Antimicrobial Stewardship in Public-Sector Hospitals in KwaZulu-Natal, South Africa
Abstract
1. Introduction
2. Results
2.1. Pilot Study
2.2. Main Study
2.3. Key Support for the AMS Committee
2.3.1. Leadership Support
2.3.2. Accountability, and Drug and Antimicrobial Expertise
2.3.3. Composition of the AMS Committee and Key Support for the AMS Programme
2.4. Actions to Support the AMS Optimal Antimicrobial Use
2.4.1. Policies and Procedures
2.4.2. Interventions
2.4.3. Broad Interventions
2.4.4. Pharmacist-Specific Interventions
2.4.5. Diagnosis and Infection-Specific Interventions
2.5. Tracking: Monitoring Antibiotic Prescribing, Use, and Resistance
2.6. Reporting of Information to Staff on Improving Antibiotic Use
2.7. Education
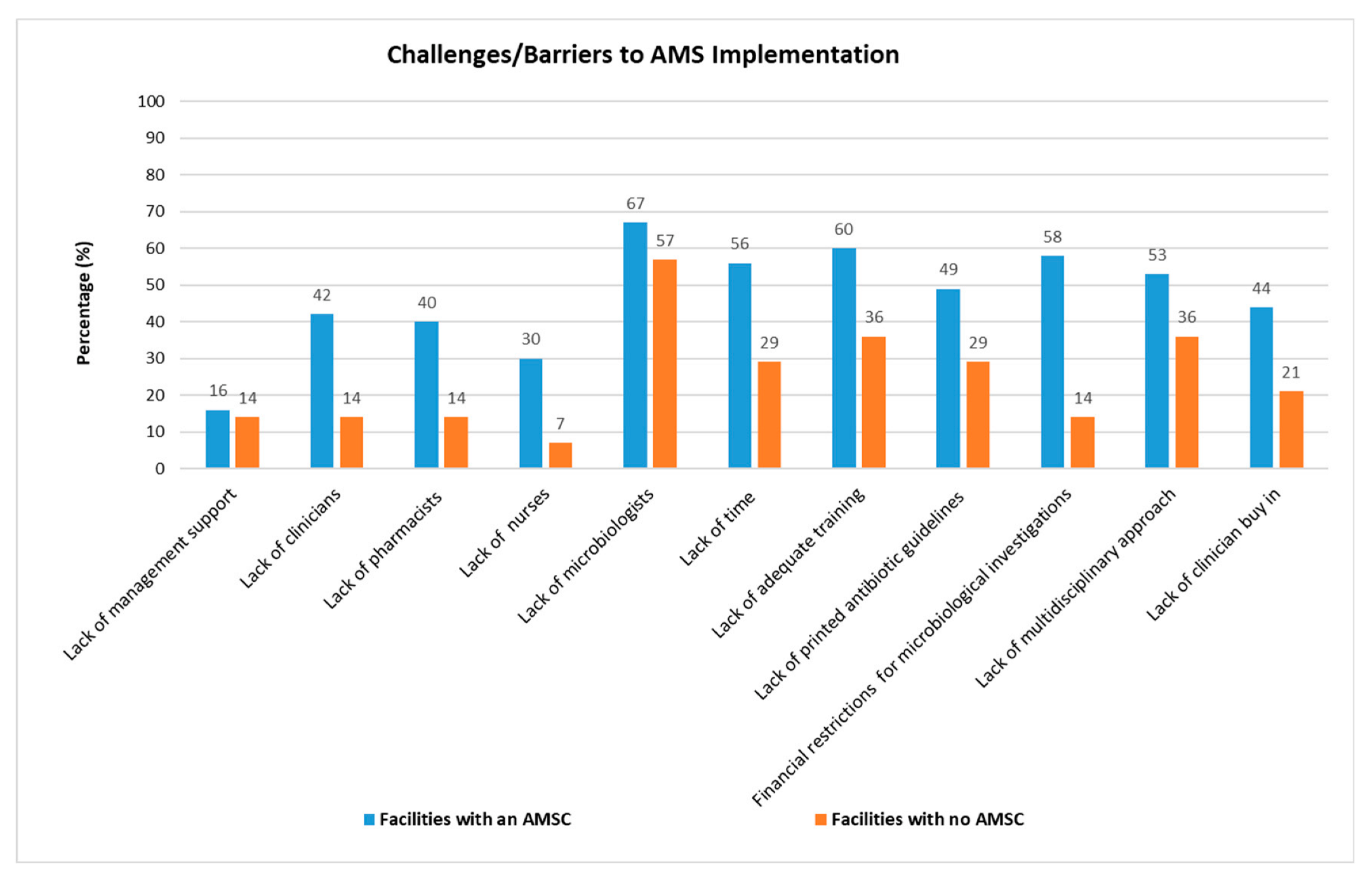
2.8. Comparing Challenges between Facilities with an AMSC vs. Facilities without an AMSC
3. Discussion
3.1. Leadership Support
3.2. Accountability
3.3. Drug and Antimicrobial Expertise
3.4. Composition of the AMS Committee and Key Support for the AMS Programme
Key Support for the AMS Program
3.5. Actions to Support Optimal Antimicrobial Use
3.5.1. Policies and Procedures
3.5.2. AMS Interventions
3.5.3. Broad Interventions
3.5.4. Pharmacist-Specific Interventions
3.5.5. Diagnosis and Infection-Specific Interventions
3.6. Tracking: Monitoring Antibiotic Prescribing, Use, and Resistance
3.6.1. Process Measures
3.6.2. Antibiotic Use and Outcome
3.6.3. Reporting Information to Staff on Improving Antibiotic Use and Education
3.7. Challenges Experienced in the Implementation of AMS Activities
4. Limitations
5. Conclusions
6. Materials and Methods
