A Survey on the Rationale Usage of Antimicrobial Agents in Small Animal Clinics and Farms in Trinidad and Jamaica
Abstract
:1. Introduction
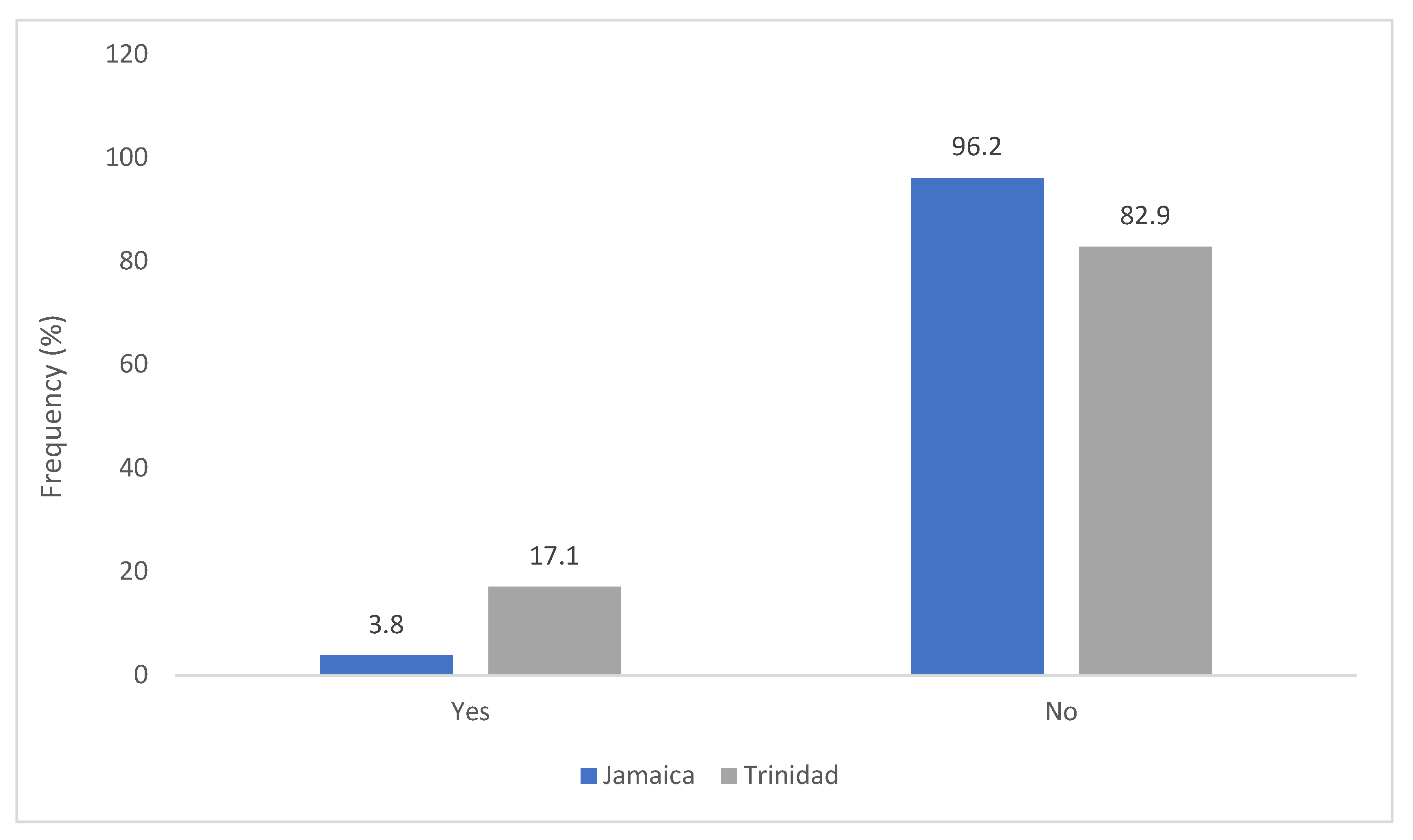
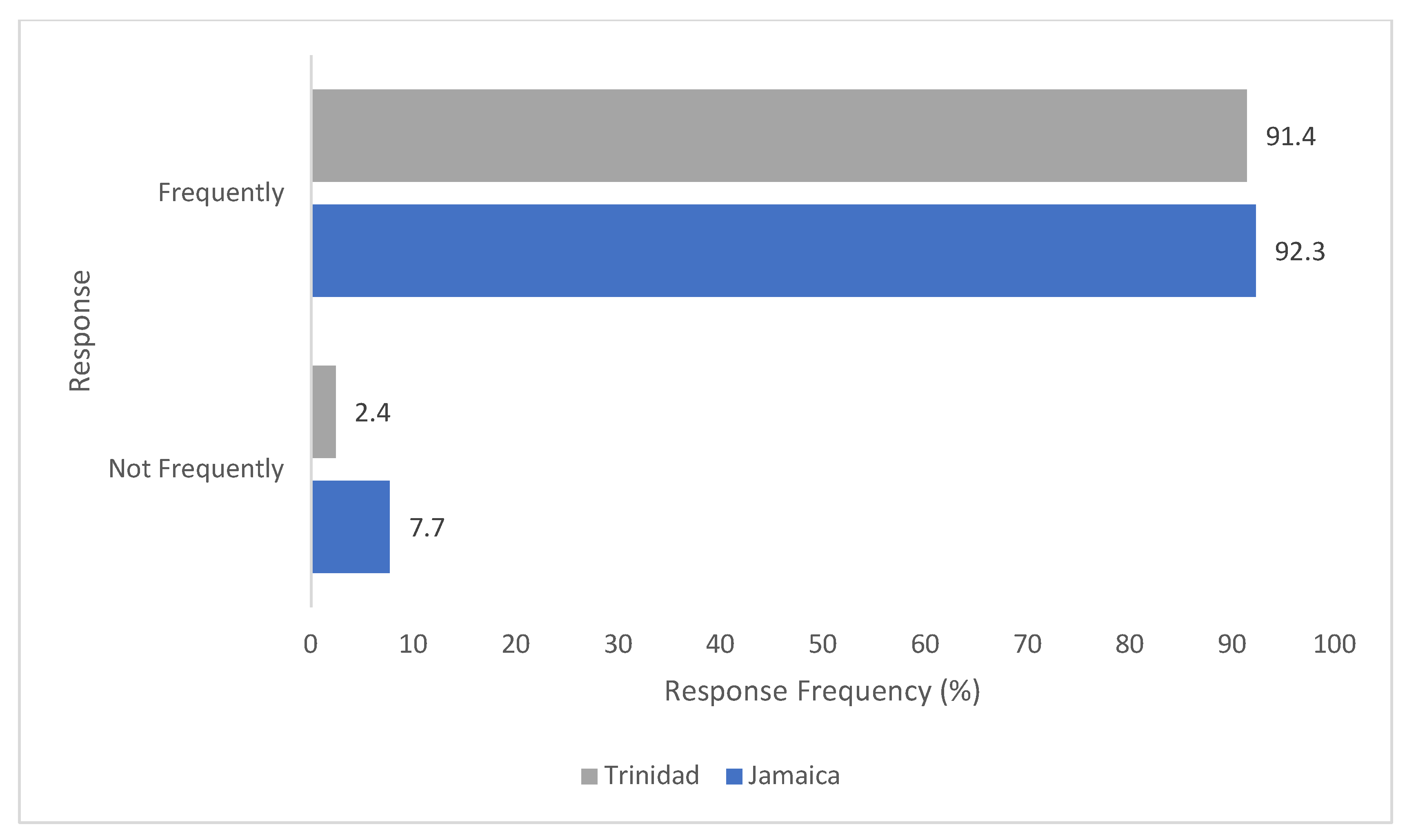
2. Results
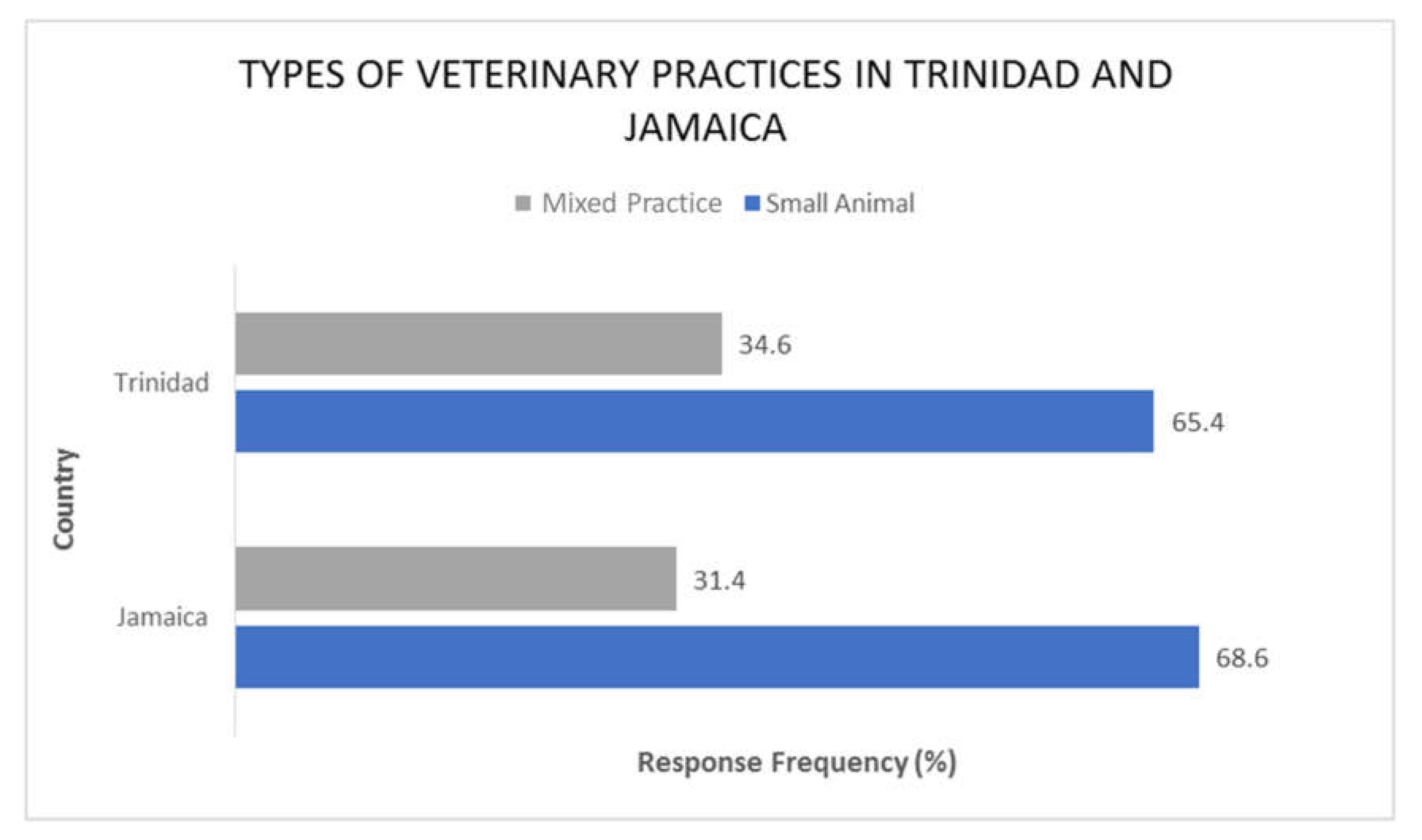
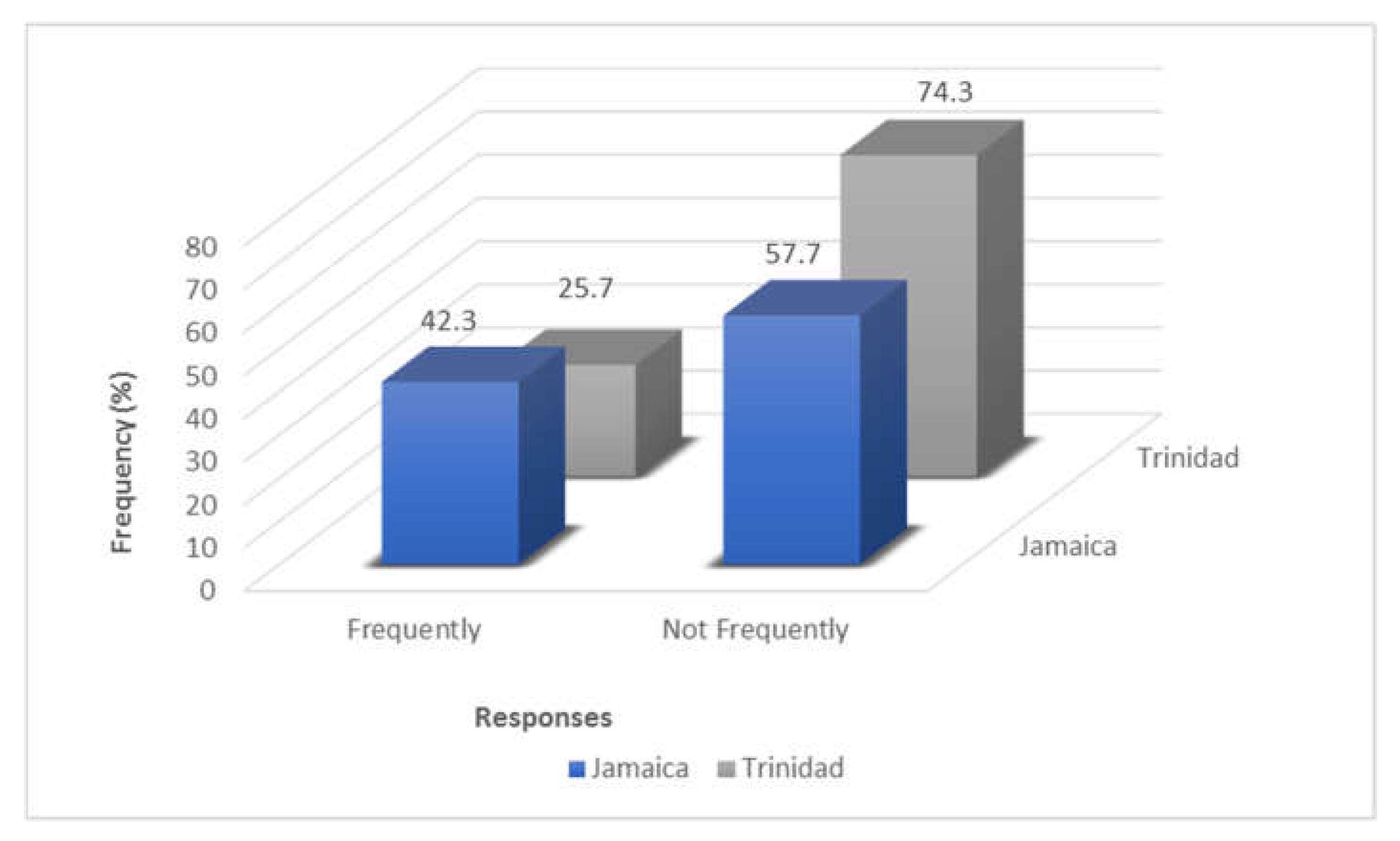
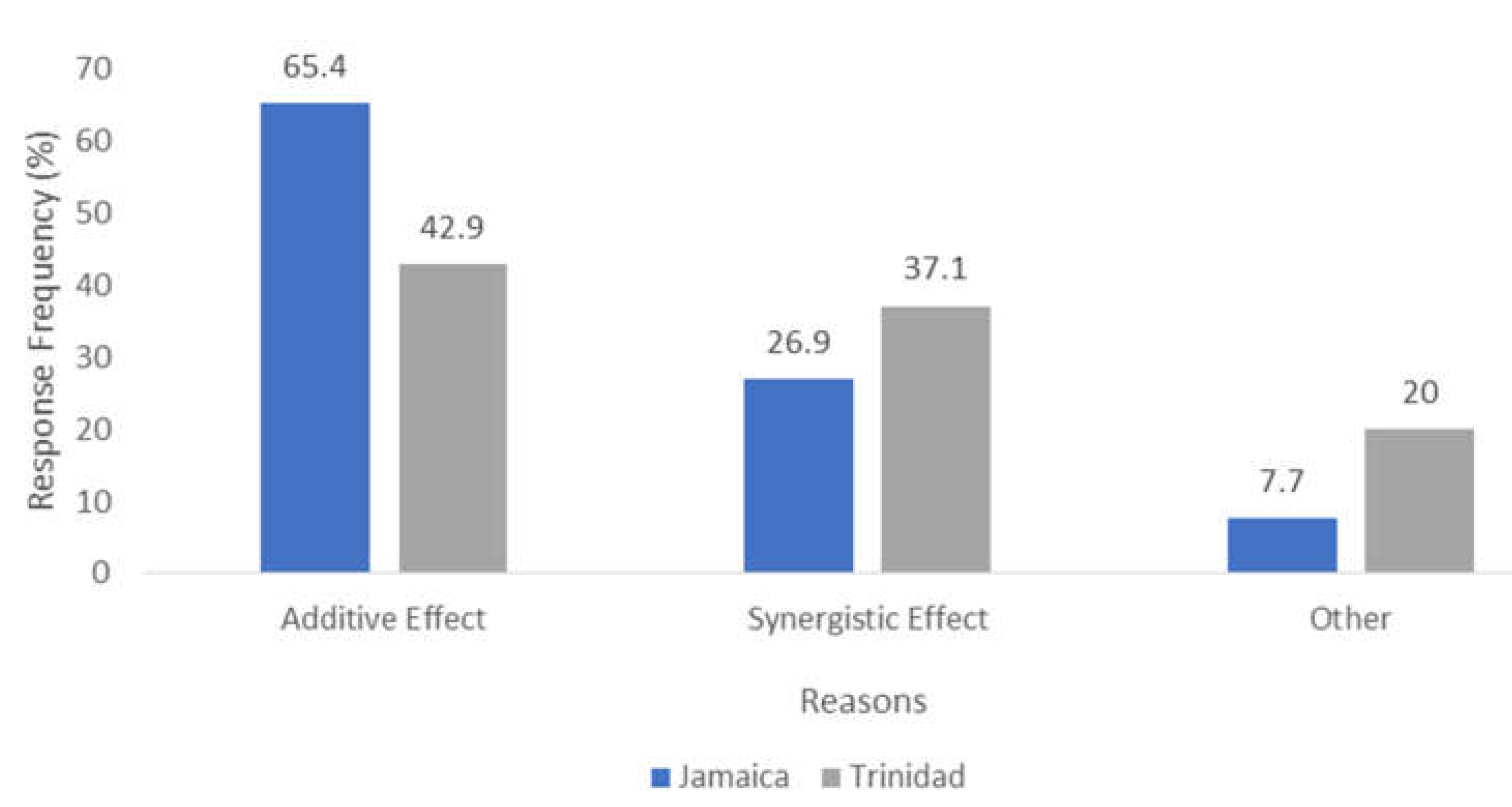
2.1. Trinidad Questionnaire Analysis
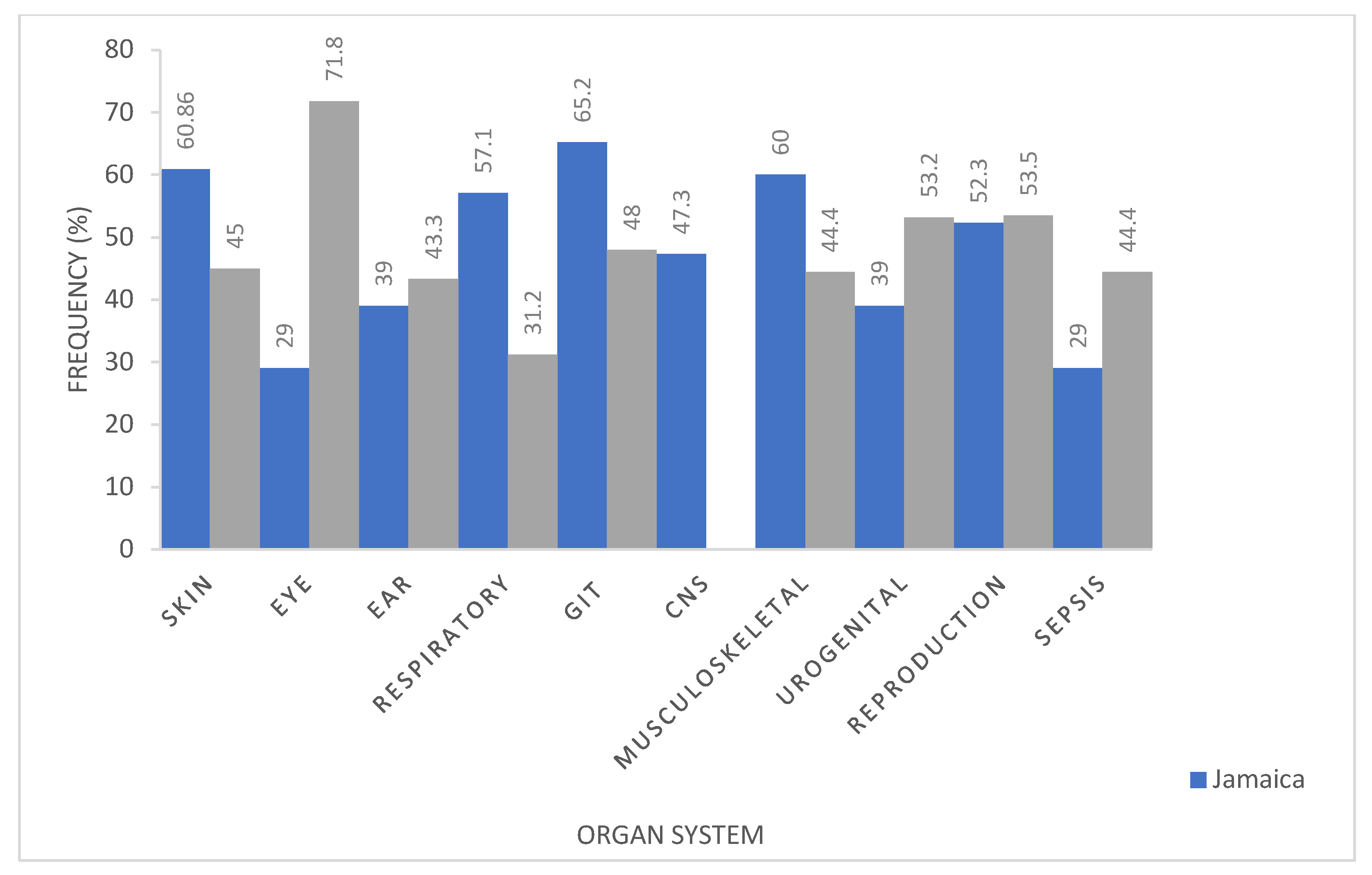
2.2. Jamaica Questionnaire Analysis
2.3. Comparative Analysis of Results—Trinidad and Jamaica
3. Discussion
3.1. Limitations of the Study
3.1.1. Discrepancy in Clinic and Farm Lists
3.1.2. Information on Drug Use on Farms in Jamaica
3.1.3. Survey Dissemination
3.1.4. Validity/Reliability/Risk of Bias of Questionnaires
4. Materials and Methods
4.1. Study Design and Questioner
4.2. Sample Size
4.3. Statistical Analyses
4.4. Ethical Consideration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilchrist, M.J.; Greko, C.; Wallinga, D.B.; Beran, G.W.; Riley, D.G.; Thorne, P.S. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ. Health Perspect. 2007, 115, 313–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungemach, F.R.; Müller-Bahrdt, D.; Abraham, G. Guidelines for prudent use of antimicrobials and their implications on antibiotic usage in veterinary medicine. Int. J. Med. Microbiol. 2006, 296, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Speksnijder, D.; Mevius, D.; Bruschke, C.; Wagenaar, J. Reduction of veterinary antimicrobial use in the Netherlands. The Dutch success model. Zoonoses Public Health 2015, 62, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr. Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- Martinez, L. Global infectious disease surveillance. Int. J. Infect. Dis. 2000, 4, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Haque, M. Antibiotic Use, Antibiotic Resistance, and Antibiotic Stewardship–A Global Public Consequences. Bangladesh J. Med. Sci. 2019, 18, 169–170. [Google Scholar] [CrossRef] [Green Version]
