Synergistic Effect of Quercetin on Antibacterial Activity of Florfenicol Against Aeromonas hydrophila In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
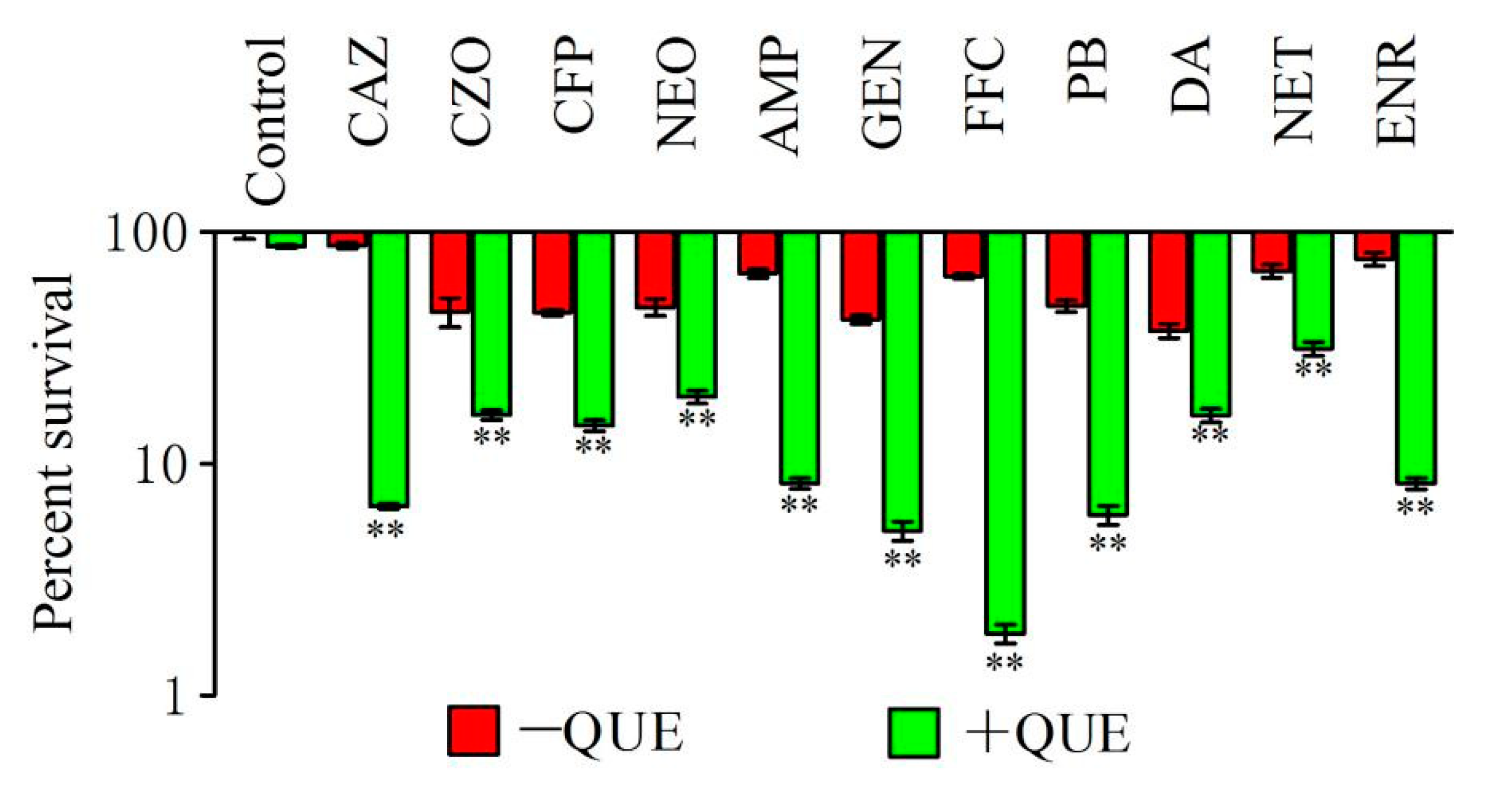
2.1. Quercetin Improved the Bactericidal Efficacy of Different Antimicrobial Agents
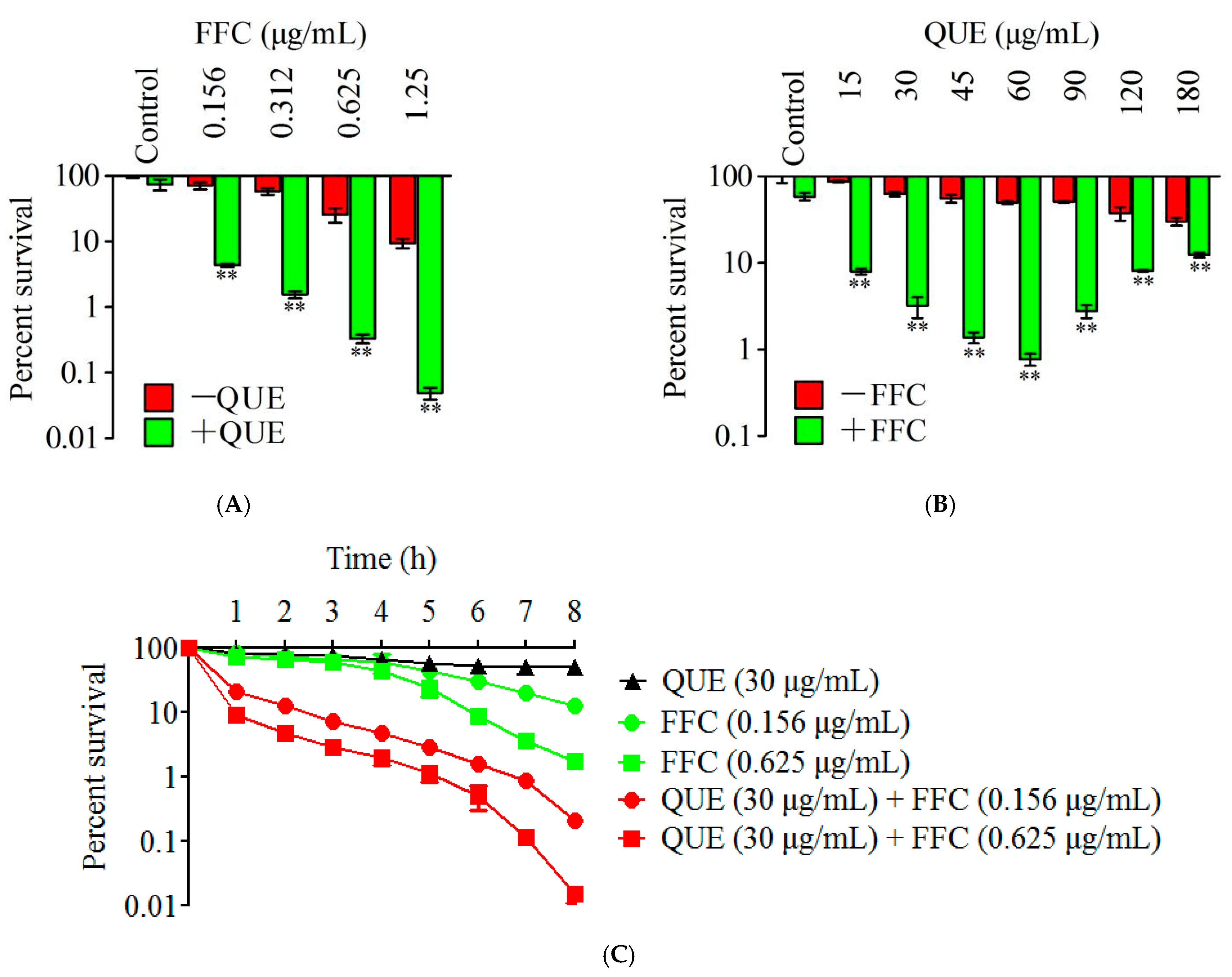
2.2. Antimicrobial Activities of Quercetin and Florfenicol
2.3. Synergistic Bactericidal Effect of Quercetin and Florfenicol against A. hydrophila In Vitro
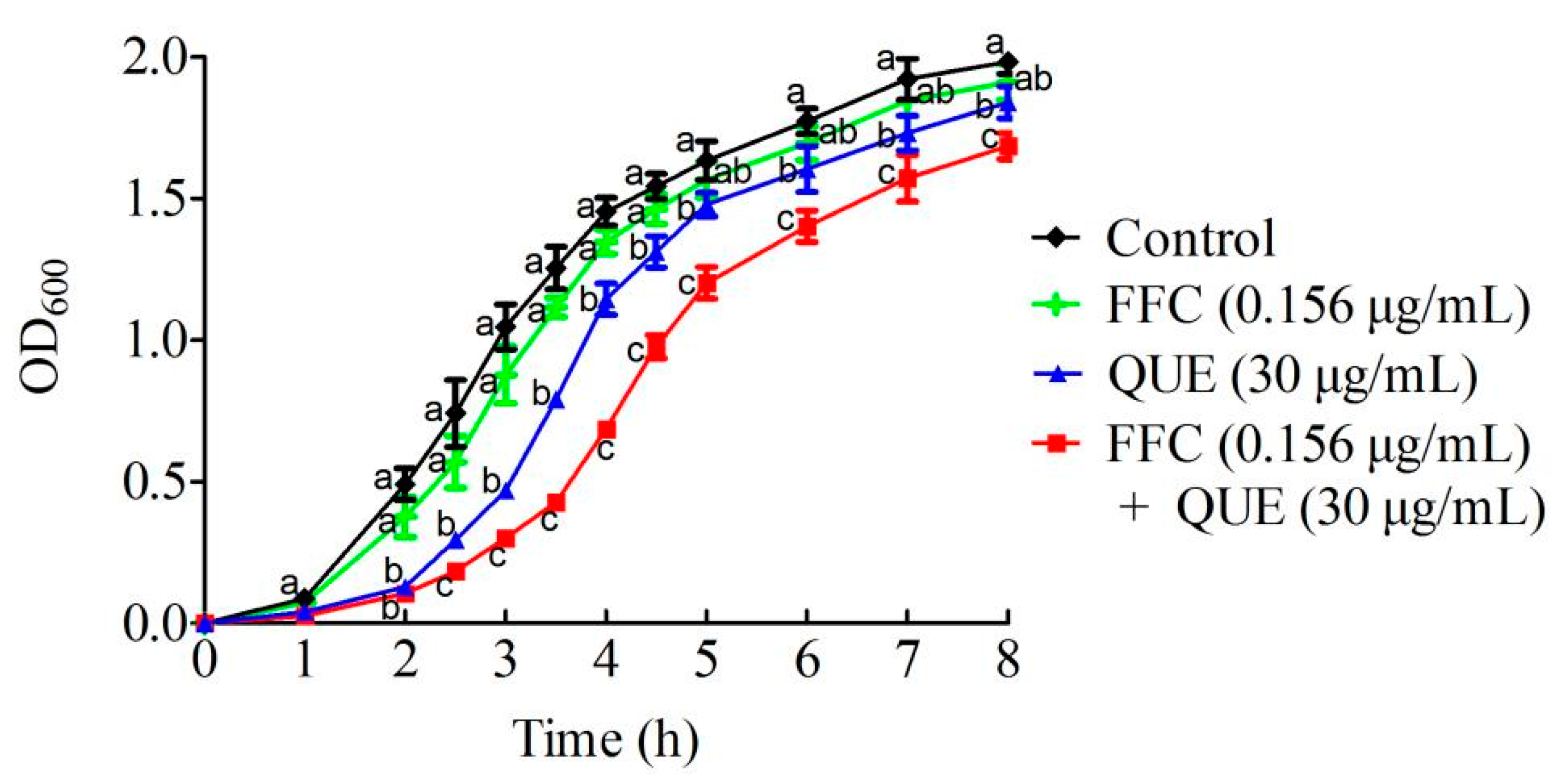
2.4. Effect of the Combination of Quercetin and Florfenicol on Bacterial Growth
2.5. Quercetin Improved the Ability of Florfenicol to Eradicate Bacteria
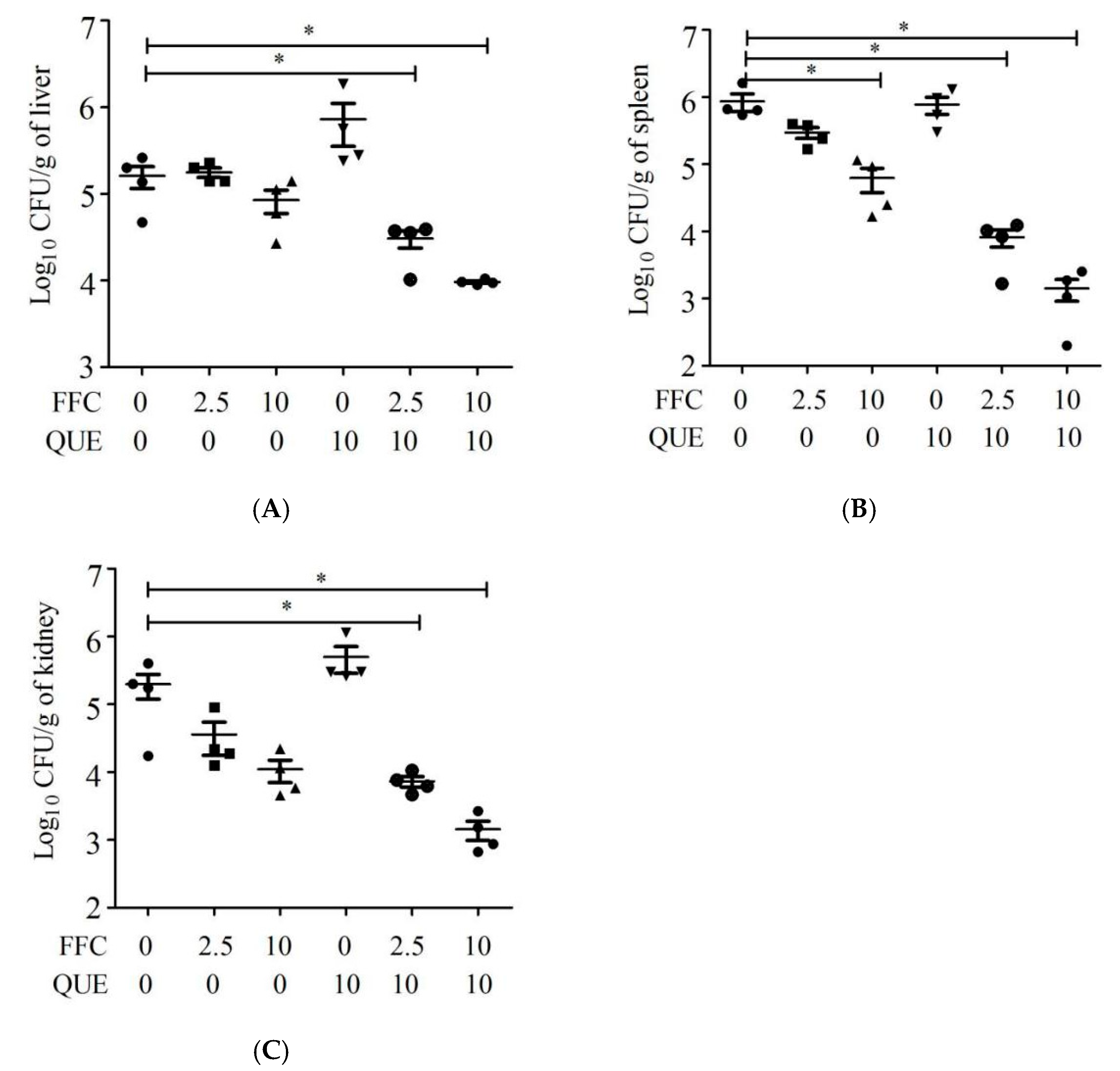
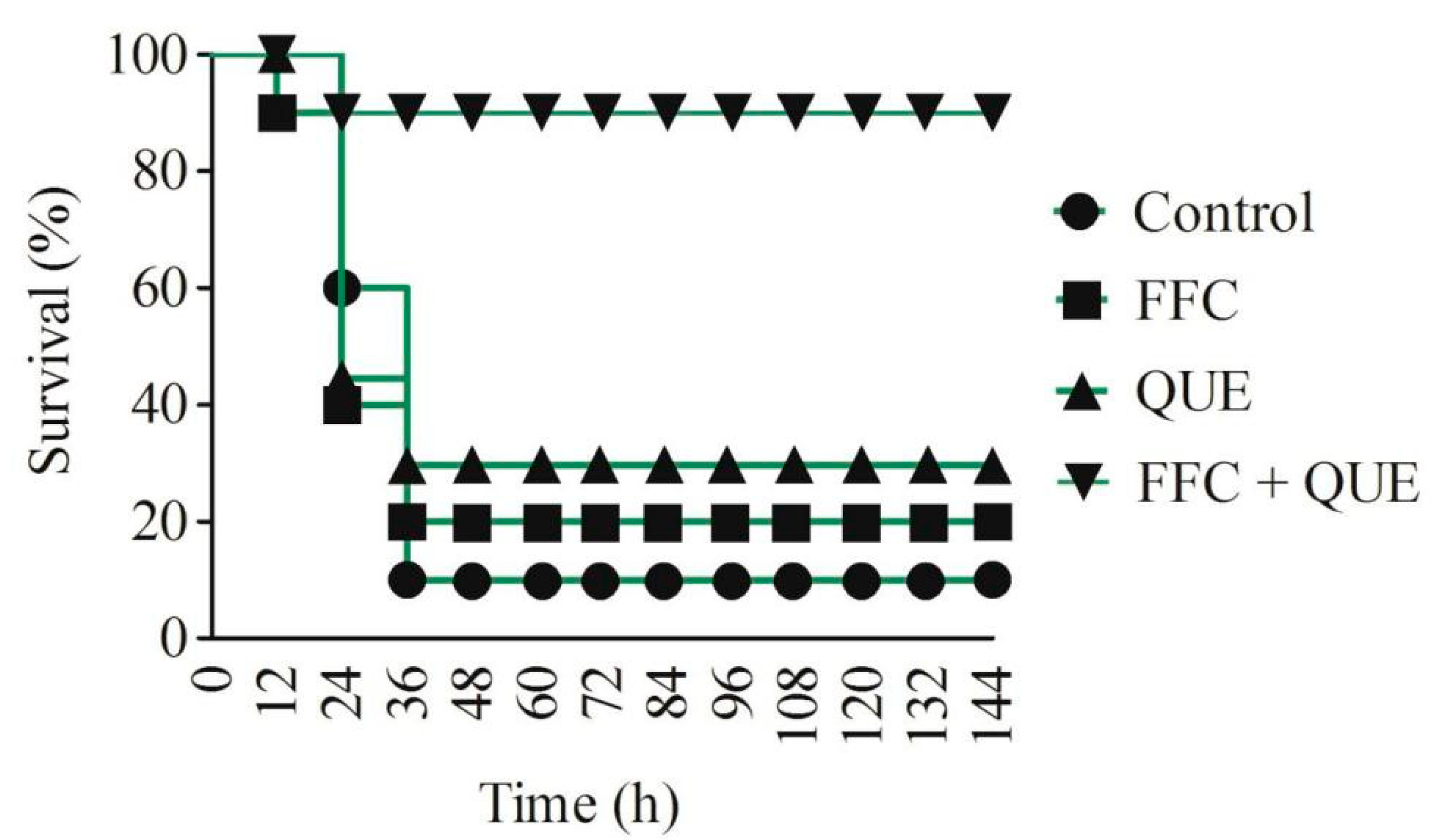
2.6. Effect of Quercetin and Florfenicol on the Anti-Infection Ability
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Quercetin and Antibiotic Bactericidal Assays In Vitro
4.3. Determination of FIC Based on MIC Determination
4.4. Bacterial Survival Growth Curve
4.5. Bacterial Eradication Assay In Vivo
4.6. Bacterial Infection and Drugs Therapy
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafferty, K.D.; Harvell, C.D.; Conrad, J.M.; Friedman, C.S.; Kent, M.L.; Kuris, A.M.; Powell, E.N.; Rondeau, D.; Saksida, S.M. Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci. 2015, 7, 471–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janda, J.M.; Abbott, S.L. The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.E.; Hoi, L.; Schmidt, A.S.; Qian, D.; Shimada, T.; Shen, J.Y.; Larsen, J.L. Is Aeromonas hydrophila the dominant motile Aeromonas species that causes disease outbreaks in aquaculture production in the Zhejiang Province of China? Dis. Aquat. Organ. 2001, 46, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Malek, A.M. Incidence and virulence characteristics of Aeromonas spp. in fish. Vet. World 2017, 10, 34–37. [Google Scholar] [CrossRef] [Green Version]
- Nhinh, D.T.; Le, D.V.; Van, K.V.; Huong Giang, N.T.; Dang, L.T.; Hoai, T.D. Prevalence, virulence gene distribution and alarming the multidrug resistance of Aeromonas hydrophila associated with disease outbreaks in freshwater aquaculture. Antibiotics 2021, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Stratev, D.; Odeyemi, O.A. Antimicrobial resistance of Aeromonas hydrophila isolated from different food sources: A mini-review. J. Infect. Public Health 2016, 9, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, L.; Wickramanayake, M.; Heo, G.J. Virulence and antimicrobial resistance potential of Aeromonas spp. associated with shellfish. Lett. Appl. Microbiol. 2021, 73, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Hatha, M.; Vivekanandhan, A.A.; Joice, G.J.; Christol. Antibiotic resistance pattern of motile aeromonads from farm raised fresh water fish. Int. J. Food Microbiol. 2005, 98, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. 