Antifungal-Loaded Acrylic Bone Cement in the Treatment of Periprosthetic Hip and Knee Joint Infections: A Review
Abstract
:1. Introduction
2. Results
2.1. Cement Loading and Pharmacokinetic Properties
2.2. Surgical Treatment, Systemic Therapy, and Outcome
3. Discussion
3.1. Surgical Treatment Recommendations
3.2. Antifungal-Loaded Bone Cement
3.2.1. Antifungal-Loaded Bone Cement—In Vitro and Animal Studies
3.2.2. Antifungal-Loaded Bone Cement—Clinical Studies
3.3. Systemic Antifungal Therapy
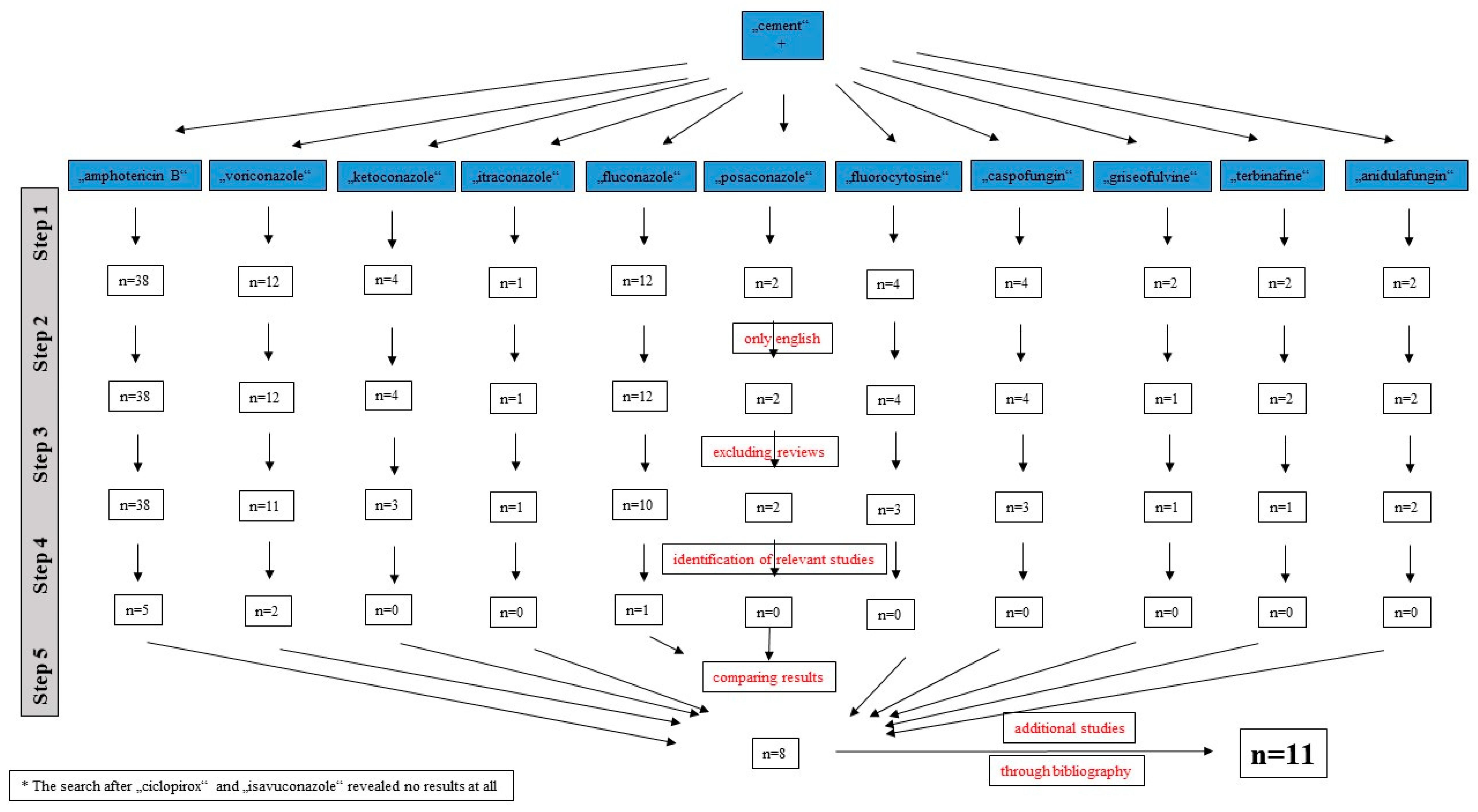
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, Y.; Chang, C.H.; Lin, Y.C.; Lee, S.H.; Hsieh, P.H.; Chang, Y. Different microbiological profiles between hip and knee prosthetic joint infections. J. Orthop. Surg. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago, L.; De Vecchi, E.; Bortolin, M.; Zagra, L.; Romano, C.L.; Cappelletti, L. Epidemiology and antibiotic resistance of late prosthetic knee and hip infections. J. Arthroplast. 2017, 32, 2496–2500. [Google Scholar] [CrossRef] [PubMed]
- Nickinson, R.S.J.; Board, T.N.; Gambhir, A.K.; Porter, M.L.; Kay, P.R. The microbiology of the infected knee arthroplasty. Int. Orthop. 2010, 34, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiq, I.; Gambhir, A.K.; Wroblewski, B.M.; Kay, P.R. The microbiology of the infected hip arthroplasty. Int. Orthop. 2006, 30, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Bjerke-Kroll, B.T.; Christ, A.B.; McLawhorn, A.S.; Sculco, P.K.; Jules-Elysee, K.M.; Sculco, T.P. Periprosthetic joint infections treated with two-stage revision over 14 years: An evolving microbiologic profile. J. Arthroplast. 2014, 29, 877–882. [Google Scholar] [CrossRef]
- Holleyman, R.J.; Deehan, D.J.; Walker, L.; Charlett, A.; Samuel, J.; Shirley, M.D.F.; Baker, P.N. Staphylococcal resistance profiles in deep infection following primary hip and knee arthroplasty: A study using the NJR dataset. Arch. Orthop. Trauma. Surg. 2019, 139, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Holleyman, R.J.; Baker, P.; Charlett, A.; Gould, K.; Deehan, D.J. Microorganisms responsible for periprosthetic knee infections in England and Wales. Knee. Surg. Sports. Traumatol. Arthosc. 2016, 24, 3080–3087. [Google Scholar] [CrossRef]
- Holleyman, R.J.; Baker, P.N.; Charlett, A.; Gould, K.; Deehan, D.J. Analysis of causative microorganisms in 248 primary hip arthroplasties revised for infection: A study using the NJR dataset. Hip. Int. 2016, 26, 82–89. [Google Scholar] [CrossRef]