- Wojkowska-Mach, J.; Godman, B.; Glassman, A.; Kurdi, A.; Pilc, A.; Rozanska, A.; Skoczyński, S.; Wałaszek, M.; Bochenek, T. Antibiotic consumption and antimicrobial resistance in Poland; findings and implications. Antimicrob. Resist. Infect. Control 2018, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Usha, P.; Jose, S.; Nisha, A. Antimicrobial Drug Resistance-A global concern. Vet. World 2010, 3, 138–139. [Google Scholar]
- Ungemach, F. Figures on quantities of antibacterials used for different purposes in the EU countries and interpretation. Acta Vet. Scand. Suppl. 2000, 93, 89–97. [Google Scholar]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ammini, P.; Kumar, S.; Varela, M.F. The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms 2017, 5, 11. [Google Scholar] [CrossRef]
- Smith, P.W.; Agbaje, M.; LeRoux-Pullen, L.; Van Dyk, D.; Debusho, L.K.; Shittu, A.; Sirdar, M.M.; Fasanmi, O.G.; Adebowale, O.; Fasina, F.O. Implication of the knowledge and perceptions of veterinary students of antimicrobial resistance for future prescription of antimicrobials in animal health, South Africa. J. S. Afr. Vet. Assoc. 2019, 90, a1576. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, M.; Dougherty, J.; Poppe, C. Antimicrobial resistance. Health Policy Res. Bull. 2003, 6, 6–9. [Google Scholar]
- Fasina, F.O.; LeRoux-Pullen, L.; Smith, P.; Debusho, L.K.; Shittu, A.; Jajere, S.M.; Adebowale, O.; Odetokun, I.; Agbaje, M.; Fasina, M.M. Knowledge, attitudes, and perceptions associated with antimicrobial stewardship among veterinary students: A multi-country survey from Nigeria, South Africa, and Sudan. Front. Public Health 2020, 8, 517964. [Google Scholar] [CrossRef]
