Does a New Antibiotic Scheme Improve the Outcome of Staphylococcus aureus-Caused Acute Prosthetic Joint Infections (PJI) Treated with Debridement, Antibiotics and Implant Retention (DAIR)?
Abstract
1. Introduction
2. Results
2.1. Demographic Results
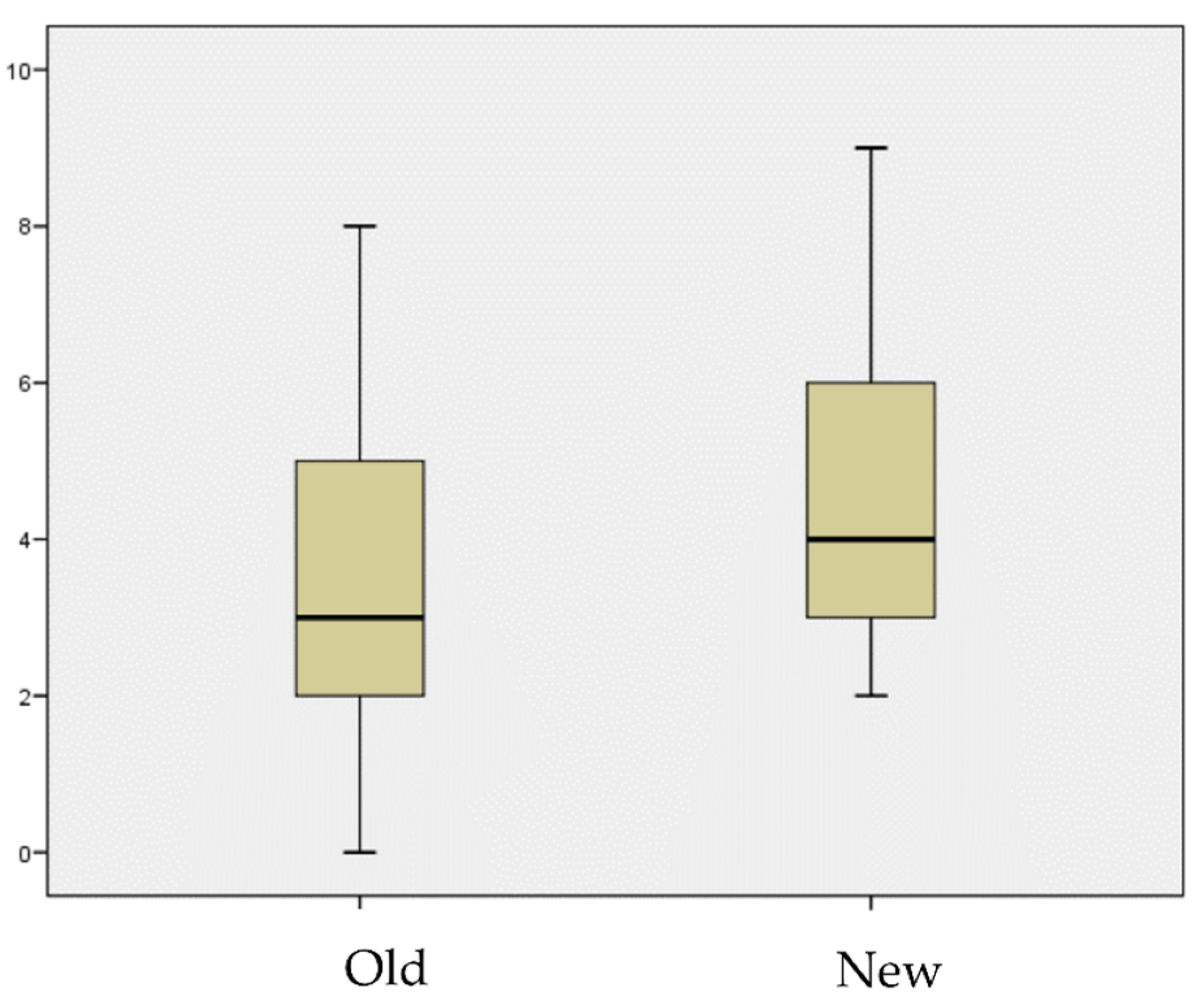
2.2. Treatment Results
3. Discussion
4. Materials and Methods
4.1. Study Design, Patients and Settings
4.2. Data Collection
4.3. Definition
4.4. Groups Division
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, Y.J.; Lee, M.S.; Lee, C.H.; Lin, P.C.; Kuo, F.C. Daptomycin treatment in patients with resistant staphylococcal periprosthetic joint infection. BMC Infect. Dis. 2017, 17, 736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; He, L.; Fang, X.Y.; Huang, Z.D.; Bai, G.C.; Li, W.B.; Zhang, W.M. Debridement, Antibiotics, and Implant Retention for Acute Periprosthetic Joint Infection. Orthop. Surg. 2020, 12, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Sorlí, L.; Horcajada, J.P. High dose daptomycin together with rifampin as salvage therapy for prosthetic joint infections. Med. Clin. 2017, 149, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Azzam, K.A.; Seeley, M.; Ghanem, E.; Austin, M.S.; Purtill, J.J.; Parvizi, J. Irrigation and debridement in the management of prosthetic joint infection: Traditional indications revisited. J. Arthroplast. 2010, 25, 1022e7. [Google Scholar] [CrossRef]
- Byren, I.; Bejon, P.; Atkins, B.L.; Angus, B.; Masters, S.; McLardy-Smith, P.; Gundle, R.; Berendt, A. One hundred and twelve infected arthroplasties treated with “DAIR” (debridement, antibiotics and implant retention): Antibiotic duration and outcome. J. Antimicrob. Chemother. 2009, 63, 1264–1271. [Google Scholar] [CrossRef]
- Letouvet, B.; Arvieux, C.; Leroy, H.; Polard, J.-L.; Chapplain, J.M.; Common, H.; Ecoffey, C.; Huten, D.; Jolivet-Gougeon, A.; Tattevin, P. Predictors of failure for prosthetic joint infections treated with debridement. Med. Mal. Infect. 2016, 46, 39–43. [Google Scholar] [CrossRef]
- Lowik, C.A.M.; Jutte, P.C.; Tornero, E.; Ploegmakers, J.J.W.; Knobben, B.A.S.; de Vries, A.J.; Zijlstra, W.P.; Dijkstra, B.; Soriano, A.; Wouthuyzen-Bakker, M.; et al. Predicting failure in early acute prosthetic joint infection treated with debridement, antibiotics, and implant retention: External validation of the KLICC Score. J. Arthroplast. 2018, 33, 2582–2587. [Google Scholar] [CrossRef]
- Soriano, A.; Garca, S.; Bori, G.; Almela, M.; Gallart, X.; Macule, F.; Sierra, J.; Martínez, J.A.; Suso, S.; Mens, J. Treatment of acute post-surgical infection of joint arthroplasty. Clin. Microbiol. Infect. 2006, 12, 930–933. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Murillo, O.; Iribarren, J.A.; Soriano, A.; Sánchez-Somolinos, M.; Baraia-Etxaburu, J.M.; Rico, A.; Palomino, J.; Rodríguez-Pardo, D.; Horcajada, J.P.; et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin. Infect. Dis. 2013, 56, 182–194. [Google Scholar] [CrossRef]
- Jugun, K.; Vaudaux, P.; Garbino, J.; Pagani, L.; Hoffmeyer, P.; Lew, D.; Uçkay, I. The safety and efficacy of high dose daptomycin combined with rifampicin for the treatment of Gram-positive osteoarticular infections. Int. Orthop. 2013, 37, 1375–1380. [Google Scholar] [CrossRef]
- Grillo, S.; Cuervo, G.; Carratalà, J.; Grau, I.; Pallarès, N.; Tebé, C.; GuillemTió, L.; Murillo, O.; Ardanuy, C.; Domínguez, M.A.; et al. Impact of β-Lactam and Daptomycin Combination Therapy on Clinical Outcomes in Methicillin-susceptible Staphylococcus aureus Bacteremia: A Propensity Score-matched Analysis. Clin. Infect. Dis. 2019, 69, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Lye, D.C.; Yahav, D.; Sud, A.; Robinson, J.O.; Nelson, J.; Archuleta, S.; Roberts, M.A.; Cass, A.; Paterson, D.L.; et al. Effect of Vancomycin or Daptomycin With vs Without an Antistaphylococcal β-Lactam on Mortality, Bacteremia, Relapse, or Treatment Failure in Patients With MRSA Bacteremia: A Randomized Clinical Trial. JAMA 2020, 323, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Dupieux, C.; Trouillet-Assant, S.; Camus, C.; Abad, L.; Bes, M.; Benito, Y.; Chidiac, C.; Lustig, S.; Ferry, T.; Valour, F.; et al. Intraosteoblastic activity of daptomycin in combination with oxacillin and ceftaroline against MSSA and MRSA. J. Antimicrob. Chemother. 