- Azzam, K.; Parvizi, J.; Jungkind, D.; Hanssen, A.; Fehring, T.; Springer, B.; Bozic, K.; Della Valle, C.; Pullido, L.; Barrack, R. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: A multi-institutional experience. J. Bone Joint. Surg. Am. 2009, 91, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Fusini, F.; Aprato, A.; Massé, A.; Bistolfi, A.; Girardo, M.; Artiaco, S. Candida periprosthetic infection of the hip: A systematic review of surgical treatment and clinical outcomes. Int. Orthop. 2020, 44, 15–22. [Google Scholar] [CrossRef]
- Kuiper, J.W.; van den Bekerom, M.P.; van der Stappen, J.; Nolte, P.A.; Colen, S. 2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infections. Acta. Orthop. 2013, 84, 517–523. [Google Scholar] [CrossRef]
- Schoof, B.; Jakobs, O.; Schmidl, S.; Klatte, T.O.; Frommelt, L.; Gehrke, T.; Gebauer, M. Fungal periprosthetic joint infection of the hip: A systematic review. Orthop. Rev. 2015, 7, 5748. [Google Scholar] [CrossRef]
- Jakobs, O.; Schoof, B.; Klatte, T.O.; Schmidl, S.; Fensky, F.; Guenther, D.; Frommelt, L.; Gehrke, T.; Gebauer, M. Fungal periprosthetic joint infection in total knee arthroplasty: A systematic review. Orthop. Rev. 2015, 7, 5623. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1-50. [Google Scholar] [CrossRef]
- Koutserimpas, C.; Chamakioti, I.; Zervakis, S.; Raptis, K.; Alpantaki, K.; Kofteridis, D.P.; Vrioni, G.; Samonis, G. Non-candida fungal prosthetic joint infections. Diagnostics 2021, 11, 1410. [Google Scholar] [CrossRef]
- Czuban, M.; Wulsten, D.; Wang, L.; Di Luca, M.; Trampuz, A. Release of different amphotericin B formulations from PMMA bone cements and their activity against Candida biofilm. Colloids. Surf. B Biointerfaces 2019, 183, 110406. [Google Scholar] [CrossRef]
- Houdek, M.T.; Greenwood-Quaintance, K.E.; Morrey, M.E.; Patel, R.; Hanssen, A.D. Elution of high dose Amphotericin B deoxycholate from polymethylmethacrylate. J. Arthroplast. 2015, 30, 2308–2310. [Google Scholar] [CrossRef]
- Roberts, J.; Bingham, J.; McLaren, A.C.; McLemore, R. Liposomal formulation decreases toxicity of Amphotericin B in vitro and in vivo. Clin. Orthop. Relat. Res. 2015, 473, 2262–2269. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.H.; Tai, C.L.; Hsu, H.Y.; Hsieh, P.H.; Lee, M.S.; Ueng, S.W. Liquid antibiotics in bone cement: An effective way to improve the efficiency of antibiotic release in antibiotic loaded bone cement. Bone Joint Res. 2014, 3, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, B.; McLaren, A.C.; Pauken, C.; McLemore, R. Liposomal formulation increases local delivery of amphotericin from bone cement: A pilot study. Clin. Orthop. Relat. Res. 2012, 470, 2671–2676. [Google Scholar] [CrossRef] [Green Version]