6.1. Ethical Considerations
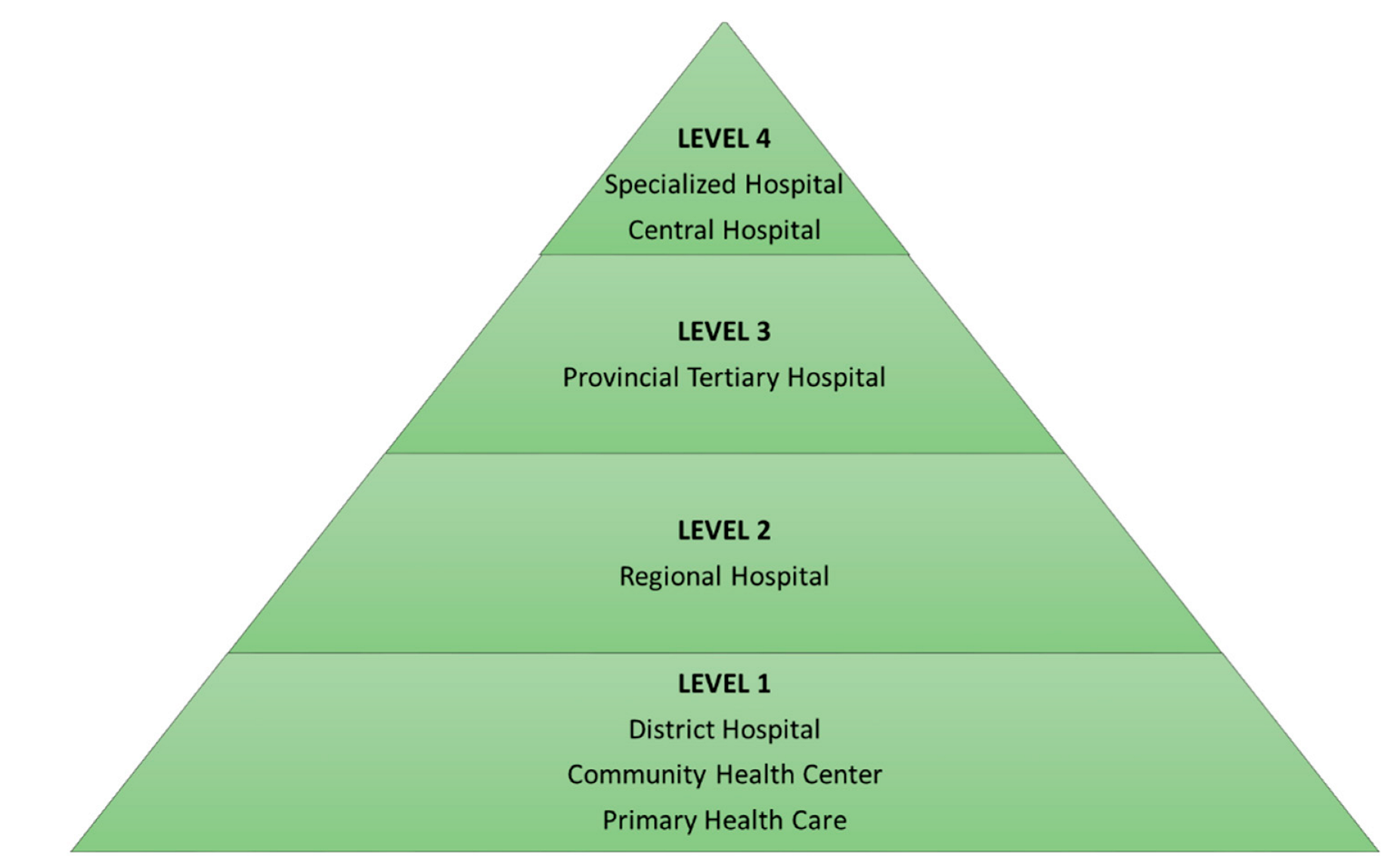
6.2. Study Setting
6.3. Study Design
6.4. Data-Collection Instrument
6.5. Sample Sites
Pilot Survey
6.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 23 August 2018).
- National Department of Health. Antimicrobial Resistance National Strategy Framework 2014–2024. 2014. Available online: https://www.health-e.org.za/wp-content/uploads/2015/09/Antimicrobial-Resistance-National-Strategy-Framework-2014-2024.pdf (accessed on 24 August 2018).
- Center for Disease Dynamics, EP. The State of the World’s Antibiotics, 2015; CDDEP: Washington, DC, USA, 2015; Available online: https://cddep.org/publications/state_worlds_antibiotics_2015/ (accessed on 24 August 2018).
- WHO. Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 16 September 2019).
- National Department of Health. Antimicrobial Resistance Backround Document. 2014. Available online: https://www.knowledgehub.org.za/elibrary/antimicrobial-resistance-background-document (accessed on 5 September 2018).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2014. Available online: http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html (accessed on 7 July 2018).
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 1 July 2020).
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events With Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Harbarth, S.; Carlet, J.; Cosgrove, S.; Goossens, H.; Holmes, A.; Jarlier, V.; Voss, A.; Pittet, D.; for the World Healthcare-Associated Infections Forum participants. Antimicrobial resistance: A global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob. Resist. Infect. Control 2013, 2, 31. [Google Scholar] [CrossRef]
