the Wild-Type and Strain DM01R
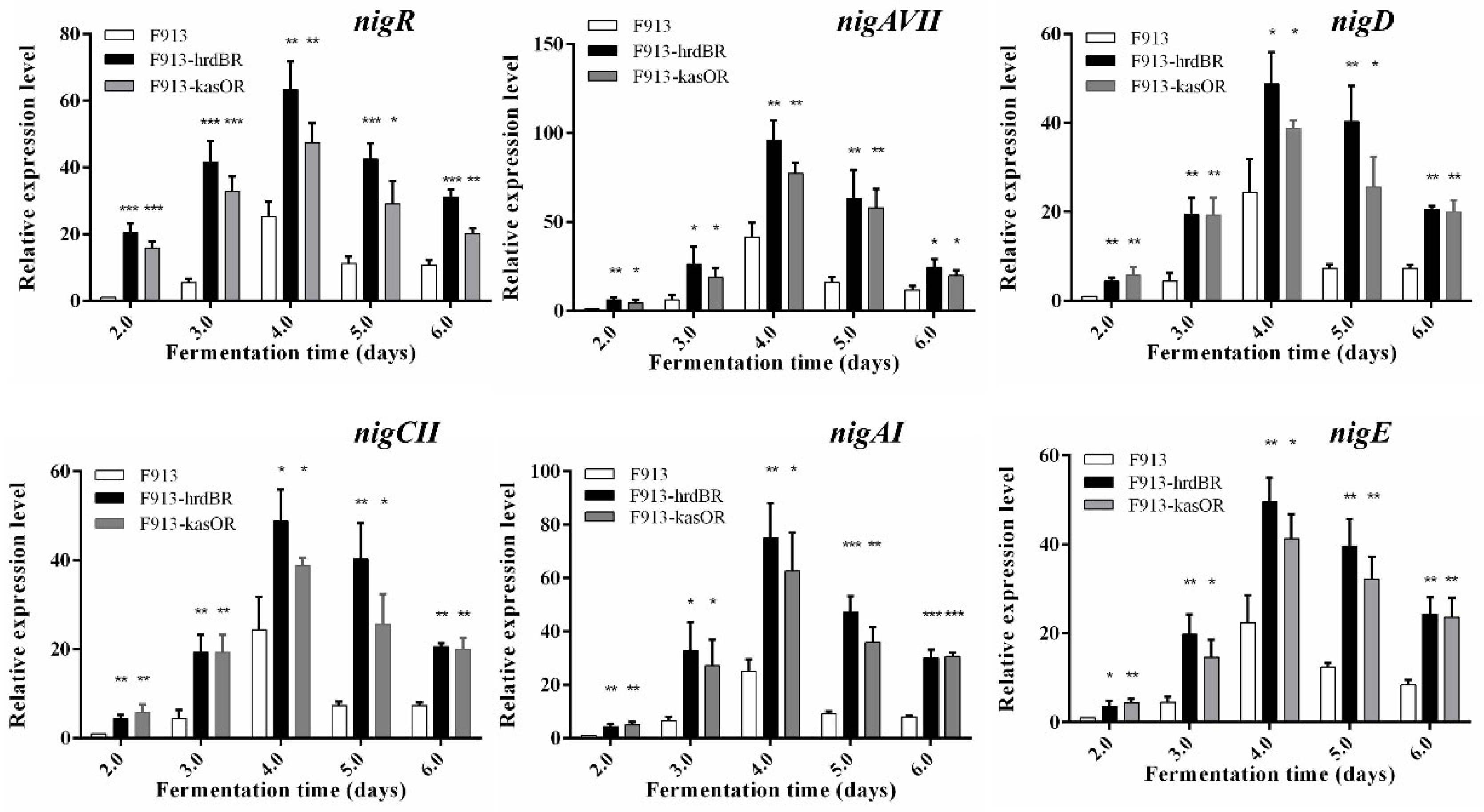
2.4. Overexpression of NigR Significantly Enhanced Production of Nigericin
2.5. Gene Expression Analysis in the Wild-Type and Overexpression Strains
3. Discussion
4. Materials and Methods
4.1. Strains, Plasmids, and Growth Conditions
4.2. Construction of NigR Mutants
4.3. Complementation and Overexpression of NigR
4.4. Production and Analysis of Nigericin
4.5. RNA Extraction, RT-PCR and qRT-PCR
4.6. Genome Sequencing, Assembly and Annotation
4.7. Nucleotide Sequence Accession Number
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Chater, K.; Chandra, G.; Niu, G.; Tan, H. Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev. 2013, 77, 112–143. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Chater, K.F.; Tian, Y.; Zhang, J.; Tan, H. Specialised metabolites regulating antibiotic biosynthesis in Streptomyces spp. FEMS Microbiol. Rev. 2016, 40, 554–573. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; He, L.; Niu, G. Regulation of antibiotic biosynthesis in actinomycetes: Perspectives and challenges. Synth. Syst. Biotechnol. 2018, 3, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreno, M.A.; Caballero, J.; Hopwood, D.A.; Malpartida, F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell 1991, 66, 769–780. [Google Scholar] [CrossRef]
- Arias, P.; Fernandez-Moreno, M.A.; Malpartida, F. Characterization of the pathway-specific positive transcriptional regulator for actinorhodin biosynthesis in Streptomyces coelicolor A3(2) as a DNA-binding protein. J. Bacteriol. 1999, 181, 6958–6968. [Google Scholar] [CrossRef]
- White, J.; Bibb, M. bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3 (2) involves a pathway-specific regulatory cascade. J. Bacteriol. 1997, 179, 627–633. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, X.; Li, R.; Pan, Y.; Liu, G.; Tan, H. SanG, a transcriptional activator, controls nikkomycin biosynthesis through binding to the sanN–sanO intergenic region in Streptomyces ansochromogenes. Microbiology 2010, 156, 828–837. [Google Scholar] [CrossRef]
- Perez-Llarena, F.J.; Liras, P.; Rodriguez-Garcia, A.; Martin, J.F. A regulatory gene (ccaR) required for cephamycin and clavulanic acid production in Streptomyces clavuligerus: Amplification results in overproduction of both beta-lactam compounds. J. Bacteriol. 1997, 179, 2053–2059. [Google Scholar] [CrossRef]
- Garg, R.P.; Parry, R.J. Regulation of valanimycin biosynthesis in Streptomyces viridifaciens: Characterization of VlmI as a Streptomyces antibiotic regulatory protein (SARP). Microbiology 2010, 156, 472–483. [Google Scholar] [CrossRef][Green Version]