- Kweon, C.; McLaren, A.C.; Leon, C.; McLemore, R. Amphotericin B delivery from bone cement increases with porosity but strength decreases. Clin. Orthop. Relat. Res. 2011, 469, 3002–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sealy, P.I.; Nguyen, C.; Tucci, M.; Benghuzzi, H.; Cleary, J.D. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann. Pharmacother. 2009, 43, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Goss, B.; Lutton, C.; Weinrauch, P.; Jabur, M.; Gillett, G.; Crawford, R. Elution and mechanical properties of antifungal bone cement. J. Arthroplast. 2007, 22, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, D.; Kodali, P.; Dipersio, J.; Acus, R.; Askew, M. In vitro analysis of antifungal impregnated polymethylmethacrylate bone cement. Clin. Orthop. Relat. Res. 2002, 403, 228–231. [Google Scholar] [CrossRef]
- Grimsrud, C.; Raven, R.; Fothergill, A.W.; Kim, H.A.T. The in vitro elution characteristics of antifungal-loaded PMMA bone cement and calcium sulfate bone substitute. Orthopedics 2011, 34, e378–e381. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; McLaren, A.; Pauken, C.; McLemore, R. Voriconazole is cytotoxic at locally delivered concentrations: A pilot study. Clin. Orthop. Relat. Res. 2013, 471, 3165–3170. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.B.; McLaren, A.C.; Pauken, C.; Clarke, H.D.; McLemore, R. Voriconazole is delivered from antifungal-loaded bone cement. Clin. Orthop. Relat. Res. 2013, 471, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Moreno, J.; Merino, V.; Nácher, A.; Rodrigo, J.L.; Bonet Yuste, B.B.; Merino-Sanjuán, M. Bioactivity of ceftazidime and fluconazole included in polymethlymethacrylate bone cement for use in arthroplasty. J. Arthroplast. 2017, 32, 3126–3133. [Google Scholar] [CrossRef]
- Escolà-Vergé, L.; Rodríguez-Pardo, D.; Lora-Tamayo, J.; Morata, L.; Murillo, O.; Vilchez, H.; Sorli, L.; Carrión, L.G.; Barbero, J.M.; Palomino-Nicás, J.; et al. Study Group on Osteoarticular Infections of the Spanish Society of Clinical Microbiology and Infectious Diseases (GEIO-SEIMC), and the Spanish Network for Research in Infectious Pathology (REIPI). Candida periprosthetic joint infection: A rare and difficult-to-treat infection. J. Infect. 2018, 77, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Deelstra, J.J.; