Clinical Features and Outcomes of Monobacterial and Polybacterial Episodes of Ventilator-Associated Pneumonia Due to Multidrug-Resistant Acinetobacter baumannii
Abstract
1. Introduction
2. Methods and Materials
Statistical Analysis
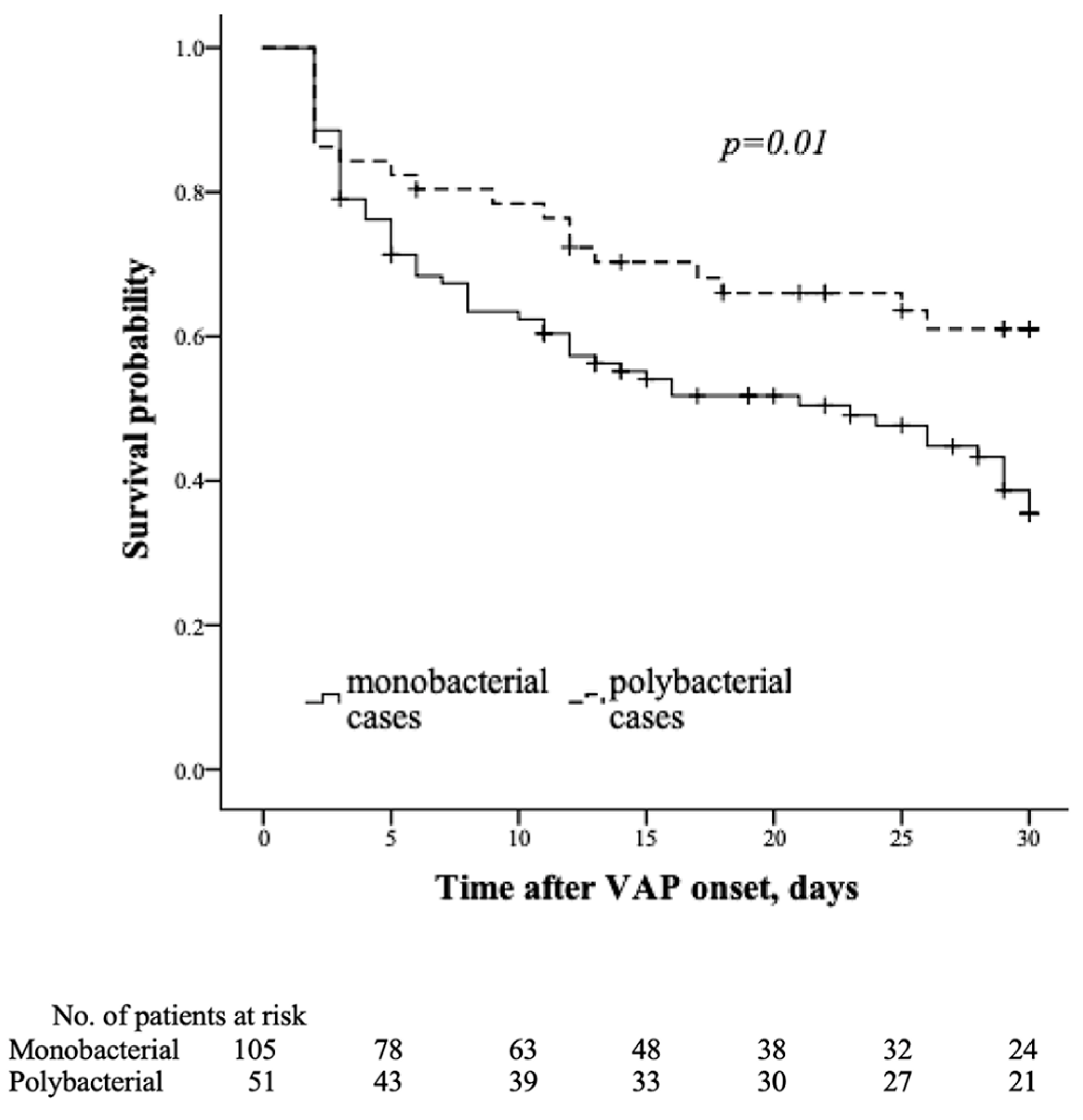
3. Results
4. Discussion
Study Novelties and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rello, J.; Lisboa, T.; Koulenti, D. Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir. Med. 2014, 2, 764–774. [Google Scholar] [CrossRef]
- Kollef, M.; Hamilton, C.W.; Ernst, F.R. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect. Control Hosp. Epidemiol. 2012, 3, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-associated pneumonia in adults: Narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Kharel, S.; Bist, A.; Mishra, S.M. Ventilator-associated pneumonia among ICU patients in WHO Southeast Asian region: A systematic review. PLoS ONE 2021, 16, e0247832. [Google Scholar] [CrossRef] [PubMed]
- Inchai, J.; Pothirat, C.; Bumroongkit, C.; Limsukon, A.; Khositsakulchai, W.; Liwsrisakun, C. Prognostic factors associated with mortality of drug-resistant Acinetobacter baumannii ventilator-associated pneumonia. J. Intensive Care 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Tsioutis, C.; Kritsotakis, E.I.; Karageorgos, S.A.; Stratakou, S.; Psarologakis, C.; Kokkini, S.; Gikas, A. Clinical epidemiology, treatment and prognostic factors of extensively drug-resistant Acinetobacter baumannii ventilator-associated pneumonia in critically ill patients. Int. J. Antimicrob. Agents 2016, 48, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Lee, S.H.; Lee, S.Y.; Yang, S.; Noh, H.; Chung, E.K.; Lee, J.I. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: A systemic review and Bayesian network meta-analysis. Crit. Care 2017, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Erdem, H.; Cag, Y.; Gencer, S.; Uysal, S.; Karakurt, Z.; Harman, R.; Aslan, A.; Mutlu-Yilmaz, E.; Karabay, O.; Uygun, Y.; et al. Treatment of ventilator-associated pneumonia (VAP) caused by Acinetobacter: Results of perspective and multicenter ID-IRI study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 45–52. [Google Scholar] [CrossRef]
- Lakbar, I.; Medam, S.; Ronfle, R.; Cassir, N.; Delamerre, L.; Hammad, E.; Lopez, A.; Lepape, A.; Machut, A.; Boucekine, M.; et al. REA RAISIN Study Group. Association between mortality and highly antimicrobial-resistant bacteria in intensive care unit-acquired pneumonia. Sci. Rep. 2021, 11, 16487. [Google Scholar] [CrossRef]
- Chang, Y.; Jeon, K.; Lee, S.M.; Cho, Y.J.; Kim, Y.S.; Chong, Y.P.; Hong, S.B. The Distribution of Multidrug-resistant Microorganisms and Treatment Status of Hospital-acquired Pneumonia/Ventilator-associated Pneumonia in Adults Intensive Care Units: A Prospective Cohort Observational Study. J. Korean Med. Sci. 2021, 36, e251. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.C.K.B.; Holder, M.W. Timing of antimicrobial therapy after identification of ventilator-associated condition is not associated with mortality in patients with ventilator-associated pneumonia: A cohort study. PLoS ONE 2014, 9, e97575. [Google Scholar] [CrossRef]