- Pollack, L.A.; Srinivasan, A. Core Elements of Hospital Antibiotic Stewardship Programs From the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59 (Suppl. 3), S97–S100. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries. A Practical Toolkit; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- National Department of Health. Ministerial Advisory Committee on Antimicrobial Resistance National Department of Health Affordable Medicines Directorate. In Guidelines on Implementation of the Antimicrobial Strategy in South Africa: One Health Approach & Governance.; 2017. Available online: https://www.icanetwork.co.za/download/guidelines/Antimicrobial-Stewardship-Guidelines-Governance_June2017.pdf (accessed on 5 May 2020).
- National Department of Health. Guidelines for the Prevention and Containment of Antimicrobial Resistance in South African Hospitals. 2018. Available online: https://www.fidssa.co.za/Content/Documents/2019SAGuidelineAMRHospitals.pdf (accessed on 5 December 2019).
- National Department of Health. Surveillance of Antimicrobial Resistance and Consumption of Antibiotics in South Africa. 2018. Available online: http://www.health.gov.za/index.php/component/phocadownload/category/199-antimicrobial-resistance (accessed on 20 May 2020).
- National Department of Health. South African Antimicrobial Resistance National Strategy Framework; A One Health Approach, 2018–2024. 2018. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/south-africa-antimicrobial-resistance-national-action-plan-2018---2024.pdf?sfvrsn=533118b0_1%0D (accessed on 20 May 2020).