2017, 72, 3353–3356. [Google Scholar] [CrossRef]
- Yang, S.J.; Xiong, Y.Q.; Boyle-Vavra, S.; Daum, R.; Jones, T.; Bayer, A.S. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-non susceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob. Agents Chemother. 2010, 54, 3161–3169. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chen, P.Y.; Wang, J.T.; Chang, S.C. A study on combination of daptomycin with selected antimicrobial agents: In vitro synergistic effect of MIC value of 1 mg/L against MRSA strains. BMC Pharmacol. Toxicol. 2019, 20, 25. [Google Scholar] [CrossRef]
- Pérez-Cardona, P.S.C.; Ojeda, V.B.; Pardo, D.R.; Serrallach, C.P.; Farfán, E.G.; Mateu, C.A.; Sanchez, X.F. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J. Antimicrob. Chemother. 2012, 67, 1749–1754. [Google Scholar] [CrossRef][Green Version]
- Byren, I.; Rege, S.; Campanaro, E.; Yankelev, S.; Anastasiou, D.; Kuropatkin, G.; Evans, R. Randomized controlled trial of the safety and efficacy of Daptomycin versus standard-of-care therapy for management of patients with osteomyelitis associated with prosthetic devices undergoing two-stage revision arthroplasty. Antimicrob. Agents Chemother. 2012, 56, 5626–5632. [Google Scholar] [CrossRef]
- García-de-la-Mària, C.; Gasch, O.; García-Gonzalez, J.; Soy, D.; Shaw, E.; Ambrosioni, J.; Almela, M.; Pericàs, J.M.; Tellez, A.; Falces, C.; et al. The Combination of Daptomycin and Fosfomycin Has Synergistic, Potent, and Rapid Bactericidal Activity against Methicillin-Resistant Staphylococcus aureus in a Rabbit Model of Experimental Endocarditis. Antimicrob. Agents Chemother. 2018, 62, e02633-17. [Google Scholar] [CrossRef]