- Mendelson, M.; Matsoso, M.P. The World Health Organization global action plan for antimicrobial resistance. SAMJ S. Afr. Med. J. 2015, 105, 325. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Migura, L.; Hendriksen, R.S.; Fraile, L.; Aarestrup, F.M. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: The missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 2014, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kools, S.A.; Moltmann, J.F.; Knacker, T. Estimating the use of veterinary medicines in the European Union. Regul. Toxicol. Pharmacol. 2008, 50, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Carmo, L.P.; Schüpbach-Regula, G.; Müntener, C.; Chevance, A.; Moulin, G.; Magouras, I. Approaches for quantifying antimicrobial consumption per animal species based on national sales data: A Swiss example, 2006 to 2013. Eurosurveillance 2017, 22, 30458. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, D.H. Reservoirs of antimicrobial resistance in pet animals. Clin. Infect. Dis. 2007, 45, S148–S152. [Google Scholar] [CrossRef] [PubMed]
- Collineau, L.; Belloc, C.; Stärk, K.D.; Hémonic, A.; Postma, M.; Dewulf, J.; Chauvin, C. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses Public Health 2017, 64, 165–184. [Google Scholar] [CrossRef] [Green Version]
- Saradamma, R.D.; Higginbotham, N.; Nichter, M. Social factors influencing the acquisition of antibiotics without prescription in Kerala State, south India. Soc. Sci. Med. 2000, 50, 891–903. [Google Scholar] [CrossRef]