- National Department of Health. Implementation Plan for The Antimicrobial Resistance Strategy Framework In South Africa: 2014–2019. 2015. Available online: https://www.health.gov.za/index.php/antimicrobial-resistance (accessed on 20 May 2020).
- Chetty, S.; Reddy, M.; Ramsamy, Y.; Naidoo, A.; Essack, S. Antimicrobial stewardship in South Africa: A scoping review of the published literature. JAC-Antimicrob. Resist. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Wasserman, S.B.T.; Mendelson, M. A Pocket Guide to Antibiotic Prescribing for Adults in South Africa. 2015. Available online: https://www.fidssa.co.za/Content/Documents/SAASP_Antibiotic_Guidelines_2015.pdf (accessed on 3 March 2020).
- National Department of Health. Standard Treatment Guidelines and Essential Medicines List for South Africa. 2015. Available online: http://www.health.gov.za/index.php/component/phocadownload/category/286-hospital-level-adults (accessed on 20 March 2020).
- CDC. The Core Elements of Human Antibiotic Stewardship Programs in Resource-Limited Settings: National and Hospital Levels; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/antibiotic-use/healthcare/implementation.html (accessed on 25 April 2020).
- Engler, D.; Meyer, J.C. Compliance with South Africa’s Antimicrobial Resistance National Strategy Framework: Are we there yet? J. Chemother. 2021, 33, 21–31. [Google Scholar] [CrossRef]
- Peters, S.M.; Sheik, S.; Werner, J.L.; Davies, M.-A.; Willems, B. Antimicrobial stewardship in the Western Cape: A situational analysis of existing facility-level initiatives. S. Afr. Med. J. 2021, 111, 421–425. [Google Scholar] [CrossRef]
- Pombo, M.H.R.G.S.; Thompson, D.; Lamkang, A.S.; Pulcini, C. Global Core Standards for Hospital Antimicrobial Stewardship Programs, International Perspectives and Future Directions. 2018. Available online: https://cddep.org/wp-content/uploads/2018/12/Global-Core-Standards-for-Hospital-Antimicrobial-Stewardship-Programs.pdf (accessed on 4 March 2019).
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef]
- Pulcini, C.; Beovic, B.; Howard, P.; Mendelson, M. Human resources estimates and funding for antibiotic stewardship teams are urgently needed: Authors’ response. Clin. Microbiol. Infect. 2018, 24, 557. [Google Scholar] [CrossRef]
- Boyles, T.H.; Naicker, V.; Rawoot, N.; Raubenheimer, P.J.; Eick, B.; Mendelson, M. Sustained reduction in antibiotic consumption in a South African public sector hospital; Four year outcomes from the Groote Schuur Hospital antibiotic stewardship program. S. Afr. Med. J. 2017, 107, 115–118. [Google Scholar] [CrossRef]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- ECDC. Proposals for EU Guidelines on the Prudent Use of Antimicrobials in Humans. 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/proposals-eu-guidelines-prudent-use-antimicrobials-humans (accessed on 3 June 2020).
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; Becker, P.J.; Goff, D.A.; Bauer, K.A.; Nathwani, D.; van den Bergh, D. Antimicrobial stewardship across 47 South African hospitals: An implementation study. Lancet Infect. Dis. 2016, 16, 1017–1025. [Google Scholar] [CrossRef]
- Junaid, E.; Jenkins, L.; Swanepoel, H.; North, Z.; Gould, T. Antimicrobial stewardship in a rural regional hospital—Growing a positive culture. S. Afr. Med. J. 2018, 108, 546–550. [Google Scholar] [CrossRef]