- Li, R.; Liu, G.; Xie, Z.; He, X.; Chen, W.; Deng, Z.; Tan, H. PolY, a transcriptional regulator with ATPase activity, directly activates transcription of polR in polyoxin biosynthesis in Streptomyces cacaoi. Mol. Microbiol. 2010, 75, 349–364. [Google Scholar] [CrossRef]
- Li, R.; Xie, Z.; Tian, Y.; Yang, H.; Chen, W.; You, D.; Liu, G.; Deng, Z.; Tan, H. polR, a pathway-specific transcriptional regulatory gene, positively controls polyoxin biosynthesis in Streptomyces cacaoi subsp. asoensis. Microbiology 2009, 155, 1819–1831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, P.C.; Umeyama, T.; Horinouchi, S. afsS is a target of AfsR, a transcriptional factor with ATPase activity that globally controls secondary metabolism in Streptomyces coelicolor A3 (2). Mol. Microbiol. 2002, 43, 1413–1430. [Google Scholar] [CrossRef] [PubMed]
- Pressman, B.C. Biological Applications of Ionophores. Annu. Rev. Biochem. 1976, 45, 501–530. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Schildknecht, E.; Marusich, W.L. Comparative anticoccidial activity and compatibility of lasalocid in broiler chickens. Poult. Sci. 1975, 54, 757–761. [Google Scholar] [CrossRef]
- Aitchison, E.M.; Ralph, I.G.; Rowe, J.B. Evaluation of feed additives for increasing wool production from Merino sheep. 1. Lasalocid, avoparcin and flavomycin included in lucerne-based pellets or oaten chaff fed at maintenance. Anim. Prod. Sci. 1989, 29, 321–325. [Google Scholar] [CrossRef]
- Harned, R.L.; Hidy, P.H.; Corum, C.J.; Jones, K.L. Nigericin a new crystalline antibiotic from an unidentified Streptomyces. Antibiot. Chemother. 1951, 1, 594–596. [Google Scholar]
- Steinrauf, L.K.; Pinkerton, M.; Chamberlin, J.W. The structure of nigericin. Biochem. Biophys. Res. Commun. 1968, 33, 29–31. [Google Scholar] [CrossRef]
- Wesley, J. Polyether Antibiotics: Naturally Occurring Acid Ionophores; Marcel Deckker: New York, NY, USA, 1982. [Google Scholar]
- Gupta, P.; Onder, T.; Jiang, T.G.; Tao, K.; Kuperwasser, C.; Weinberg, R.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Baibakov, B.; Frank, G.; Margolis, L.; Skulachev, V. Antitumor effect of K+/H+-antiporter nigericin on human lung-carcinoma grown in in-vivo-like histocultures. Int. J. Oncol. 1993, 3, 1127–1129. [Google Scholar] [CrossRef]
