The Impact of Antimicrobial Stewardship and Infection Control Interventions on Acinetobacter baumannii Resistance Rates in the ICU of a Tertiary Care Center in Lebanon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hospital Setting
2.2. Antimicrobial Stewardship
2.2.1. Antimicrobial Stewardship Interventions
2.2.2. Antimicrobial Stewardship Measures
2.3. Infection Control
3. Results
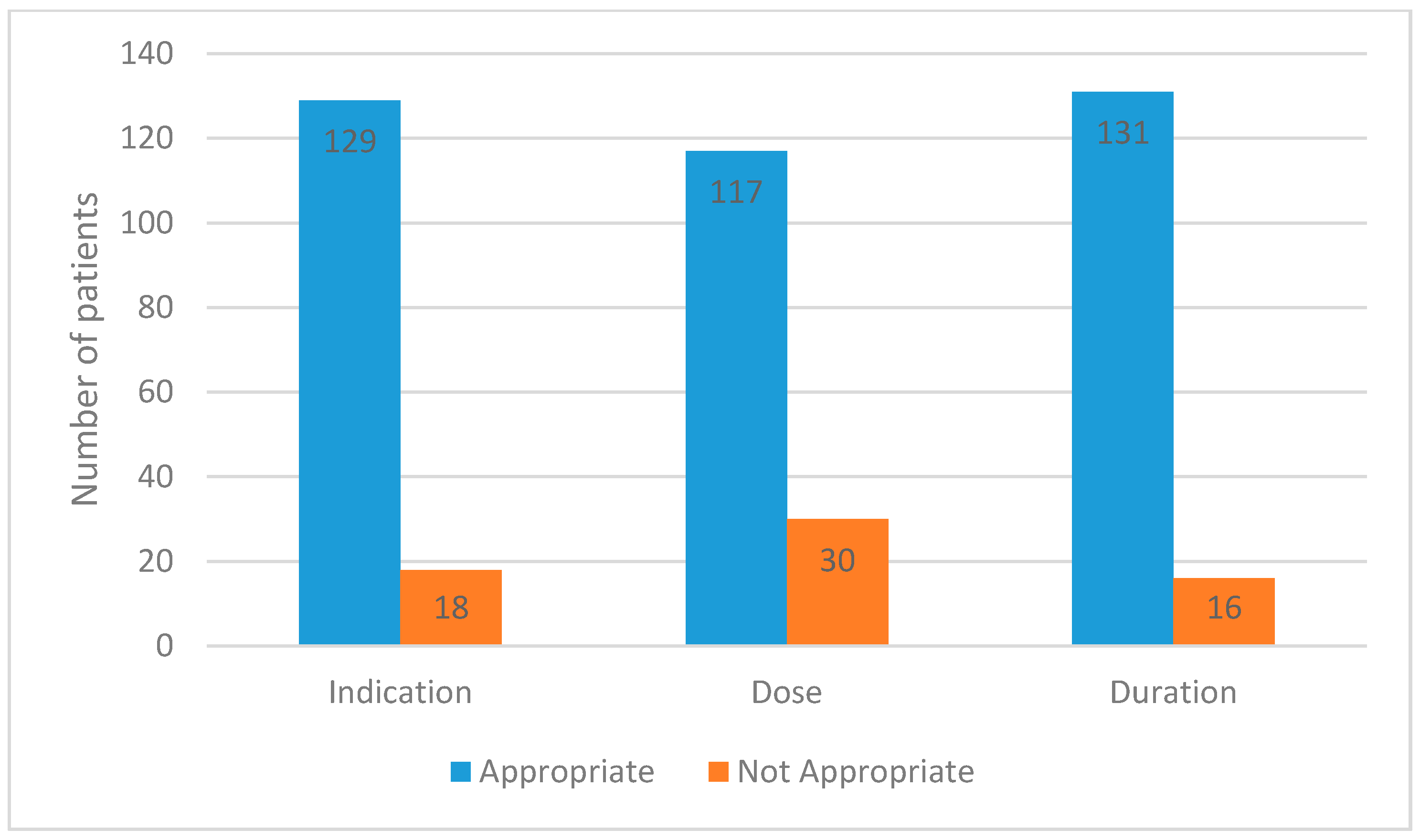
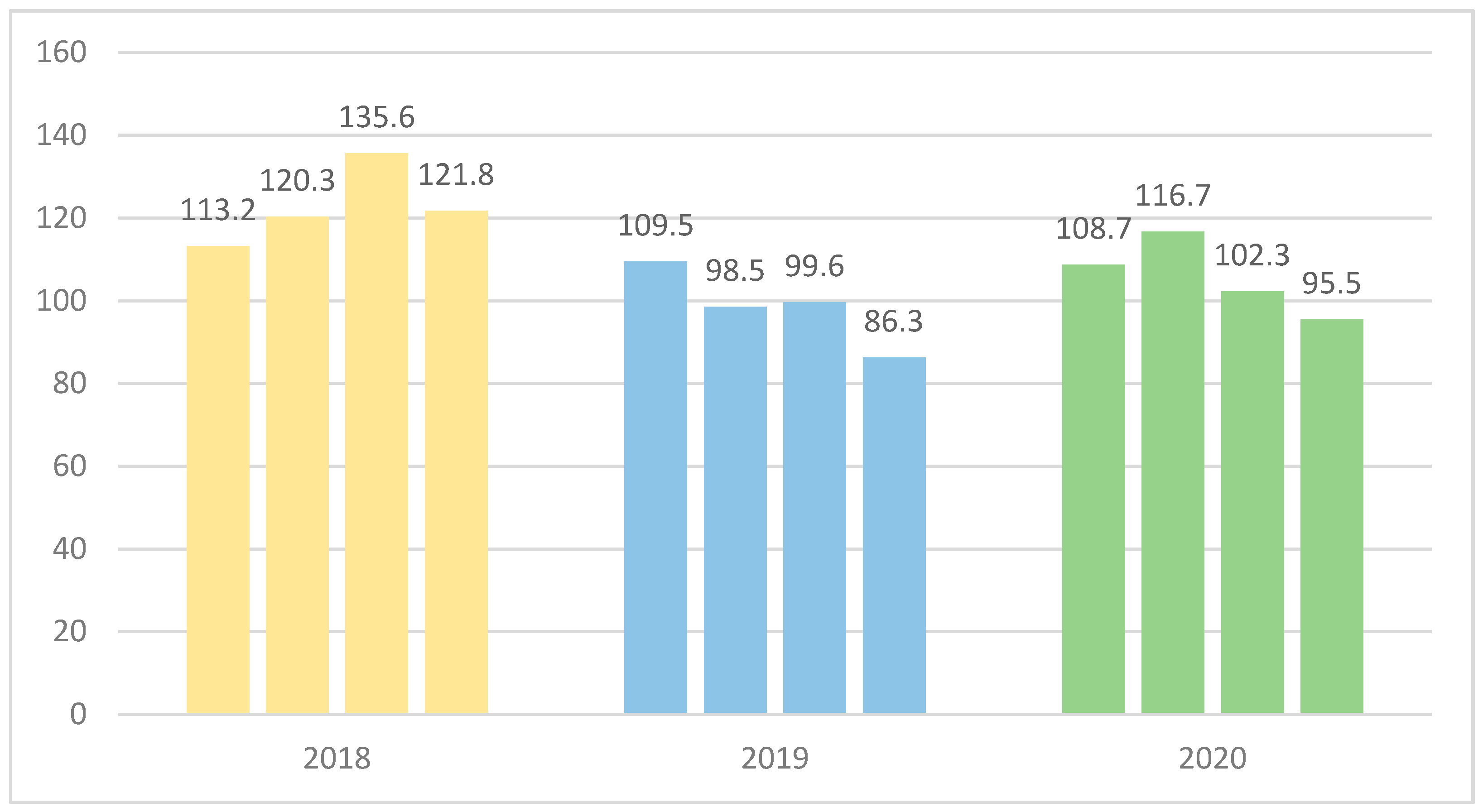
3.1. Antimicrobial Stewardship Results
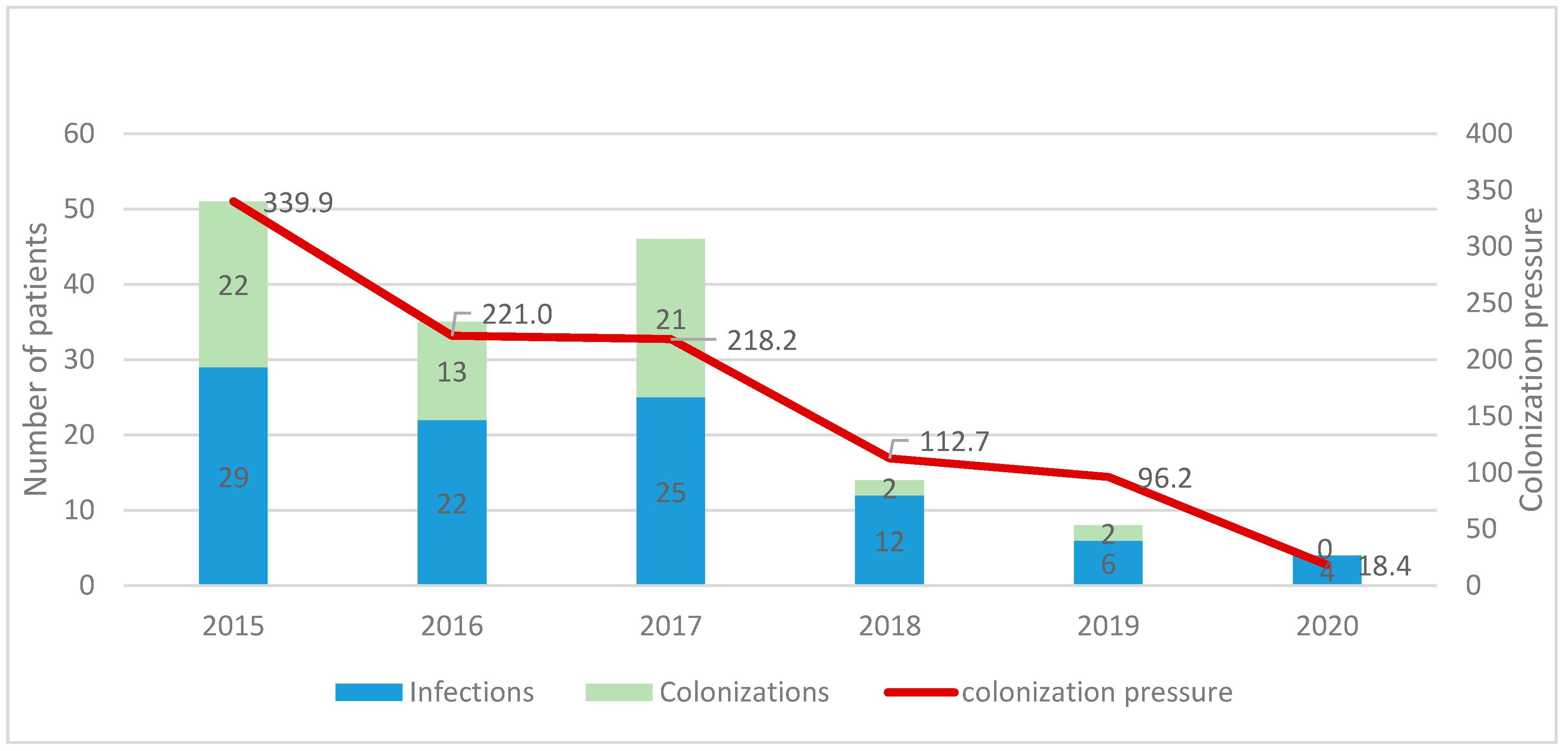
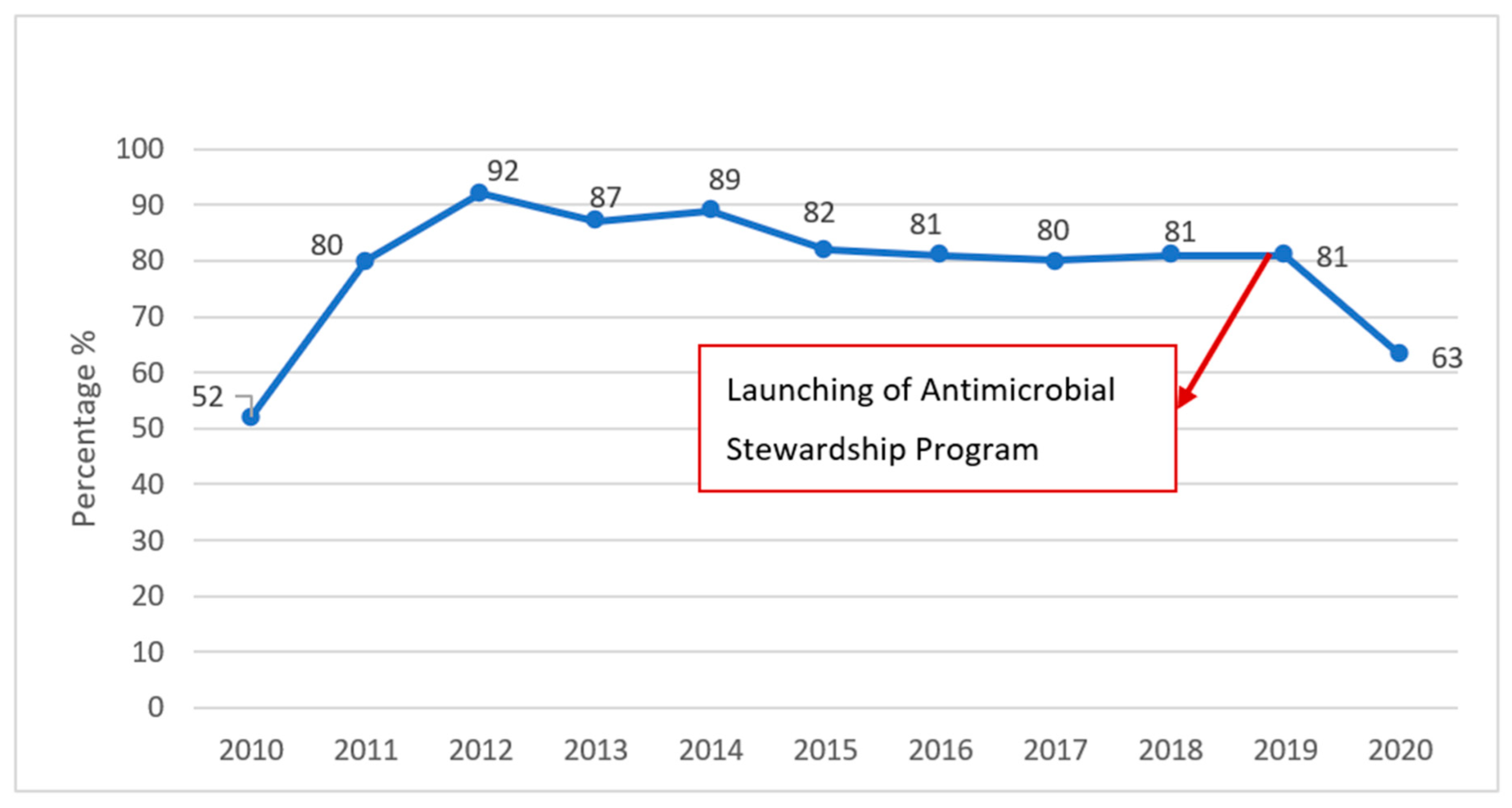
3.2. Infection Control Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- At UN, Global Leaders Commit to Act on Antimicrobial Resistance. 2016. Available online: https://www.who.int/news/item/21-09-2016-at-un-global-leaders-commit-to-act-on-antimicrobial-resistance (accessed on 17 March 2022).