- Joseph, N.M.; Sistla, S.; Dutta, T.K.; Badhe, A.S.; Rasitha, D.; Parija, S.C. Outcome of ventilator-associated pneumonia: Impact of antibiotic therapy and other factors. Australas. Med. J. 2012, 5, 135–140. [Google Scholar] [CrossRef]
- Nowak, J.; Zander, E.; Stefanik, D.; Higgins, P.G.; Roca, I.; Vila, J.; McConnell, M.J.; Cisneros, J.M.; Seifert, H.; MagicBullet Working Group WP4. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J. Antimicrob. Chemother. 2017, 72, 3277–3282. [Google Scholar] [CrossRef] [PubMed]
- Papathanakos, G.; Andrianopoulos, I.; Papathanasiou, A.; Priavali, E.; Koulenti, D.; Koulouras, V. Colistin-resistant Acinetobacter baumannii bacteremia: A serious threat for critically ill patients. Microorganisms 2020, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Papathanakos, G.; Andrianopoulos, I.; Papathanasiou, A.; Koulenti, D.; Gartzonika, K.; Koulouras, V. Pandrug-resistant Acinetobacter baumannii treatment: Still a debatable topic with no definite solutions. J. Antimicrob. Chemother. 2020, 75, 3081. [Google Scholar] [CrossRef]
- Čiginskienė, A.; Dambrauskienė, A.; Rello, J.; Adukauskienė, D. Ventilator-Associated Pneumonia due to Drug-Resistant Acinetobacter baumannii: Risk Factors and Mortality Relation with Resistance Profiles, and Independent Predictors of In-Hospital Mortality. Medicina 2019, 55, 49. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Ioannou, P.; Samonis, G.; Kofteridis, D.P. Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii. Antibiotics 2021, 10, 1344. [Google Scholar] [CrossRef]
- Kumar, S.; Anwer, R.; Azzi, A. Virulence potential and treatment options of multidrug-resistant (MDR) Acinetobacter baumannii. Microorganisms 2021, 9, 2104. [Google Scholar] [CrossRef]
- Rello, J.; Ulldemolins, M.; Lisboa, T.; Koulenti, D.; Mañez, R.; Martin-Loeches, I.; De Waele, J.J.; Putensen, C.; Guven, M.; Deja, M.; et al. EU-VAP/CAP Study Group. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur. Respir. J. 2011, 37, 1332–1339. [Google Scholar] [CrossRef]
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Pérez-Torres, D.; Fragkou, P.C.; Zahar, J.R.; Koulenti, D. Nosocomial pneumonia in the era of multidrug-resistance: Updates in diagnosis and management. Microorganisms 2021, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [CrossRef] [PubMed]
- Dellinger, R.F.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 6.0. 2016. Available online: http://www.eucast.org (accessed on 18 May 2021).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Ollson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Difrancesco, L.F.; Liapikou, A.; Rinaudo, M.; Carbonara, M.; Li Bassi, G.; Gabarrus, A.; Torres, A. Polymicrobial intensive care unit-acquired pneumonia: Prevalence, microbiology and outcome. Crit. Care 2015, 19, 450. [Google Scholar] [CrossRef]