2021, 8, e2100749. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Fadda, L.; Attia, H.A.; Sharaf, I.A.; Mohamed, A.M. Pulmonary prophylactic impact of melatonin and/or quercetin: A novel therapy for inflammatory hypoxic stress in rats. Acta Pharm. 2017, 67, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, H.; Bai, R.; Bi, F.; Liu, J.; Qin, Y. Synthesis, characterization, antioxidant and antimicrobial activities of starch aldehyde-quercetin conjugate. Int. J. Biol. Macromol. 2020, 156, 462–470. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W.A.; de Aguiar Cordeiro, R.; Castelo-Branco, D.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal effects of the flavonoids kaempferol and quercetin: A possible alternative for the control of fungal biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch. Pharm. Res. 2008, 31, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Yao, J.Y.; Zhou, B.; Yang, J.X.; Chaudry, M.T.; Wang, M.; Xiao, F.L.; Li, Y.; Yin, W.Z. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Protect. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Memariani, H.; Memariani, M.; Ghasemian, A. An overview on anti-biofilm properties of quercetin against bacterial pathogens. World J. Microbiol. Biotechnol. 2019, 35, 143. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhang, J.; Xie, J.; Yang, L.; Xing, Y.; Li, Z. The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2020, 46, 759–770. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Javahery, S.; Doan, H.V. Effects of dietary vitamin C, thyme essential oil, and quercetin on the immunological and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2022, 553, 738053. [Google Scholar] [CrossRef]
- Zhao, X.L.; Chen, H.; Zhong, K.K.; Li, L.; Kong, X.H. Myo-inositol as an adjuvant to florfenicol against Aeromonas hydrophila infection in common carp Cyprinus carpio. FEMS Microbiol. Lett. 2018, 365, fny212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, M.B.; Brookings, E.S.; Moser, S.A.; Sonke, P.B.; Waites, K.B. Comparative in vitro antimicrobial susceptibilities of nosocomial isolates of Acinetobacter baumannii and synergistic activities of nine antimicrobial combinations. Antimicrob. Agents Chemother. 1997, 41, 881–885. [Google Scholar] [CrossRef] [Green Version]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.J.; Gilbert, M.; Van Boeckel, T.P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021, 12, 5384. [Google Scholar] [CrossRef] [PubMed]
- Al-Saif, S.S.; Abdel-Raouf, N.; El-Wazanani, H.A.; Aref, I.A. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Chen, L.; Liu, Y.; Shi, L.; Wan, S.; Wang, L. Evaluation of antimicrobial activity of water-soluble flavonoids extract from Vaccinium bracteatum Thunb. leaves. Food Sci. Biotechnol. 