- Morency-Potvin, P.; Schwartz, D.N.; Weinstein, R.A. Antimicrobial Stewardship: How the Microbiology Laboratory Can Right the Ship. Clin. Microbiol. Rev. 2017, 30, 381–407. [Google Scholar] [CrossRef]
- Ostrowsky, B.; Banerjee, R.; Bonomo, R.A.; Cosgrove, S.E.; Davidson, L.; Doron, S.; Gilbert, D.N.; Jezek, A.; Lynch, J.B.; Septimus, E.J.; et al. Infectious Diseases Physicians: Leading the Way in Antimicrobial Stewardship. Clin. Infect. Dis. 2018, 66, 995–1003. [Google Scholar] [CrossRef]
- Yam, P.; Fales, D.; Jemison, J.; Gillum, M.; Bernstein, M. Implementation of an antimicrobial stewardship program in a rural hospital. Am. J. Health Syst. Pharm. 2012, 69, 1142–1148. [Google Scholar] [CrossRef]
- Nansook, H.B.; Parbhoo, N.; Steele, G.; Mhlongo, T.; Naidoo, N.; Ramjee, R.J. Antimicrobial stewardship at Prince Mshiyeni Memorial Hospital in KwaZulu-Natal—A pharmacist’s perspective on this multidisciplinary strategy. SA Pharm. J. 2019, 86, 15–16. [Google Scholar]
- Boyles, T.H.; Whitelaw, A.; Bamford, C.; Moodley, M.; Bonorchis, K.; Morris, V.; Rawoot, N.; Naicker, V.; Lusakiewicz, I.; Black, J.; et al. Antibiotic stewardship ward rounds and a dedicated prescription chart reduce antibiotic consumption and pharmacy costs without affecting inpatient mortality or re-admission rates. PLoS ONE 2013, 8, e79747. [Google Scholar] [CrossRef]
- Dik, J.-W.; Poelman, R.; Friedrich, A.; Nannan Panday, P.; Lo-Ten-Foe, J.; Assen, S.; van Gemert-Pijnen, J.E.; Niesters, H.G.; Hendrix, R.; Sinha, B. An integrated stewardship model: Antimicrobial, infection prevention and diagnostic (AID). Future Microbiol. 2015, 11, 93–102. [Google Scholar] [CrossRef]
- Rout, J.; Brysiewicz, P. Exploring the role of the ICU nurse in the antimicrobial stewardship team at a private hospital in KwaZulu-Natal. S. Afr. J. Crit. Care 2017, 33, 46–50. [Google Scholar] [CrossRef][Green Version]
- Messina, A.P.; van den Bergh, D.; Goff, D.A. Antimicrobial Stewardship with Pharmacist Intervention Improves Timeliness of Antimicrobials Across Thirty-three Hospitals in South Africa. Infect. Dis. Ther. 2015, 4 (Suppl. 1), 5–14. [Google Scholar] [CrossRef]
- Antimicrobial Resistance. Available online: https://www.nicd.ac.za/centres/centre-for-healthcare-associated-infections-antimicrobial-resistance-and-mycoses/ (accessed on 2 June 2020).
- Ford, B.A.; Martello, J.L.; Wietholter, J.P.; Piechowski, K.L. Antibiotic de-escalation on internal medicine services with rounding pharmacists compared to services without. Int. J. Clin. Pharm. 2020, 42, 772–776. [Google Scholar] [CrossRef]
- Ramsamy, Y.; Muckart, D.J.; Han, K.S. Microbiological surveillance and antimicrobial stewardship minimise the need for ultrabroad-spectrum combination therapy for treatment of nosocomial infections in a trauma intensive care unit: An audit of an evidence-based empiric antimicrobial policy. S. Afr. Med. J. 2013, 103, 371–376. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Sanchez, G.V.; Fleming-Dutra, K.E.; Roberts, R.M.; Hicks, L.A. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm. Rep. 2016, 65, 1–12. Available online: https://www.cdc.gov/mmwr/volumes/65/rr/rr6506a1.html (accessed on 23 August 2018). [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Levy Hara, G. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef]
- World Health Organization. Diagnostic Stewardship: A Guide to Implementation in Antimicrobial Resistance Surveillance Sites 2016. Available online: https://www.who.int/publications/i/item/WHO-DGO-AMR-2016.3 (accessed on 10 June 2020).