- Sarda, C.; Fazal, F.; Rello, J. Management of ventilator-associated pneumonia (VAP) caused by resistant gram-negative bacteria: Which is the best strategy to treat? Expert Rev. Respir. Med. 2019, 13, 789–798. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Rafailidis, P.I.; Konstantelias, A.A.; Falagas, M.E. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: The study, the patient, the bug or the drug? J. Infect. 2013, 66, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Lee, Y.J.; Wi, Y.M.; Kwan, B.S.; Jung, K.H.; Hong, W.P.; Kim, J.M. Predictors of mortality in patients with extensively drug-resistant Acinetobacter baumannii pneumonia receiving colistin therapy. Int. J. Antimicrob. Agents 2016, 48, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.C.; Wunderink, R.G.; Jones, C.B.; Leeper, K.V. Ventilator-associated pneumonia due to Pseudomonas Aeruginosa. Chest 1996, 109, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Figliolini, C.; Trouillet, J.L.; Kassis, N.; Wolff, M.; Gibert, C.; Chastre, J. Incidence and outcomes of polymicrobial ventilator-associated pneumonia. Chest 2002, 121, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Lasa, I.; Solano, C. Polymicrobial infections: Do bacteria behave differently depending on their neighbours? Virulence 2018, 9, 895–897. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, A.N.; de Jong, A.; Junker, S.; Becher, D.; Chlebowicz, M.A.; Duipmans, J.C.; Jonkman, M.F.; van Dijl, J.M. From the wound to the bench: Exoproteome interplay between wound-colonizing Staphylococcus aureus strains and co-existing bacteria. Virulence 2018, 9, 363–378. [Google Scholar] [CrossRef]
- Short, F.L.; Murdoch, S.L.; Ryan, R.P. Polybacterial human disease: The ills of social networking. Trends Microbiol. 2014, 32, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Fields, F.R.; Lee, S.W.; McConnell, M.J. Using bacterial genomes and essential genes for the development of new antibiotics. Biochem. Pharmacol. 2017, 134, 74–86. [Google Scholar] [CrossRef]
- Butler, D.A.; Biagi, M.; Tan, X.; Qasmieh, S.; Bulman, Z.P.; Wenzler, E. Multidrug Resistant Acinetobacter baumannii: Resistance by Any Other Name Would Still be Hard to Treat. Curr. Infect. Dis. Rep. 2019, 21, 46. [Google Scholar] [CrossRef]
- Almomani, B.A.; McCullough, A.; Gharaibeh, R.; Samrah, S.; Mahasneh, F. Incidence and predictors of 14-day mortality in multidrug-resistant Acinetobacter baumannii in ventilator-associated pneumonia. J. Infect. Dev. Ctries. 2015, 9, 1323–1330. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlet, J.; Carratala, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Wang, J.; Niu, H.; Wang, R.; Cai, Y. Safety and efficacy of colistin alone or in combination in adults with Acinetobacter baumannii infection: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2019, 53, 383–400. [Google Scholar] [CrossRef]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2019, 63, e01110-18. [Google Scholar] [CrossRef]