2019, 28, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Cascaes, M.M.; Guilhon, G.; Zoghbi, M.D.G.; Andrade, E.H.A.; Santos, L.S.; Kelly, R.d.S.J.; Trovatti Uetanabaro, A.P.; Araujo, I.S. Flavonoids, antioxidant potential and antimicrobial activity of Myrcia rufipila mcvaugh leaves (myrtaceae). Nat. Prod. Res. 2021, 35, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, B.M.; Arora, S.; Lim, C.S. Bactericidal antibiotic-phytochemical combinations against methicillin resistant Staphylococcus aureus. Braz. J. Microbiol. 2012, 43, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Donoso, F.; Ramirez, V.T.; Golubeva, A.V.; Moloney, G.M.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Naturally derived polyphenols protect against corticosterone-induced changes in primary cortical neurons. Int. J. Neuropsychopharmacol. 2019, 22, 765–777. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Ho, S.P.; Hsu, T.Y.; Che, M.H.; Wang, W.S. Antibacterial effect of chloramphenicol, thiamphenicol and florfenicol against aquatic animal bacteria. J. Vet. Med. Sci. 2000, 62, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef]
- Siriwong, S.; Thumanu, K.; Hengpratom, T.; Eumkeb, G. Synergy and mode of action of ceftazidime plus quercetin or luteolin on Streptococcus pyogenes. Evid. Based Complement. Altern. Med. 2015, 2015, 759459. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.U.; Khurram, M.; Khan, T.A.; Faidah, H.S.; Shah, Z.U.; Rahman, S.U.; Haseeb, A.; Ilyas, M.; Ullah, N.; Khayam, S.M.U.; et al. Effects of luteolin and quercetin in