- Matsitse, T.B.; Helberg, E.; Meyer, J.C.; Godman, B.; Massele, A.; Schellack, N. Compliance with the primary health care treatment guidelines and the essential medicines list in the management of sexually transmitted infections in correctional centres in South Africa: Findings and implications. Expert Rev. Anti-Infect. Ther. 2017, 15, 963–972. [Google Scholar] [CrossRef]
- Chunnilall, D.; Peer, A.; Naidoo, I.; Essack, S. An evaluation of antibiotic prescribing patterns in adult intensive care units in a private hospital in KwaZulu-Natal. S. Afr. J. Infect. Dis. 2015, 30, 17–22. [Google Scholar] [CrossRef]
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; van den Bergh, D. From guidelines to practice: A pharmacist-driven prospective audit and feedback improvement model for peri-operative antibiotic prophylaxis in 34 South African hospitals. J. Antimicrob. Chemother. 2017, 72, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.; Davey, P.; Harbarth, S.; Nathwani, D.; Sneddon, J.; Marwick, C.A. Impact of antimicrobial stewardship interventions on Clostridium difficile infection and clinical outcomes: Segmented regression analyses. J. Antimicrob. Chemother. 2018, 73, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Measuring Antimicrobial Consumption and Use. Available online: https://www.who.int/medicines/areas/rational_use/AMU_Surveillance/en/ (accessed on 2 June 2020).
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- Global PPS. Available online: https://www.global-pps.com/ (accessed on 2 June 2020).
- Carling, P.; Fung, T.; Killion, A.; Terrin, N.; Barza, M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect. Control Hosp. Epidemiol. 2003, 24, 699–706. [Google Scholar] [CrossRef]
- Labi, A.-K.; Obeng-Nkrumah, N.; Bjerrum, S.; Aryee, N.A.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Physicians’ knowledge, attitudes, and perceptions concerning antibiotic resistance: A survey in a Ghanaian tertiary care hospital. BMC Health Serv. Res. 2018, 18, 126. [Google Scholar] [CrossRef]
- Fathi, I.; Sameh, O.; Abu-Ollo, M.; Naguib, A.; Alaa-Eldin, R.; Ghoneim, D.; Elhabashi, S.; Taha, A.; Ibrahim, Y.; Radwan, R.; et al. Knowledge, Attitudes, and Beliefs Regarding Antimicrobial Therapy and Resistance Among Physicians in Alexandria University Teaching Hospitals and the Associated Prescription Habits. Microb. Drug Resist. 2017, 23, 71–78. [Google Scholar] [CrossRef]
- Abera, B.; Kibret, M.; Mulu, W. Knowledge and beliefs on antimicrobial resistance among physicians and nurses in hospitals in Amhara Region, Ethiopia. BMC Pharmacol. Toxicol. 2014, 15, 26. [Google Scholar] [CrossRef]
- Teixeira Rodrigues, A.; Roque, F.; Falcão, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef]
- Wasserman, S.; Potgieter, S.; Shoul, E.; Constant, D.; Stewart, A.; Mendelson, M.; Boyles, T.H. South African medical students’ perceptions and knowledge about antibiotic resistance and appropriate prescribing: Are we providing adequate training to future prescribers? S. Afr. Med. J. 2017, 107, 405–410. [Google Scholar] [CrossRef]
- Bulabula, A.N.H.; Jenkins, A.; Mehtar, S.; Nathwani, D. Education and management of antimicrobials amongst nurses in Africa-a situation analysis: An Infection Control Africa Network (ICAN)/BSAC online survey. J. Antimicrob. Chemother. 2018, 73, 1408–1415. [Google Scholar] [CrossRef]
- Burger, M.; Fourie, J.; Loots, D.; Mnisi, T.; Schellack, N.; Bezuidenhout, S.; Meyer, J.C. Knowledge and perceptions of antimicrobial stewardship concepts among final year pharmacy students in pharmacy schools across South Africa. S. Afr. J. Infect. Dis. 2016, 31, 84–90. [Google Scholar]
- Antimicrobial Stewardship: A Competency-Based Approach. Available online: https://openwho.org/courses/AMR-competency (accessed on 2 June 2020).
- Courses & Educational Material. Available online: https://www.fidssa.co.za/SAASP/Edu_Material (accessed on 2 June 2020).
- Referral System: Levels of Health Care. Available online: http://www.kznhealth.gov.za/Referral-system.htm (accessed on 27 March 2019).
- National Department of Health. Regulations Relating to Categories of Hospitals (R185/2012). 2003. Available online: http://www.health.gov.za/index.php/2014-03-17-09-09-38/legislation/joomla-split-menu/category/84-2012r (accessed on 8 May 2020).