- Harvey, B.M.; Mironenko, T.; Sun, Y.H.; Hong, H.; Deng, Z.; Leadlay, P.; Weissman, K.; Haydock, S. Insights into polyether biosynthesis from analysis of the nigericin biosynthetic gene cluster in Streptomyces sp. DSM4137. Chem. Biol. 2007, 14, 703–714. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Pan, G.; Zhou, Z. Isolation and identification of an actinomycete strain with algicidal activity against Microcystis aeruginosa. J. Southwest Univ. (Nat. Sci. Ed.) 2015, 37, 9. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zheng, J.; Guan, H.; Liu, W.; Tan, H. Co-expression of a SARP Family Activator ChlF2 and a Type II Thioesterase ChlK Led to High Production of Chlorothricin in Streptomyces antibioticus DSM 40725. Front. Bioeng. Biotechnol. 2020, 8, 1030. [Google Scholar] [CrossRef] [PubMed]
- Oliynyk, M.; Stark, C.B.; Bhatt, A.; Jones, M.A.; Hughes-Thomas, Z.A.; Wilkinson, C.; Oliynyk, Z.; Demydchuk, Y.; Staunton, J.; Leadlay, P.F. Analysis of the biosynthetic gene cluster for the polyether antibiotic monensin in Streptomyces cinnamonensis and evidence for the role of monB and monC genes in oxidative cyclization. Mol. Microbiol. 2003, 49, 1179–1190. [Google Scholar] [CrossRef]
- Yu, Q.; Du, A.; Liu, T.; Deng, Z.; He, X. The biosynthesis of the polyether antibiotic nanchangmycin is controlled by two pathway-specific transcriptional activators. Arch. Microbiol. 2012, 194, 415–426. [Google Scholar] [CrossRef]
- Stratigopoulos, G.; Bate, N.; Cundliffe, E. Positive control of tylosin biosynthesis: Pivotal role of TylR. Mol. Microbiol. 2004, 54, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Princival, I.M.R.; Princival, J.L.; Dias da Silva, E.; de Arruda Lima, S.M.; Carregosa, J.C.; Wisniewski, A., Jr.; de Lucena, C.C.O.; Halwass, F.; Alves Franca, J.A.; Ferreira, L.; et al. Streptomyces hygroscopicus UFPEDA 3370: A valuable source of the potent cytotoxic agent nigericin and its evaluation against human colorectal cancer cells. Chem. Biol. Interact. 2021, 333, 109316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, H.; Liu, H.; Wang, H.; Wang, X.; Xiang, W. Characterization of a pathway-specific activator of milbemycin biosynthesis and improved milbemycin production by its overexpression in Streptomyces bingchenggensis. Microb. Cell Factories 2016, 15, 152. [Google Scholar] [CrossRef]
- Leulmi, N.; Sighel, D.; Defant, A.; Khenaka, K.; Mancini, I. Enhanced production and quantitative evaluation of nigericin from the algerian soil-living Streptomyces youssoufiensis SF10 Strain. Fermentation 2019, 5, 13. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, W. Improvement of fermentation conditions for nigericin produced by Streptomyces hygroscopicus NND-52. Chin. J. Pharm. 2002, 33, 375–377. [Google Scholar]