- WHO. Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- By 2050, Drug-Resistant Infections Could Cause Global Economic Damage on par with 2008 Financial Crisis. 2016. Available online: https://www.worldbank.org/en/news/press-release/2016/09/18/by-2050-drug-resistant-infections-could-cause-global-economic-damage-on-par-with-2008-financial-crisis (accessed on 5 May 2022).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Maragakis, L.L.; Perencevich, E.N.; Cosgrove, S.E. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2008, 6, 751–763. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandachi, I.; Chaddad, A.; Hanna, J.; Matta, J.; Daoud, Z. Understanding the Epidemiology of Multi-Drug Resistant Gram-Negative Bacilli in the Middle East Using a One Health Approach. Front. Microbiol. 2019, 10, 1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, H.W. Bad bugs, no drugs 2002–2020: Progress, challenges, and call to action. Trans. Am. Clin. Climatol. Assoc. 2020, 131, 65–71. [Google Scholar] [PubMed]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Rello, J.; Marshall, J.K.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [Green Version]
- Talaat, M.; Zayed, B.; Tolba, S.; Abdou, E.; Gomaa, M.; Itani, D.; Hutin, Y.; Hajjeh, R. Increasing Antimicrobial Resistance in World Health Organization Eastern Mediterranean Region, 2017–2019. Emerg. Infect. Dis. 2022, 28, 717–724. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Teerawattanapong, N.; Kengkla, K.; Dilokthornsakul, P.; Saokaew, S.; Apisarnthanarak, A.; Chaiyakunapruk, N. Prevention and Control of Multidrug-Resistant Gram-Negative Bacteria in Adult Intensive Care Units: A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. 2017, 64, S51–S60. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Hays, J.P.; Kemp, A.; Okechukwu, R.; Murugaiyan, J.; Ekwanzala, M.D.; Alvarez, M.J.R.; Paul-Satyaseela, M.; Iwu, C.D.; Balleste-Delpierre, C.; et al. The potential impact of the COVID-19 pandemic on global antimicrobial and biocide resistance: An AMR Insights global perspective. JAC-Antimicrob. Resist. 2021, 3, dlab038. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, S.; Taylor, A.; Brown, A.; de Kraker, M.E.A.; El-Saed, A.; Alshamrani, M.; Hendriksen, R.S.; Jacob, M.; Löfmark, S.; Perovic, O.; et al. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: A global survey. J. Antimicrob. Chemother. 2021, 76, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- CDC. COVID-19 & Antibiotic Resistance. 2022. Available online: https://www.cdc.gov/drugresistance/covid19.html (accessed on 27 May 2022).
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2020, 27, 83–88. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Pattabiraman, V.; Konnor, R.Y.; Patel, P.R.; Wong, E.; Xu, S.Y.; Smith, B.; Edwards, J.R.; Dudeck, M.A. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2021, 43, 12–25. [Google Scholar] [CrossRef]
- Knight, G.M.; E Glover, R.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Genet. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- WHO. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Theuretzbacher, U. Global antimicrobial resistance in Gram-negative pathogens and clinical need. Curr. Opin. Microbiol. 2017, 39, 106–112. [Google Scholar] [CrossRef]
- Clark, N.M.; Zhanel, G.G.; Lynch, J.P.I. Emergence of antimicrobial resistance among Acinetobacter species: A global threat. Curr. Opin. Crit. Care 2016, 22, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Moghnieh, R.A.; Kanafani, Z.A.; Tabaja, H.Z.; Sharara, S.L.; Awad, L.S.; Kanj, S.S. Epidemiology of common resistant bacterial pathogens in the countries of the Arab League. Lancet Infect. Dis. 2018, 18, e379–e394. [Google Scholar] [CrossRef]
- Dandachi, I.; Azar, E.; Hamouch, R.; Maliha, P.; Abdallah, S.; Kanaan, E.; Badawi, R.; Khairallah, T.; Matar, G.M.; Daoud, Z. Acinetobacter spp. in a Third World Country with Socio-economic and Immigrants Challenges. J. Infect. Dev. Ctries. 2019, 13, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Abbara, A.; Rawson, T.M.; Karah, N.; El-Amin, W.; Hatcher, J.; Tajaldin, B.; Dar, O.; Dewachi, O.; Abu Sitta, G.; Uhlin, B.E.; et al. Antimicrobial resistance in the context of the Syrian conflict: Drivers before and after the onset of conflict and key recommendations. Int. J. Infect. Dis. 2018, 73, 1–6. [Google Scholar] [CrossRef] [Green Version]
- WHO. Regional Operational Framework for Implementation of the Global Action Plan on Antimicrobial Resistance. 2016. Available online: https://applications.emro.who.int/docs/Fact_Sheet_2016_EN_19200.pdf?ua=1URL (accessed on 23 March 2022).
- Al Salman, J.; Al Dabal, L.; Bassetti, M.; Alfouzan, W.A.; Al Maslamani, M.; Alraddadi, B.; Elhoufi, A.; Enani, M.; Khamis, F.A.; Mokkadas, E.; et al. Management of infections caused by WHO critical priority Gram-negative pathogens in Arab countries of the Middle East: A consensus paper. Int. J. Antimicrob. Agents 2020, 56, 106104. [Google Scholar] [CrossRef]
- Henig, O.; Weber, G.; Hoshen, M.B.; Paul, M.; German, L.; Neuberger, A.; Gluzman, I.; Berlin, A.; Shapira, C.; Balicer, R.D. Risk factors for and impact of carbapenem-resistant Acinetobacter baumannii colonization and infection: Matched case–control study. Eur. J. Clin. Microbiol. 2015, 34, 2063–2068. [Google Scholar] [CrossRef]
- Matar, G.M.; Gay, E.; Cooksey, R.C.; Elliott, J.A.; Heneine, W.M.; Uwaydah, M.M.; Matossian, R.M.; Tenover, F.C. Identification of an epidemic strain of Acinetobacter baumannii using electrophoretic typing methods. Eur. J. Epidemiol. 1992, 8, 9–14. [Google Scholar] [CrossRef]
- Sleiman, A.; Fayad, A.G.A.; Banna, H.; Matar, G.M. Prevalence and molecular epidemiology of carbapenem-resistant Gram-negative bacilli and their resistance determinants in the Eastern Mediterranean Region over the last decade. J. Glob. Antimicrob. Resist. 2021, 25, 209–221. [Google Scholar] [CrossRef]
- Chamoun, K.; Farah, M.; Araj, G.; Daoud, Z.; Moghnieh, R.; Salameh, P.; Saade, D.; Mokhbat, J.; Abboud, E.; Hamze, M.; et al. Surveillance of antimicrobial resistance in Lebanese hospitals: Retrospective nationwide compiled data. Int. J. Infect. Dis. 2016, 46, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Moghnieh, R.; Araj, G.F.; Awad, L.; Daoud, Z.; Mokhbat, J.E.; Jisr, T.; Abdallah, D.; Azar, N.; Irani-Hakimeh, N.; Balkis, M.M.; et al. A compilation of antimicrobial susceptibility data from a network of 13 Lebanese hospitals reflecting the national situation during 2015–2016. Antimicrob. Resist. Infect. Control 2019, 8, 41. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Zahreddine, N.; Tayyar, R.; Sfeir, J.; Araj, G.F.; Matar, G.M.; Kanj, S.S. Multi-drug resistant Acinetobacter species: A seven-year experience from a tertiary care center in Lebanon. Antimicrob. Resist. Infect. Control 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Atrouni, A.; Hamze, M.; Jisr, T.; Lemarié, C.; Eveillard, M.; Joly-Guillou, M.-L.; Kempf, M. Wide spread of OXA-23-producing carbapenem-resistant Acinetobacter baumannii belonging to clonal complex II in different hospitals in Lebanon. Int. J. Infect. Dis. 2016, 52, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghnieh, R.; Siblani, L.; Ghadban, D.; El Mchad, H.; Zeineddine, R.; Abdallah, D.; Ziade, F.; Sinno, L.; Kiwan, O.; Kerbaj, F.; et al. Extensively drug-resistant Acinetobacter baumannii in a Lebanese intensive care unit: Risk factors for acquisition and determination of a colonization score. J. Hosp. Infect. 2015, 92, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Makke, G.; Bitar, I.; Salloum, T.; Panossian, B.; Alousi, S.; Arabaghian, H.; Medvecky, M.; Hrabak, J.; Merheb-Ghoussoub, S.; Tokajian, S. Whole-Genome-Sequence-Based Characterization of Extensively Drug-Resistant Acinetobacter baumannii Hospital Outbreak. mSphere 2020, 5, e00934-19. [Google Scholar] [CrossRef] [Green Version]