- Gurjar, M. Colistin for lung infection: An update. J. Intensive Care 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.J.; Wang, F.; Tang, L.; Bakker, J.; Liu, J.C. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2014, 44, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Thet, K.T.; Lunha, K.; Srisrattakarn, A.; Lulitanond, A.; Tavichakorntrakool, R.; Kuwatjanakul, W.; Charoensri, N.; Chanawong, A. Colistin heteroresistance in carbapenem-resistant Acinetobacter baumannii isolates from a Thai university hospital. World. J. Microbiol. Biotechnol. 2020, 36, 102. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Butler, D.; Tan, X.; Qasmieh, S.; Wenzler, E. A Breath of Fresh Air in the Fog of Antimicrobial Resistance: Inhaled Polymyxins for Gram-Negative Pneumonia. Antibiotics 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Florescu, D.F.; Qiu, F.; McCartan, M.A.; Mindru, C.; Fey, P.D.; Kalil, A.C. What is the efficacy and safety of colistin for the treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin. Infect. Dis. 2012, 54, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Valachis, A.; Samonis, G.; Kofteridis, D.P. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: A systematic review and metaanalysis. Crit. Care Med. 2015, 43, 527–533. [Google Scholar] [CrossRef]
- Sole-Lleonart, C.; Rouby, J.J.; Blot, S.; Poulakou, G.; Chastre, J.; Palmer, L.B.; Bassetti, M.; Luyt, C.E.; Pereira, J.M.; Riera, J.; et al. Nebulization of antiinfective agents in invasively mechanically ventilated adults: A systematic review and meta-analysis. Anesthesiology 2017, 126, 890–908. [Google Scholar] [CrossRef]
- Rello, J.; Solé-Lleonart, C.; Rouby, J.J.; Chastre, J.; Blot, S.; Poulakou, G.; Luyt, C.E.; Riera, J.; Palmer, L.B.; Pereira, J.M.; et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: A position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2017, 23, 629–639. [Google Scholar] [CrossRef]
- Rello, J.; Rouby, J.J.; Sole-Lleonart, C.; Chastre, J.; Blot, S.; Luyt, C.E.; Riera, J.; Vos, M.C.; Monsel, A.; Dhanani, J.; et al. Key considerations on nebulization of antimicrobial agents to mechanically ventilated patients. Clin. Microbiol. Infect. 2017, 23, 640–646. [Google Scholar] [CrossRef]
- Mei, H.; Yang, T.; Wang, J.; Wang, R.; Cai, Y. Efficacy and safety of tigecycline in treatment of pneumonia caused by MDR Acinetobacter baumannii: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2019, 74, 3423–3431. [Google Scholar] [CrossRef]