- Provisional Hospital Contact Details. Available online: http://www.kznhealth.gov.za/hospitals.htm (accessed on 10 November 2018).
| Study Setting | Number | Percentage |
|---|---|---|
| District | ||
| Amajuba | 3 | 5% |
| Ethekwini | 16 | 28% |
| Harry Gwala | 6 | 11% |
| Ilembe | 4 | 7% |
| King Cetshwayo | 3 | 5% |
| Ugu | 2 | 3% |
| Umgungundlovu | 9 | 16% |
| Umkhanyakude | 4 | 7% |
| Umzinyathi | 4 | 7% |
| Uthukela | 2 | 4% |
| Zululand | 4 | 7% |
| Hospital Type | ||
| District | 30 | 53% |
| Regional | 10 | 17% |
| Tertiary | 3 | 5% |
| Central | 1 | 2% |
| Specialized | 12 | 21% |
| District and Specialized TB | 1 | 2% |
| Specialized Hospital | 12 | 21% |
| Psychiatry | 4 | 7% |
| TB | 5 | 9% |
| Chronic Rehabilitation | 2 | 3.50% |
| Ophthalmology | 1 | 2% |
| Facilities that had an antimicrobial stewardship committee (AMSC) | 43 | 75% |
| Antimicrobial stewardship (AMS) Meetings | N = 43 | |
| Bi-monthly | 1 | 2% |
| Monthly | 12 | 28% |
| Quarterly | 8 | 19% |
| Quarterly AMS combined with PTC meetings | 2 | 5% |
| Interventions | Drug and Antimicrobial Expertise, n (%) | aOR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Is There a Clinical Microbiologist on-Site or Is There off-Site Support from a Clinical Microbiologist? | Chi-Square p-Value | ||||
| Yes | No | ||||
| Microbiologist input on pathogen surveillance data | |||||
| Yes | 11 (84.6) | 2 (15.4) | 0.000 ** | 5.12 (4.08–22.02) | 0.001 ** |
| No | 5 (16.7) | 25 (83.3) | 1 | ||
| Microbiological investigations prior to commencement of antibiotics | |||||
| Yes | 10 (62.5) | 6 (37.5) | 0.011 * | 6.73 (1.08–42.01) | 0.041 * |
| No | 6 (23.1) | 20 (76.9) | 1 | ||
| Interventions | Composition of the Antimicrobial Stewardship Committee, n (%) | aOR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Is there a Representative from Microbiology | Chi-Square p-Value | ||||
| Yes | No | ||||
| Availability of AMR surveillance reports | |||||
| Yes | 12 (54.5%) | 10 (45.5%) | 0.051 * | 3.21 (0.22–46.52) | 0.389 |
| No | 5 (25%) | 15 (75%) | 1 | ||
| Interrogation of AMR surveillance reports | |||||
| Yes | 9 (60%) | 6 (40%) | 0.036 * | 0.51 (0.03–9.18) | 0.647 |
| No | 7 (26.9%) | 19 (73.1%) | 1 | ||
| Microbiologist input on pathogen surveillance data | |||||
| Yes | 12 (92.3) | 1 (7.7%) | 0.000 ** | 43.54 (4.03–147.65) | 0.002 |
| No | 5 (17.2%) | 24 (82.8%) | 1 | ||
| TRACKING: MONITORING ANTIBIOTIC PRESCRIBING, USE, AND RESISTANCE | ||
|---|---|---|
| PROCESS MEASURES | N | Responses n (%) |
| Does your stewardship program monitor adherence to a documentation policy (dose, duration, and indication)? | 43 | 25 (58%) |
| Does your stewardship program monitor adherence to facility-specific treatment recommendations? | 43 | 19 (44%) |
| Does your stewardship program monitor compliance with one or more of the specific interventions in place? | 42 | 23 (55%) |
| ANTIBIOTIC USE AND OUTCOME MEASURES | ||
| Does your facility track rates of C. difficile infection? | 41 | 14 (34%) |
| Does your facility produce an antibiogram (cumulative antibiotic susceptibility report?) | 42 | 20 (48%) |
| Does your facility monitor antibiotic use (consumption) at the unit and/or facility-wide level by one of the following metrics? | ||
| By counts of antibiotic(s) administered to patients per day (Days of Therapy; Directly Observed Therapy)? | 43 | 14 (33%) |
| By number of grams of antibiotics used Defined Daily Dose (DDD),Anatomical Therapeutic Classification)? | 43 | 10 (23%) |
| By direct expenditure for antibiotics (purchasing costs)? | 43 | 24 (56%) |