- Snydman, D.R.; McDermott, L.A.; Jacobus, N.V. Evaluation of in vitro interaction of daptomycin with gentamicin or beta-lactam antibiotics against Staphylococcus aureus and Enterococci by FIC index and timed-kill curves. J. Chemother. 2005, 17, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.; Sakoulas, G. Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin. Ther. 2014, 36, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.J.; Tiscareno-Grajeda, I.; Misenhiemer, G.; Heyderman, J. Use of daptomycin in the treatment of prosthetic joint infections: A prospective observational study of 30 patients with infected prosthetic joint infections. J. Infect. Dis. Internet 2009, 7, 1–6. [Google Scholar]
- Licitra, C.M.; Crespo, A.; Licitra, D.; Wallis-Crespo, M. Daptomycin for the treatment of osteomyelitis and prosthetic joint infection: Retrospective analysis of efficacy and safety in an outpatient infusion center. J. Infect. Dis. Internet 2011, 9, 11566. [Google Scholar]
- Rao, N.; Regalla, D.M. Uncertain efficacy of daptomycin for prosthetic joint infections: A prospective case series. Clin. Orthop Relat Res. 2006, 451, 34–37. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Parra-Ruiz, J.; Rodríguez-Pardo, D.; Barberán, J.; Ribera, A.; Tornero, E.; Pigrau, C.; Mensa, J.; Ariza, J.; Soriano, A. High doses of daptomycin (10 mg/kg/d) plus rifampin for the treatment of staphylococcal prosthetic joint infection managed with implant retention: A comparative study. Diagn Microbiol Infect. Dis. 2014, 80, 66–71. [Google Scholar] [CrossRef]
- Macias-Valcayo, A.; Pfang, B.G.; Auñón, A.; Esteban, J. Pharmacotherapy options and drug development in managing periprosthetic joint infections in the elderly. Expert Opin Pharm. 2019, 20, 1109–1121. [Google Scholar] [CrossRef]
- Perez-Jorge, C.; Gomez-Barrena, E.; Horcajada, J.P.; Puig-Verdie, L.; Esteban, J. Drug treatments for prosthetic joint infections in the era of multidrug resistance. Expert Opin Pharm. 2016, 17, 1233–1246. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Valle, C.D.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef]

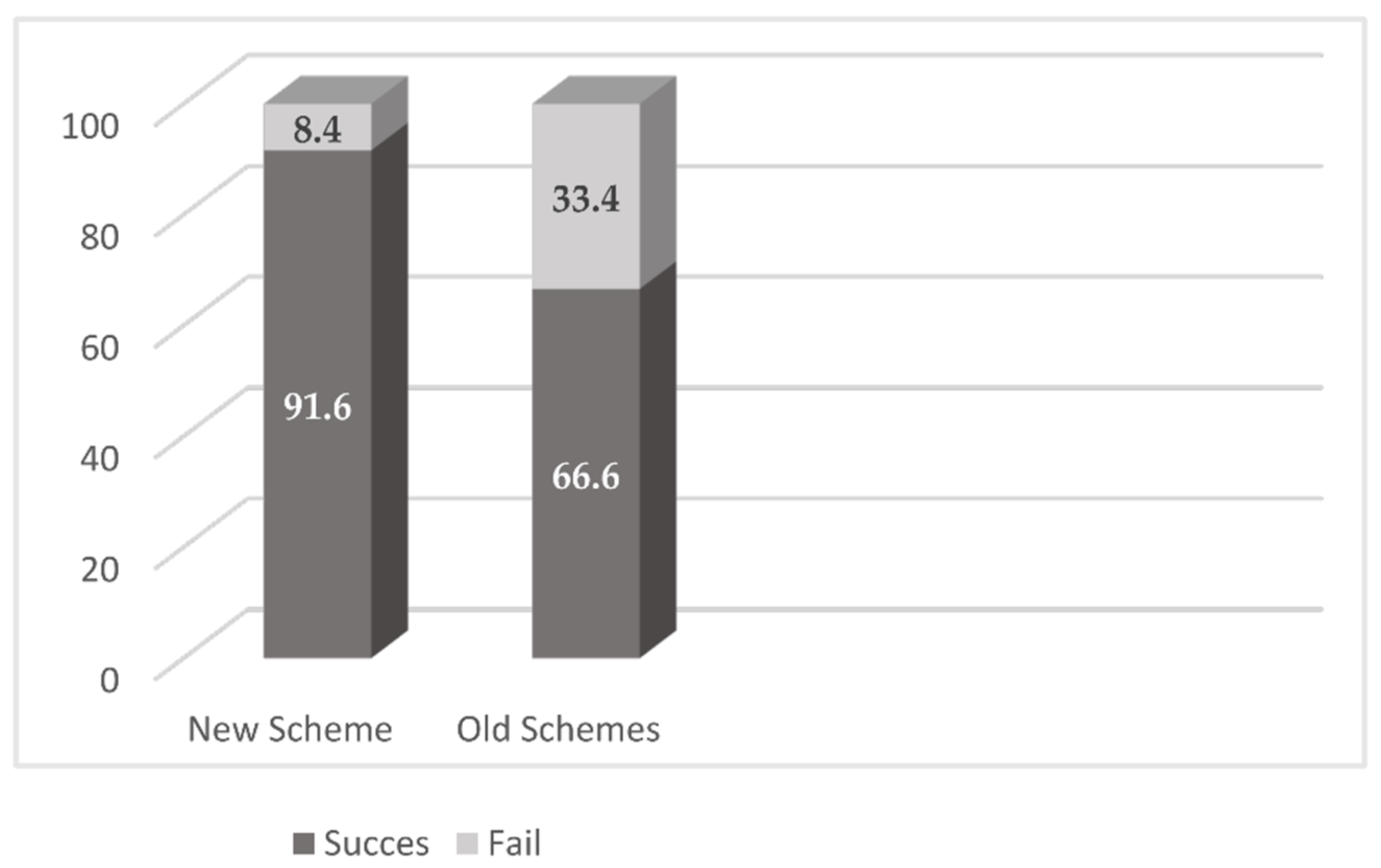
| Daptomycin-Cloxacillin | Other Treatments | |
|---|---|---|
| Number of patients | 24 | 21 |
| Mean Age (years) | 70.4 | 68.7 |
| Sex (M:F) | 1:1 | 1.6:1 |
| Charlson | 4.63 | 3.43 |
| THA | 6 | 7 |
| TKA | 18 | 14 |
| Days of treatment (SD) | 87.5 (28.6) | 102 (42.3) |
| Failure | 2 | 7 |
| Follow-up (months) | 15 | 31.2 |
| Patient | Age | Sex | Pathogen | Charlson Score | Antibiotic Scheme | Duration (Days) | Clinical Cure |
|---|---|---|---|---|---|---|---|
| 1 | 67 | F | MSSA | 3 | Old | 90 | Yes |
| 2 | 65 | M | MSSA | 3 | Old | 90 | No |
| 3 | 55 | M | MSSA | 4 | Old | 90 | Yes |
| 4 | 71 | M | MSSA | 4 | Old | 180 | Yes |
| 5 | 75 | F | MSSA | 2 | Old | 111 | Yes |
| 6 | 76 | M | MSSA | 6 | Old | 90 | No |
| 7 | 51 | M | MSSA | 4 | Old | 90 | No |
| 8 | 78 | F | MSSA | 4 | Old | 180 | No |
| 9 | 88 | M | MSSA | 3 | Old | 30 | Yes |
| 10 | 49 | M | MSSA | 4 | Old | 90 | Yes |
| 11 | 51 | M | MSSA | 6 | Old | 90 | Yes |
| 12 | 66 | F | MSSA | 8 | Old | 60 | Yes |
| 13 | 76 | M | MSSA | 5 | Old | 180 | No |
| 14 | 85 | F | MSSA | 6 | Old | 35 | No |
| 15 | 81 | F | MRSA | 6 | Old | 90 | Yes |
| 16 | 67 | F | MRSA | 3 | Old | 104 | Yes |
| 17 | 77 | F | MSSA | 7 | Old | 180 | Yes |
| 18 | 44 | M | MSSA | 4 | Old | 90 | Yes |
| 19 | 58 | M | MSSA | 3 | Old | 90 | No |
| 20 | 84 | M | MSSA | 4 | Old | 90 | Yes |
| 21 | 78 | M | MSSA | 4 | Old | 90 | Yes |
| 22 | 79 | M | MSSA | 3 | New | 90 | Yes |
| 23 | 71 | M | MSSA | 9 | New | 90 | Yes |
| 24 | 67 | F | MSSA | 6 | New | 60 | Yes |
| 25 | 59 | M | MSSA | 4 | New | 45 | Yes |
| 26 | 53 | M | MSSA | 3 | New | 45 | Yes |
| 27 | 94 | M | MSSA | 1 | New | 90 | Yes |
| 28 | 73 | F | MRSA | 4 | New | 90 | Yes |
| 29 | 71 | F | MSSA | 5 | New | 90 | Yes |
| 30 | 65 | F | MRSA | 3 | New | 90 | Yes |
| 31 | 77 | F | MSSA | 2 | New | 120 | Yes |
| 32 | 83 | F | MSSA | 3 | New | 90 | Yes |
| 33 | 83 | F | MRSA | 5 | New | 60 | Yes |
| 34 | 47 | M | MSSA | 0 | New | 60 | Yes |