- Ozturk, Y.; Celik, S.; Sahin, E.; Acik, M.N.; Cetinkaya, B. Assessment of farmers’ knowledge, attitudes and practices on antibiotics and antimicrobial resistance. Animals 2019, 9, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipangura, J.K.; Eagar, H.; Kgoete, M.; Abernethy, D.; Naidoo, V. An investigation of antimicrobial usage patterns by small animal veterinarians in South Africa. Prev. Vet. Med. 2017, 136, 29–38. [Google Scholar] [CrossRef]
- Bonelli, R.R.; Moreira, B.M.; Picão, R.C. Antimicrobial resistance among Enterobacteriaceae in South America: History, current dissemination status and associated socioeconomic factors. Drug Resist. Updates 2014, 17, 24–36. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization report: Current crisis of antibiotic resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tree, M.; McDougall, S.; Beggs, D.S.; Robertson, I.D.; Lam, T.J.; Aleri, J.W. Antimicrobial use on Australian dairy cattle farms–A survey of veterinarians. Prev. Vet. Med. 2022, 202, 105610. [Google Scholar] [CrossRef] [PubMed]
- Servia-Dopazo, M.; Taracido-Trunk, M.; Figueiras, A. Non-Clinical Factors Determining the Prescription of Antibiotics by Veterinarians: A Systematic Review. Antibiotics 2021, 10, 133. [Google Scholar] [CrossRef]
- Hardefeldt, L.Y.; Browning, G.F.; Thursky, K.A.; Gilkerson, J.R.; Billman-Jacobe, H.; Stevenson, M.A.; Bailey, K.E. Cross-sectional study of antimicrobials used for surgical prophylaxis by bovine veterinary practitioners in Australia. Vet. Rec. 2017, 181, 426. [Google Scholar] [CrossRef]
- Wayne, A.; McCarthy, R.; Lindenmayer, J. Therapeutic antibiotic use patterns in dogs: Observations from a veterinary teaching hospital. J. Small Anim. Pract. 