| Tracking: Monitoring Antibiotic Prescribing, Use, and Resistance | Composition of the Antimicrobial Stewardship Committee, n (%) | aOR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Is There a Representative from Microbiology | Chi-Square p-Value | ||||
| Yes | No | ||||
| Does your facility produce an antibiogram | |||||
| Yes | 12 (60%) | 8 (40%) | 0.019 ** | 4.80 (1.25–18.42) | 0.022 * |
| No | 5 (23.8%) | 16 (76.2%) | 1 | ||
| Reporting Information to Staff on Improving Antibiotic Use | Drug and Antimicrobial Expertise, n (%) | ||
|---|---|---|---|
| Is There an Infectious Disease Physician on Site | Chi-Square p-Value | ||
| Yes | No | ||
| Has a current antibiogram been distributed to prescribers at the facility? | |||
| Yes | 2 (20%) | 8 (80%) | 0.010 ** |
| No | 0 (0%) | 32 (100%) | |
| Do prescribers ever receive direct, personalized communication about how they can improve their antibiotic prescribing? | |||
| Yes | 0 (0%) | 27 (100%) | 0.044 * |
| No | 2 (14.3%) | 12 (85.7%) | |
| Individual Comments Recorded Verbatim | Themes |
|---|---|
| Lack of clear guidelines from DOH regarding antimicrobials. Limitations of the EDL | Limitations in guidelines and EDL |
| Lack of drug availability | Inadequate drug availability |
| The AMS Sub Committee is part of the Pharmacy and Therapeutics Committee (PTC), no dedicated AMS committee. Discussion takes place in PTC meetings | AMS meetings are combined with PTC meetings |
| Leadership has not been strong and doctor attendance has been minimal. | Inadequate strong leadership |
| Lack of nursing buy-in | Inadequate nursing support |
| Not the appropriate expertise available to have a fully functional AMS committee. | Inadequate expertise |
| Challenge has been time to have all members for a meeting to draft terms of reference due to clashing responsibilities. | Inadequate time |
| Stakeholders do not have the time due to many other facility meetings held daily. AMS is a standing item on the PTC and IPC agenda. There is no standalone AMS committee | Competing responsibilities |
| The AMS program exists in terms of what the pharmacy staff can contribute. | The responsibility lies with pharmacists |
| An AMS program with guidelines was designed and presented by the clinical pharmacist to the PTC with very underwhelming response. Although we do not have a functional AMS program, the clinical pharmacist does carry out some of the pharmacy-related activities | Suboptimal PTC buy-in |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chetty, S.; Reddy, M.; Ramsamy, Y.; Dlamini, V.C.; Reddy-Naidoo, R.; Essack, S.Y. Antimicrobial Stewardship in Public-Sector Hospitals in KwaZulu-Natal, South Africa. Antibiotics 2022, 11, 881. https://doi.org/10.3390/antibiotics11070881
Chetty S, Reddy M, Ramsamy Y, Dlamini VC, Reddy-Naidoo R, Essack SY. Antimicrobial Stewardship in Public-Sector Hospitals in KwaZulu-Natal, South Africa. Antibiotics. 2022; 11(7):881. https://doi.org/10.3390/antibiotics11070881
Chicago/Turabian StyleChetty, Sarentha, Millidhashni Reddy, Yogandree Ramsamy, Vusi C. Dlamini, Rahendhree Reddy-Naidoo, and Sabiha Y. Essack. 2022. "Antimicrobial Stewardship in Public-Sector Hospitals in KwaZulu-Natal, South Africa" Antibiotics 11, no. 7: 881. https://doi.org/10.3390/antibiotics11070881
APA StyleChetty, S., Reddy, M., Ramsamy, Y., Dlamini, V. C., Reddy-Naidoo, R., & Essack, S. Y. (2022). Antimicrobial Stewardship in Public-Sector Hospitals in KwaZulu-Natal, South Africa. Antibiotics, 11(7), 881. https://doi.org/10.3390/antibiotics11070881