- Liu, J.; Shu, Y.; Zhu, F.; Feng, B.; Zhang, Z.; Liu, L.; Wang, G. Comparative efficacy and safety of combination therapy with high-dose sulbactam or colistin with additional antibacterial agents for multiple drug-resistant and extensively drug-resistant Acinetobacter baumannii infections: A systematic review and network meta-analysis. J. Glob. Antimicrob. Resist. 2021, 24, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Zalts, R.; Neuberger, A.; Hussein, K.; Raz-Pasteur, A.; Geffen, Y.; Mashiach, T.; Finkelstein, R. Treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia: Retrospective comparison between intravenous colistin and intravenous ampicillin-sulbactam. Am. J. Ther. 2016, 23, e78–e85. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, J.M.; Rosso-Fernández, C.M.; Roca-Oporto, C.; De Pascale, G.; Jiménez-Jorge, S.; Fernández-Hinojosa, E.; Matthaiou, D.K.; Ramírez, P.; Díaz-Miguel, R.O.; Estella, A.; et al. Colistin versus meropenem in the empirical treatment of ventilator-associated pneumonia (Magic Bullet study): An investigator-driven, open-label, randomized, noninferiority controlled trial. Crit. Care 2019, 23, 383. [Google Scholar] [CrossRef] [PubMed]
- Salloju, V.; Venapally, S.; Putti, N.; Priyanka, A. Safety and effectiveness of colistin compared with non-colistin combinations in the treatment of multi drug resistant bacterial infections. Int. J. Basic. Clin. Pharmacol. 2017, 6, 1137. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Zhang, J.; Chen, Y.; Wu, J.; Guo, B.; Wu, X.; Zhang, Y.; Wang, M.; Ya, R.; Huang, H. Combined PK/PD Index May Be a More Appropriate PK/PD Index for Cefoperazone/Sulbactam against Acinetobacter baumannii in Patients with Hospital-Acquired Pneumonia. Antibiotics 2022, 11, 703. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Poulakou, G.; Blizou, A.; Blizou, M.; Rapti, V.; Karageorgopoulos, D.E.; Koulenti, D.; Papadopoulos, A.; Matthaiou, D.K.; Tsiodras, S. The Role of Minocycline in the Treatment of Nosocomial Infections Caused by Multidrug, Extensively Drug and Pandrug Resistant Acinetobacter baumannii: A Systematic Review of Clinical Evidence. Microorganisms 2019, 7, 159. [Google Scholar] [CrossRef]
- Koulenti, D.; Song, A.; Ellingboe, A.; Abdul-Aziz, M.H.; Harris, P.; Gavey, E.; Lipman, J. Infections by multidrug-resistant Gram-negative Bacteria: What’s new in our arsenal and what’s in the pipeline? Int. J. Antimicrob. Agents 2019, 53, 211–224. [Google Scholar] [CrossRef]
- Paramythiotou, E.; Routsi, C. Association between infections caused by multidrug-resistant gram-negative bacteria and mortality in critically ill patients. World. J. Crit. Care Med. 2016, 5, 111–120. [Google Scholar] [CrossRef]
- Natarajan, R.; Ramanathan, V.; Sistla, S. Poor sensorium at the time of intubation predicts polymicrobial ventilator associated pneumonia. Ther. Clin. Risk Manag. 2022, 18, 125–133. [Google Scholar] [CrossRef]
- Ketter, P.; Yu, J.; Guentzel, M.; May, H.; Gupta, R.; Eppinger, M.; Klose, K.E.; Seshu, J.; Chambers, J.P.; Cap, A.P.; et al. Acinetobacter baumannii gastrointestinal colonization is facilitated by secretory IgA which is reductively dissociated by bacterial thioredoxin A. MBio 2018, 9, e01298-18. [Google Scholar] [CrossRef]
- Hernandez, G.; Rico, P.; Diaz, E.; Rello, J. Nosocomial lung infections in adult intensive care units. Microbes Infect. 2004, 6, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.; Niederman, M.S. Aspiration pneumonia. N. Engl. J. Med. 2019, 380, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Marin-Corral, J.; Pascual-Guardia, S.; Amati, F.; Aliberti, S.; Masclans, J.R.; Soni, N.; Rodriguez, A.; Sibila, O.; Sanz, F.; Sotgiu, N.; et al. Aspiration risk factors, microbiology, and empiric antibiotics for patients hospitalized with community-acquired pneumonia. Chest 2021, 159, 58–72. [Google Scholar] [CrossRef] [PubMed]
| Variable | VAP Origin | ||
|---|---|---|---|
| Monobacterial n = 105 | Polybacterial * n = 51 | p Value | |
| Age, years, median (IQR) | 63 (54–72) | 59 (52–67) | 0.22 |
| Sex, male, n (%) | 61 (58.1) | 32 (62.7) | 0.61 |
| Prior hospitalization within 90 days, n (%) | 61 (58.1) | 16 (31.4) | <0.01 |
| Disease severity on ICU admission, median (IQR): | |||
| 7 (4–9) | 7 (5–8) | 0.64 |
| 40.5 (33.0–56.0) | 44.0 (35.0–54.0) | 0.66 |
| Admission to ICU from, n (%): | |||
| 39 (37.1) | 23 (45.1) | 0.62 |
| 37 (35.2) | 15 (29.4) | |
| 29 (27.6) | 13 (25.5) | |
| Duration of hospital stay prior to VAP onset, days, median (IQR) | 13.0 (6.25–20.0) | 11.0 (6.0–16.75) | 1.00 |
| Duration of ICU stay prior to VAP onset, days, median, IQR | 8.5 (5.0–14.0) | 9.0 (5.0- 13.75) | 0.26 |
| Admission, n (%): | |||
| 66 (62.9) | 30 (58.8) | 0.73 |
| 39 (37.1) | 21 (41.2) | |
| CCI ≥ 3, n (%) | 71 (67.6) | 24 (47.1) | 0.01 |
| Chronic illness, n (%): | 86 (81.9) | 39 (76.5) | 0.43 |
| 73 (69.5) | 33 (64.7) | 0.55 |
| 20 (19.0) | 3 (5.9) | 0.03 |
| 8 (7.6) | 2 (3.9) | 0.50 |
| 22 (21.0) | 7 (13.7) | 0.28 |
| 9 (8.6) | 4 (7.8) | 0.89 |
| 18 (17.1) | 7 (13.7) | 0.59 |
| 17 (16.2) | 4 (7.8) | 0.15 |
| 29 (27.6) | 5 (9.8) | 0.01 |
| Organ failure on ICU admission, n (%): | |||
| 75 (71.4) | 26 (50.0) | 0.01 |
| 42 (40.0) | 20 (39.2) | 0.93 |
| 15 (14.3) | 15 (29.4) | 0.03 |
| 17 (16.2) | 6 (11.8) | 0.47 |
| 2 (1.9) | 0 (0) | 0.56 |
| 6 (5.7) | 3 (5.9) | 0.97 |
| 53 (34) | 22 (14.1) | 0.39 |
| Tracheostomy before VAP, n (%) | 15 (14.3) | 10 (19.6) | 0.49 |
| Reintubation before VAP, n (%) | 13 (12.4) | 4 (7.8) | 0.39 |
| RBC transfusion before VAP, n (%) | 55 (51.9) | 28 (57.1) | 0.54 |
| Use of IV antibiotics within 90 days, n (%): | 104 (99) | 47 (92.2) | 0.04 |
| 44 (41.9) | 16 (31.4) | 0.21 |
| 86 (81.9) | 39 (76.5) | 0.43 |
| 21 (20.0) | 6 (11.8) | 0.20 |
| 5 (4.8) | 1 (2.0) | 0.66 |
| 9 (37.1) | 9 (17.6) | 0.02 |
| 16 (15.2) | 1 (2.0) | 0.01 |
| Disease severity on VAP onset, median (IQR): | |||
| 45 (32.5–54.5) | 43 (33.0–51.0) | 0.40 |
| 6 (4–10) | 5 (4–8) | 0.53 |
| Organ failure on VAP onset, n (%): | |||
| 86 (81.9) | 35 (68.6) | 0.06 |
| 41 (39.0) | 14 (27.5) | 0.16 |
| 12 (11.4) | 15 (29.4) | 0.01 |
| 18 (17.1) | 6 (11.8) | 0.38 |