- Moghnieh, R.; Tamim, H.; Jadayel, M.; Abdallah, D.; Al-Kassem, R.; Kadiri, H.; Hafez, H.; Al-Hassan, S.; Ajjour, L.; Lakkis, R.; et al. The effect of temporary closure and enhanced terminal disinfection using aerosolized hydrogen peroxide of an open-bay intensive care unit on the acquisition of extensively drug-resistant Acinetobacter baumannii. Antimicrob. Resist. Infect. Control 2020, 9, 108. [Google Scholar] [CrossRef]
- Chamieh, A.; Nawfal, T.D.; Ballouz, T.; Afif, C.; Juvelekian, G.; Hlais, S.; Rolain, J.M.; Azar, E. Control and Elimination of Extensively Drug-Resistant Acinetobacter baumanii in an Intensive Care Unit. Emerg. Infect. Dis. 2019, 25, 1928–1931. [Google Scholar] [CrossRef] [Green Version]
- Moghnieh, R.; Awad, L.; Abdallah, D.; Jadayel, M.; Sinno, L.; Tamim, H.; Jisr, T.; El-Hassan, S.; Lakkis, R.; Dabbagh, R.; et al. Effect of a “handshake” stewardship program versus a formulary restriction policy on High-End antibiotic use, expenditure, antibiotic resistance, and patient outcome. J. Chemother. 2020, 32, 368–384. [Google Scholar] [CrossRef]
- Kanj, S.S.; Tayyar, R.; Shehab, M.; El-Hafi, B.; Rasheed, S.S.; Kissoyan, K.A.B.; A Kanafani, Z.; Wakim, R.H.; Zahreddine, N.K.; Araj, G.F.; et al. Increased blaOXA-23-like prevalence in Acinetobacter baumannii at a tertiary care center in Lebanon (2007–2013). J. Infect. Dev. Ctries. 2018, 12, 228–234. [Google Scholar] [CrossRef]
- Araj, G. Antimicrobial Susceptibility Profiles of Bacterial Isolates at the American University of Beirut Medical Center. 2021. Available online: https://www.aub.edu.lb/fm/PLM/PublishingImages/Pages/AntimicrobialSusceptibilityProfiles/2017-AMR%20-%20AUBMC%20brochure.pdf (accessed on 17 March 2022).
- Brink, A.J. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr. Opin. Infect. Dis. 2019, 32, 609–616. [Google Scholar] [CrossRef]
- Moussally, M.; Zahreddine, N.; Kazma, J.; Ahmadieh, R.; Kan, S.S.; Kanafan, Z.A. Prevalence of antibiotic-resistant organisms among hospitalized patients at a tertiary care center in Lebanon, 2010–2018. J. Infect. Public Health 2020, 14, 12–16. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; El Zakhem, A.; Zahreddine, N.; Ahmadieh, R.; Kanj, S.S. Ten-year surveillance study of ventilator-associated pneumonia at a tertiary care center in Lebanon. J. Infect. Public Health 2019, 12, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Lebanon Goes Live with Epic. 2019. Available online: http://www.aubmc.org/Pages/AUBMC-launches-AUBHealth-a-new-comprehensive-health-record-system.aspx#sthash.uulttxN6.b3aSfvPw.dpbs (accessed on 17 March 2022).
- Karanika, S.; Paudel, S.; Grigoras, C.; Kalbasi, A.; Mylonakis, E. Systematic Review and Meta-analysis of Clinical and Economic Outcomes from the Implementation of Hospital-Based Antimicrobial Stewardship Programs. Antimicrob. Agents Chemother. 2016, 60, 4840–4852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogan, C.; Marchaim, D. The role of antimicrobial stewardship in curbing carbapenem resistance. Future Microbiol. 2013, 8, 979–991. [Google Scholar] [CrossRef] [PubMed]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs/Antibiotic Use; US Department of Health and Human Services: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf (accessed on 17 March 2022).
- El Masri, M.; Haddad, N.; Saad, T.; Rizk, N.A.; Zakhour, R.; Kanj, S.S.; Zeenny, R.M. Evaluation of Carbapenem Use Before and After Implementation of an Antimicrobial Stewardship-Led Carbapenem-Sparing Strategy in a Lebanese Tertiary Hospital: A Retrospective Study. Front. Cell. Infect. Microbiol. 2022, 12, 729491. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- The Joint Commission Antimicrobial Stewardship Resources Related to Antimicrobial Stewardship for Health Care Settings. Available online: https://www.jointcommission.org/resources/patient-safety-topics/infection-prevention-and-control/antimicrobial-stewardship/ (accessed on 17 March 2022).
- Morris, A.M. Antimicrobial Stewardship Programs: Appropriate Measures and Metrics to Study their Impact. Curr. Treat. Options Infect. Dis. 2014, 6, 101–112. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Collaborating Centre for Drug Statistics Methodology: ATC Classification Index with DDDs and Guidelines for ATC Classification and DDD Assignment; Norwegian Institute of Public Health: Oslo, Norway, 2006.
- Wieczorkiewicz, S.M.; Sincak, C. The Pharmacist’s Guide to Antimicrobial Therapy and Stewardship; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2015. [Google Scholar]
- Frost, S.A.; Alogso, M.-C.; Metcalfe, L.; Lynch, J.M.; Hunt, L.; Sanghavi, R.; Alexandrou, E.; Hillman, K.M. Chlorhexidine bathing and health care-associated infections among adult intensive care patients: A systematic review and meta-analysis. Crit. Care 2016, 20, 379. [Google Scholar] [CrossRef] [Green Version]
- Pittet, D.; Hugonnet, S.; Harbarth, S.; Mourouga, P.; Sauvan, V.; Touveneau, S.; Perneger, T.V. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Lancet 2000, 356, 1307–1312. [Google Scholar] [CrossRef]
- Talbot, T.R.; Johnson, J.G.; Fergus, C.; Domenico, J.H.; Schaffner, W.; Daniels, T.L.; Wilson, G.; Slayton, J.; Feistritzer, N.; Hickson, G.B. Sustained Improvement in Hand Hygiene Adherence: Utilizing Shared Accountability and Financial Incentives. Infect. Control Hosp. Epidemiol. 2013, 34, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Karageorgopoulos, D.E.; Falagas, M.E. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect. Dis. 2008, 8, 751–762. [Google Scholar] [CrossRef]
- Gonzalez-Villoria, A.M.; Valverde-Garduno, V. Antibiotic-Resistant Acinetobacter baumannii Increasing Success Remains a Challenge as a Nosocomial Pathogen. J. Pathog. 2016, 2016, 7318075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Lozano, J.-M.; THRESHOLDS study group; Lawes, T.; Nebot, C.; Beyaert, A.; Bertrand, X.; Hocquet, D.; Aldeyab, M.; Scott, M.; Conlon-Bingham, G.; et al. A nonlinear time-series analysis approach to identify thresholds in associations between population antibiotic use and rates of resistance. Nat. Microbiol. 2019, 4, 1160–1172. [Google Scholar] [CrossRef]
- Lvarez-Marín, R.; López-Cerero, L.; Guerrero-Sánchez, F.; Palop-Borras, B.; Rojo-Martín, M.D.; Ruiz-Sancho, A.; Herrero-Rodríguez, C.; García, M.V.; Lazo-Torres, A.M.; López, I.; et al. Do specific antimicrobial stewardship interventions have an impact on carbapenem resistance in Gram-negative bacilli? A multicentre quasi-experimental ecological study: Time-trend analysis and characterization of carbapenemases. J. Antimicrob. Chemother. 2021, 76, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Yusef, D.; A Hayajneh, W.; Issa, A.B.; Haddad, R.; Al-Azzam, S.; A Lattyak, E.; Lattyak, W.J.; Gould, I.; Conway, B.R.; Bond, S.; et al. Impact of an antimicrobial stewardship programme on reducing broad-spectrum antibiotic use and its effect on carbapenem-resistant Acinetobacter baumannii (CRAb) in hospitals in Jordan. J. Antimicrob. Chemother. 2020, 76, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ogutlu, A.; Guclu, E.; Karabay, O.; Utku, A.C.; Tuna, N.; Yahyaoglu, M. Effects of Carbapenem consumption on the prevalence of Acinetobacter infection in intensive care unit patients. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-H.; Lin, L.-C.; Chang, Y.-J.; Chen, Y.-M.; Chang, C.-Y.; Huang, C.-C. Infection Control Programs and Antibiotic Control Programs to Limit Transmission of Multi-Drug Resistant Acinetobacter baumannii Infections: Evolution of Old Problems and New Challenges for Institutes. Int. J. Environ. Res. Public Health 2015, 12, 8871–8882. [Google Scholar] [CrossRef]
- Wilks, M.; Wilson, A.; Warwick, S.; Price, E.; Kennedy, D.; Ely, A.; Millar, M.R. Control of an Outbreak of Multidrug-Resistant Acinetobacter baumannii-calcoaceticus Colonization and Infection in an Intensive Care Unit (ICU) Without Closing the ICU or Placing Patients in Isolation. Infect. Control Hosp. Epidemiol. 2006, 27, 654–658. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; García, L.; Ramírez, E.; Martínez-Martínez, L.; Muniain, M.A.; Fernández-Cuenca, F. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive “bundle” approach. Am. J. Infect. Control. 2009, 37, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Cheon, S.; Kim, M.-J.; Yun, S.-J.; Moon, J.Y.; Kim, Y.-S. Controlling endemic multidrug-resistant Acinetobacter baumannii in Intensive Care Units using antimicrobial stewardship and infection control. Korean J. Intern. Med. 2016, 31, 367–374. [Google Scholar] [CrossRef]