2011, 52, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- van den Bogaard, A.E.; Stobberingh, E.E. Epidemiology of resistance to antibiotics: Links between animals and humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Morar, A.; Ban-Cucerzan, A.; Herman, V.; Tîrziu, E.; Sallam, K.I.; Abd-Elghany, S.M.; Imre, K. Multidrug resistant coagulase-positive Staphylococcus aureus and their enterotoxins detection in traditional cheeses marketed in Banat Region, Romania. Antibiotics 2021, 10, 1458. [Google Scholar] [CrossRef]
- Chambers, H.F. General principles of antimicrobial therapy. In Goodman Gilman’s the Pharmacological Basis of Therapeutics; McGraw-Hill: New York, NY, USA, 2006; pp. 1095–1111. [Google Scholar]
- Lew, T.; Putsathit, P.; Sohn, K.M.; Wu, Y.; Ouchi, K.; Ishii, Y.; Tateda, K.; Riley, T.V.; Collins, D.A. Antimicrobial susceptibilities of Clostridium difficile isolates from 12 Asia-Pacific countries in 2014 and 2015. Antimicrob. Agents Chemother. 2020, 64, e00296-20. [Google Scholar] [CrossRef] [PubMed]
- Scott Weese, J.; Page, S.W.; Prescott, J.F. Antimicrobial Stewardship in Animals. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2013; pp. 117–132. [Google Scholar]
- Jessen, L.R.; Sørensen, T.M.; Lilja, Z.L.; Kristensen, M.; Hald, T.; Damborg, P. Cross-sectional survey on the use and impact of the Danish national antibiotic use guidelines for companion animal practice. Acta Vet. Scand. 2017, 59, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djebala, S.; Moula, N.; Bayrou, C.; Sartelet, A.; Bossaert, P. Prophylactic antibiotic usage by Belgian veterinarians during elective caesarean section in Belgian blue cattle. Prev. Vet. Med. 2019, 172, 104785. [Google Scholar] [CrossRef] [PubMed]
| Type of Practice | Most Commonly Used Antimicrobial Agents | |
|---|---|---|
| Small Animal Practice | Trinidad | Jamaica |
| Dogs | Cephalexin (8; 25%), Trimethoprim-Sulfamethoxazole, (7; 21.9%), (Amoxicillin 11; 34.4%), Procaine Penicillin and Streptomycin (5; 15.6%), Doxycycline (21; 65.6%), Enrofloxacin, (12; 37.5%) Combikel (procaine benzylpenicillin, benzathine benzylpenicillin, dihydrostreptomycin sulfate (3; 9.4%), Ciprofloxacin, (5; 15.6%); Amoxicillin/ Clavulanic Acid, (23; 71.9%), Metronidazole (5; 15.6%); Terramycin (2; 6.3%), Gentamycin (2; 6.3%), Itraconazole (2; 6.3%) Ketoconazole (2; 6.3%) | Penicillin, (3;11.5%) Gentamicin, (7; 26.9%), Cephalosporins, (3; 11.5%) Amoxicillin Clavulanic (12; 46.2%) Acid, Trimethoprim Sulfa, (5; 19.2%) Cefadroxil, (2; 7.7% Cefuroxime (3; 11.5%) Metronidazole (6; 23.1%); Amoxicillin (14; 53.8%) Azithromycin (1; 3.8%), Ciprofloxacin (1; 3.8%), Doxycycline (5; 19.2%) Enrofloxacin, (4; 15.4%) Clindamycin (1; 3.8%), Ketoconazole (3; 11.5%), Avermectins (1; 3.8%) Fenbendazole (3; 11.5%);, Tetracyclines, (1; 3.8%), Ceftriaxone (1; 3.8%), Cefadroxil (2; 7.7%), |
| Cats | Amoxicillin (15; 46.9%), Amoxicillin/Clavulanic Acid, (25; 78.1%), Doxycycline (4; 12.5%), Metronidazole (3; 9.4%), Enrofloxacin (5; 15.6%), Trimethoprim-Sulfamethoxazole (2; 6.25%) Ciprofloxacin (2; 6.25%), Cephalexin (3; 9.4) Clotrimazole (1; 3.1%) Gentamycin (1; 3.1%), | Penicillins (2; 7.7%), Cefovecin (1; 3.8), Amoxicillin Clavulanic Acid, (10; 38.5%) Trimethoprim Sulfa, (5; 19.2%) Cefadroxil (1; 3.8%), Cefuroxime (3; 11.5%), Amoxicillin, (17; 65.9%) Ciprofloxacin(2; 7.7%) Doxycycline, (3; 11.5%) Enroflox-acin, (4; 15.4%), Gentamicin (4; 15.4%), Ceftriaxone, (1; 3.8%) Tetracyclines, (1; 3.8%) Ketoconazole, (1; 3.8%) Metronidazole (1; 3.8%) |
| Mixed practices | ||
| Pigs | Procaine Penicillin and Streptomycin (1; 10%), Combikel (procaine benzylpenicillin, benzathine benzylpenicillin, dihydrostreptomycin sulfate) (2; 20%), Amoxicillin (1; 10%), Enrofloxacin (1; 10%) | Quinolones (1; 11.1%), Amoxicillin (2; 22.2%), Oxytetracycline (2; 22.2%) Neomycin-Tetracycline combination (1; 11.1%) |
| Poultry | Trimethoprim-Sulfamethoxazole (2; 20%) Piperazine (1; 10%), Amoxicillin (2; 20%) | Sulphonamide (2; 22.2%) |
| Sheep | Penstrep (Procaine Penicillin and Streptomycin) (5; 50%), Combikel (procaine benzylpenicillin, benzathine benzylpenicillin, dihydrostreptomycin sulfate) (2; 20%), Penbendazole (1; 10%); Sulphonamides (1; 10%) | Amoxicillin (2; 22.2%), Oxytetracycline,(1; 11.1%) |
| Cattle | Penstrep (Procaine Penicillin and Streptomycin), (6; 60%); Oxytetracycline (3; 30%); Combikel (procaine benzylpenicillin, benzathine benzylpenicillin, dihydrostreptomycin sulfate) (2; 20%) | Tetracycline, (1; 11.1%) Amoxicillin (3; 33.3%), Oxytetracycline, (1; 11.1%) Gentamicin, (1; 11.1%) Cephapirin (1; 11.1%) |
| Goats | Penstrep (Procaine Penicillin and Streptomycin), (6; 60%) Combikel (procaine benzylpenicillin, benzathine benzylpenicillin, dihydrostreptomycin sulfate), (2; 20%) Oxytetracycline,(2; 20%) Trimethoprim-Sulfamethoxazole, (1; 10%) | Amoxicillin, (4; 44.4% Oxytetracycline, (1; 11.1%) Trimethoprim-Sulfa (1; 11.1%) |
| Horses | Penicillins, (1; 11.1%) Gentamicin, (2; 22.2%) Oxytetracycline (1; 11.1%) | |
| Porcupine | Enrofloxacin (1; 10%) | |
| Drug | Country | Response | p-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Amoxicillin-Clavulanate | Trinidad Jamaica | 26 16 | 9 10 | 0.43 |
| Amoxicillin | Trinidad Jamaica | 22 21 | 13 5 | 0.16 |
| Sulphonamides | Trinidad Jamaica | 13 8 | 22 18 | 0.81 |
| Cephalosporin | Trinidad Jamaica | 17 14 | 18 12 | 0.89 |
| Fluoroquinolones | Trinidad Jamaica | 13 14 | 22 12 | 0.30 |
| Tetracycline | Trinidad Jamaica | 21 10 | 14 16 | 0.16 |
| Aminoglycoside | Trinidad Jamaica | 7 11 | 28 15 | 0.11 |