| 4 (3.8) | 0 (0) | 0.30 |
| 9 (8.6) | 2 (3.9) | 0.29 |
| 51 (48.6) | 17 (33.3) | 0.07 |
| Sepsis on VAP onset, n (%) | 93 (88.6) | 39 (76.5) | 0.049 |
| Septic shock on VAP onset, n (%) | 54 (51.9) | 18 (34.6) | 0.041 |
| Temperature on VAP onset, n (%): | |||
| 13 (12.4) | 6 (11.8) | 0.91 |
| 48 (45.7) | 22 (43.1) | 0.76 |
| Oxygenation index on VAP onset, n (%) | |||
| 19 (18.1) | 14 (27.5) | 0.18 |
| 62 (59.0) | 34 (64.7) | 0.36 |
| 24 (22.9) | 3 (5.9) | <0.01 |
| Inflammatory markers on VAP onset, median (IQR): | |||
| 12.2 (8.7–17.9) | 10.9 (7.3–13.4) | 0.03 |
| 172 (113–241) | 172 (119–235) | 0.88 |
| Acidosis on VAP onset, metabolic, n (%) | 39 (37.1) | 17 (33.3) | 0.64 |
| RRT during the ICU stay, n (%) | 39 (37.1) | 9 (17.6) | 0.01 |
| Variable | 30-Day Mortality | ||
|---|---|---|---|
| VAP Origin | p Value | ||
| Monobacterial, n/Total (%) ** | Polybacterial *, n/Total (%) ** | ||
| All sample | 60/105 (57.1) | 19/51 (37.3) | 0.02 |
| Severity on VAP diagnosis | |||
| 28/64 (43.8) | 9/35 (25.7) | 0.08 |
| 32/41 (78.0) | 10/16 (62.5) | 0.23 |
| Appropriateness of antibacterial treatment | |||
| 35/69 (50.7) | 8/34 (23.5) | <0.01 |
| 25/36 (69.4) | 11/17 (64.7) | 0.73 |
| Appropriate treatment and severity on VAP diagnosis | |||
| 18/43 (41.9) | 4/21 (19.0) | 0.07 |
| 17/26 (65.4) | 4/13 (10.3) | 0.04 |
| Time of appropriate antibacterial treatment | |||
| 26/47 (55.3) | 6/18 (33.3) | 0.11 |
| 34/58 (58.6) | 13/33 (39.4) | 0.08 |
| Antibacterial resistance profile of A. baumannii strains | |||
| 9/16 (56.3) | 3/7 (42.9) | 0.68 |
| 51/89 (57.3) | 16/44 (36.4) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adukauskiene, D.; Ciginskiene, A.; Adukauskaite, A.; Koulenti, D.; Rello, J. Clinical Features and Outcomes of Monobacterial and Polybacterial Episodes of Ventilator-Associated Pneumonia Due to Multidrug-Resistant Acinetobacter baumannii. Antibiotics 2022, 11, 892. https://doi.org/10.3390/antibiotics11070892
Adukauskiene D, Ciginskiene A, Adukauskaite A, Koulenti D, Rello J. Clinical Features and Outcomes of Monobacterial and Polybacterial Episodes of Ventilator-Associated Pneumonia Due to Multidrug-Resistant Acinetobacter baumannii. Antibiotics. 2022; 11(7):892. https://doi.org/10.3390/antibiotics11070892
Chicago/Turabian StyleAdukauskiene, Dalia, Ausra Ciginskiene, Agne Adukauskaite, Despoina Koulenti, and Jordi Rello. 2022. "Clinical Features and Outcomes of Monobacterial and Polybacterial Episodes of Ventilator-Associated Pneumonia Due to Multidrug-Resistant Acinetobacter baumannii" Antibiotics 11, no. 7: 892. https://doi.org/10.3390/antibiotics11070892
APA StyleAdukauskiene, D., Ciginskiene, A., Adukauskaite, A., Koulenti, D., & Rello, J. (2022). Clinical Features and Outcomes of Monobacterial and Polybacterial Episodes of Ventilator-Associated Pneumonia Due to Multidrug-Resistant Acinetobacter baumannii. Antibiotics, 11(7), 892. https://doi.org/10.3390/antibiotics11070892