- Valencia-Martín, R.; Gonzalez-Galan, V.; Alvarez-Marín, R.; Cazalla-Foncueva, A.M.; Aldabó, T.; Gil-Navarro, M.V.; Alonso-Araujo, I.; Martin, C.; Gordon, R.; García-Nuñez, E.J.; et al. A multimodal intervention program to control a long-term Acinetobacter baumannii endemic in a tertiary care hospital. Antimicrob. Resist. Infect. Control 2019, 8, 199. [Google Scholar] [CrossRef]
- Abouzeid, M.; Habib, R.R.; Jabbour, S.; Mokdad, A.H.; Nuwayhid, I. Lebanon’s humanitarian crisis escalates after the Beirut blast. Lancet 2020, 396, 1380–1382. [Google Scholar] [CrossRef]
- Jabbour, R.; Harakeh, M.; Sailan, S.D.; Nassar, V.; Tashjian, H.; Massouh, J.; Massouh, A.; Puzantian, H.; Darwish, H. Nurses’ stories from Beirut: The 2020 explosive disaster on top of a pandemic and economic crises. Int. Nurs. Rev. 2021, 68, 1–8. [Google Scholar] [CrossRef]
- Gebran, A.; Abou Khalil, E.; El Moheb, M.; Albaini, O.; El Warea, M.; Ibrahim, R.; Karam, K.; El Helou, M.O.; Ramly, E.P.; El Hechi, M.; et al. The Beirut Port Explosion Injuries and Lessons Learned: Results of the Beirut Blast Assessment for Surgical Services (BASS) Multicenter Study. Ann. Surg. 2022, 275, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Bizri, A.R.; Khalil, P.B. A Lebanese physician’s dilemma: Not how, but with what? Lancet 2021, 398, 841. [Google Scholar] [CrossRef]
- Zahreddine, N.K.; Haddad, S.F.; Kerbage, A.; Kanj, S.S. Challenges of coronavirus disease 2019 (COVID-19) in Lebanon in the midst of the economic collapse. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, E67. [Google Scholar] [CrossRef]
- Rizk, N.A.; Moghnieh, R.; Haddad, N.; Rebeiz, M.-C.; Zeenny, R.M.; Hindy, J.-R.; Orlando, G.; Kanj, S.S. Challenges to Antimicrobial Stewardship in the Countries of the Arab League: Concerns of Worsening Resistance during the COVID-19 Pandemic and Proposed Solutions. Antibiotics 2021, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Allaw, F.; Zahreddine, N.K.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.; Dbaibo, G.; Kanj, S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens 2021, 10, 157. [Google Scholar] [CrossRef]



| Metrics | Equations |
|---|---|
| DDD per 1000 patient days | |
| DOT per 1000 patient days | Days on which patients received more than one carbapenem are counted only once |
| CP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizk, N.A.; Zahreddine, N.; Haddad, N.; Ahmadieh, R.; Hannun, A.; Bou Harb, S.; Haddad, S.F.; Zeenny, R.M.; Kanj, S.S. The Impact of Antimicrobial Stewardship and Infection Control Interventions on Acinetobacter baumannii Resistance Rates in the ICU of a Tertiary Care Center in Lebanon. Antibiotics 2022, 11, 911. https://doi.org/10.3390/antibiotics11070911
Rizk NA, Zahreddine N, Haddad N, Ahmadieh R, Hannun A, Bou Harb S, Haddad SF, Zeenny RM, Kanj SS. The Impact of Antimicrobial Stewardship and Infection Control Interventions on Acinetobacter baumannii Resistance Rates in the ICU of a Tertiary Care Center in Lebanon. Antibiotics. 2022; 11(7):911. https://doi.org/10.3390/antibiotics11070911
Chicago/Turabian StyleRizk, Nesrine A., Nada Zahreddine, Nisrine Haddad, Rihab Ahmadieh, Audra Hannun, Souad Bou Harb, Sara F. Haddad, Rony M. Zeenny, and Souha S. Kanj. 2022. "The Impact of Antimicrobial Stewardship and Infection Control Interventions on Acinetobacter baumannii Resistance Rates in the ICU of a Tertiary Care Center in Lebanon" Antibiotics 11, no. 7: 911. https://doi.org/10.3390/antibiotics11070911
APA StyleRizk, N. A., Zahreddine, N., Haddad, N., Ahmadieh, R., Hannun, A., Bou Harb, S., Haddad, S. F., Zeenny, R. M., & Kanj, S. S. (2022). The Impact of Antimicrobial Stewardship and Infection Control Interventions on Acinetobacter baumannii Resistance Rates in the ICU of a Tertiary Care Center in Lebanon. Antibiotics, 11(7), 911. https://doi.org/10.3390/antibiotics11070911