combination with some conventional antibiotics against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Sci. 2016, 17, 1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.K.; Tousif, S.; Bhaskar, A.; Devi, A.; Negi, K.; Moitra, B.; Ranganathan, A.; Dwivedi, V.P.; Das, G. Luteolin as a potential host-directed immunotherapy adjunct to isoniazid treatment of tuberculosis. PLoS Pathog. 2021, 17, e1009805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Yang, Y.P.; Wang, L.; Chen, H.; Zhu, L.; Li, L.; Kong, X.H. Aspartate induces metabolic changes and improves the host’s ability to fight against Aeromonas hydrophila infection in Cyprinus carpio. Aquac. Res. 2022, 53, 1050–1061. [Google Scholar] [CrossRef]
- Wang, W.Y.; Sun, C.X.; Mao, L.K.; Ma, P.H.; Liu, F.G.; Yang, J.; Gao, Y.X. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Zhao, X.L.; Jin, Z.H.; Di, G.L.; Li, L.; Kong, X.H. Molecular characteristics, pathogenicity and medication regimen of Aeromonas hydrophila isolated from common carp (Cyprinus carpio L.). J. Vet. Med. Sci. 2019, 81, 1769–1775. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.L.; Chen, Z.G.; Yang, T.C.; Jiang, M.; Wang, J.; Cheng, Z.X.; Yang, M.J.; Zhu, J.X.; Zhang, T.T.; Li, H.; et al. Glutamine promotes antibiotic uptake to kill multidrug-resistant uropathogenic bacteria. Sci. Transl. Med. 2021, 13, eabj0716. [Google Scholar] [CrossRef]