| 35 | 48 | M | MSSA | 8 | New | 45 | Yes |
| 36 | 78 | F | MSSA | 2 | New | 90 | Yes |
| 37 | 61 | M | MSSA | 6 | New | 90 | No |
| 38 | 79 | F | MSSA | 5 | New | 90 | Yes |
| 39 | 81 | M | MSSA | 5 | New | 120 | No |
| 40 | 62 | M | MSSA | 2 | New | 90 | Yes |
| 41 | 74 | M | MSSA | 3 | New | 105 | Yes |
| 42 | 61 | M | MSSA | 0 | New | 180 | Yes |
| 43 | 59 | F | MSSA | 1 | New | 90 | Yes |
| 44 | 80 | M | MSSA | 7 | New | 90 | Yes |
| 45 | 85 | M | MRSA | 3 | New | 90 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auñón, Á.; Tovar-Bazaga, M.; Blanco-García, A.; García-Cañete, J.; Parrón, R.; Esteban, J. Does a New Antibiotic Scheme Improve the Outcome of Staphylococcus aureus-Caused Acute Prosthetic Joint Infections (PJI) Treated with Debridement, Antibiotics and Implant Retention (DAIR)? Antibiotics 2022, 11, 922. https://doi.org/10.3390/antibiotics11070922
Auñón Á, Tovar-Bazaga M, Blanco-García A, García-Cañete J, Parrón R, Esteban J. Does a New Antibiotic Scheme Improve the Outcome of Staphylococcus aureus-Caused Acute Prosthetic Joint Infections (PJI) Treated with Debridement, Antibiotics and Implant Retention (DAIR)? Antibiotics. 2022; 11(7):922. https://doi.org/10.3390/antibiotics11070922
Chicago/Turabian StyleAuñón, Álvaro, Miguel Tovar-Bazaga, Antonio Blanco-García, Joaquín García-Cañete, Raúl Parrón, and Jaime Esteban. 2022. "Does a New Antibiotic Scheme Improve the Outcome of Staphylococcus aureus-Caused Acute Prosthetic Joint Infections (PJI) Treated with Debridement, Antibiotics and Implant Retention (DAIR)?" Antibiotics 11, no. 7: 922. https://doi.org/10.3390/antibiotics11070922
APA StyleAuñón, Á., Tovar-Bazaga, M., Blanco-García, A., García-Cañete, J., Parrón, R., & Esteban, J. (2022). Does a New Antibiotic Scheme Improve the Outcome of Staphylococcus aureus-Caused Acute Prosthetic Joint Infections (PJI) Treated with Debridement, Antibiotics and Implant Retention (DAIR)? Antibiotics, 11(7), 922. https://doi.org/10.3390/antibiotics11070922