| Drug | Country | No. of Response | p-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Penicillin combinations | Trinidad Jamaica | 10 0 | 25 26 | <0.01 |
| Amoxicillin | Trinidad Jamaica | 4 5 | 31 21 | 0.48 |
| Sulphonamides | Trinidad Jamaica | 3 3 | 32 23 | 1 |
| Tetracyclines | Trinidad Jamaica | 3 3 | 32 23 | 1 |
| Aminoglycosides | Trinidad Jamaica | 0 2 | 35 24 | 0.38 |
| Fluoroquinolones | Trinidad Jamaica | 13 9 | 22 17 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismaila, M.S.; Thomas-Rhoden, A.; Neptune, A.; Sookram, K.; Gopaul, S.; Padarath, T.; Persad, A.; Georges, K.; Sundaram, V. A Survey on the Rationale Usage of Antimicrobial Agents in Small Animal Clinics and Farms in Trinidad and Jamaica. Antibiotics 2022, 11, 885. https://doi.org/10.3390/antibiotics11070885
Ismaila MS, Thomas-Rhoden A, Neptune A, Sookram K, Gopaul S, Padarath T, Persad A, Georges K, Sundaram V. A Survey on the Rationale Usage of Antimicrobial Agents in Small Animal Clinics and Farms in Trinidad and Jamaica. Antibiotics. 2022; 11(7):885. https://doi.org/10.3390/antibiotics11070885
Chicago/Turabian StyleIsmaila, Muhammad Sani, Alexandra Thomas-Rhoden, Angel Neptune, Kezia Sookram, Samantha Gopaul, Travis Padarath, Anil Persad, Karla Georges, and Venkatesan Sundaram. 2022. "A Survey on the Rationale Usage of Antimicrobial Agents in Small Animal Clinics and Farms in Trinidad and Jamaica" Antibiotics 11, no. 7: 885. https://doi.org/10.3390/antibiotics11070885
APA StyleIsmaila, M. S., Thomas-Rhoden, A., Neptune, A., Sookram, K., Gopaul, S., Padarath, T., Persad, A., Georges, K., & Sundaram, V. (2022). A Survey on the Rationale Usage of Antimicrobial Agents in Small Animal Clinics and Farms in Trinidad and Jamaica. Antibiotics, 11(7), 885. https://doi.org/10.3390/antibiotics11070885