- Zhao, X.L.; Chen, H.; Jin, Z.H.; Li, L.; Zhang, J.; Kong, X.H. GC-MS-based metabolomics analysis reveals L-aspartate enhances the antibiotic sensitivity of neomycin sulfate-resistant Aeromonas hydrophila. J. Fish Dis. 2018, 41, 1831–1841. [Google Scholar] [CrossRef]
| Strain | MICQUE (μg/mL) | MICFFC (μg/mL) | FICQUE+FFC (μg/mL) | FICI |
|---|---|---|---|---|
| A. hydrophila | 360 | 2.5 | 90 + 0.078 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Cui, X.; Yang, Y.; Zhu, L.; Li, L.; Kong, X. Synergistic Effect of Quercetin on Antibacterial Activity of Florfenicol Against Aeromonas hydrophila In Vitro and In Vivo. Antibiotics 2022, 11, 929. https://doi.org/10.3390/antibiotics11070929
Zhao X, Cui X, Yang Y, Zhu L, Li L, Kong X. Synergistic Effect of Quercetin on Antibacterial Activity of Florfenicol Against Aeromonas hydrophila In Vitro and In Vivo. Antibiotics. 2022; 11(7):929. https://doi.org/10.3390/antibiotics11070929
Chicago/Turabian StyleZhao, Xianliang, Xiuying Cui, Yunpeng Yang, Lei Zhu, Li Li, and Xianghui Kong. 2022. "Synergistic Effect of Quercetin on Antibacterial Activity of Florfenicol Against Aeromonas hydrophila In Vitro and In Vivo" Antibiotics 11, no. 7: 929. https://doi.org/10.3390/antibiotics11070929
APA StyleZhao, X., Cui, X., Yang, Y., Zhu, L., Li, L., & Kong, X. (2022). Synergistic Effect of Quercetin on Antibacterial Activity of Florfenicol Against Aeromonas hydrophila In Vitro and In Vivo. Antibiotics, 11(7), 929. https://doi.org/10.3390/antibiotics11070929