- Liu, G.; Tian, Y.; Yang, H.; Tan, H. A pathway-specific transcriptional regulatory gene for nikkomycin biosynthesis in Streptomyces ansochromogenes that also influences colony development. Mol. Microbiol. 2005, 55, 1855–1866. [Google Scholar] [CrossRef]
- Bibb, M.J.; Janssen, G.R.; Ward, J.M. Cloning and analysis of the promoter region of the erythromycin resistance gene (ermE) of Streptomyces erythraeus. Gene 1985, 38, 215–226. [Google Scholar] [CrossRef]
- Du, D.; Zhu, Y.; Wei, J.; Tian, Y.; Niu, G.; Tan, H. Improvement of gougerotin and nikkomycin production by engineering their biosynthetic gene clusters. Appl. Microbiol. Biotechnol. 2013, 97, 6383–6396. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Wang, J.; Xiang, S.; Feng, X.; Yang, K. An engineered strong promoter for streptomycetes. Appl. Environ. Microbiol. 2013, 79, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Muller, R.; Wohlleben, W.; et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef]
- Paget, M.S.B.; Chamberlin, L.; Atrih, A.; Foster, S.J.; Buttner, M.J. Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 1999, 181, 204–211. [Google Scholar] [CrossRef]
- Bierman, M.; Logan, R.; Obrien, K.; Seno, E.T.; Rao, R.N.; Schoner, B.E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 1992, 116, 43–49. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Ma, M.; Guo, S.; Xu, Y.; Xie, J.; Pan, G.; Zhou, Z. Characterization of Pathway-Specific Regulator NigR for High Yield Production of Nigericin in Streptomyces malaysiensis F913. Antibiotics 2022, 11, 938. https://doi.org/10.3390/antibiotics11070938
Wei J, Ma M, Guo S, Xu Y, Xie J, Pan G, Zhou Z. Characterization of Pathway-Specific Regulator NigR for High Yield Production of Nigericin in Streptomyces malaysiensis F913. Antibiotics. 2022; 11(7):938. https://doi.org/10.3390/antibiotics11070938
Chicago/Turabian StyleWei, Junhong, Mengting Ma, Senwen Guo, Yaobo Xu, Jie Xie, Guoqing Pan, and Zeyang Zhou. 2022. "Characterization of Pathway-Specific Regulator NigR for High Yield Production of Nigericin in Streptomyces malaysiensis F913" Antibiotics 11, no. 7: 938. https://doi.org/10.3390/antibiotics11070938
APA StyleWei, J., Ma, M., Guo, S., Xu, Y., Xie, J., Pan, G., & Zhou, Z. (2022). Characterization of Pathway-Specific Regulator NigR for High Yield Production of Nigericin in Streptomyces malaysiensis F913. Antibiotics, 11(7), 938. https://doi.org/10.3390/antibiotics11070938






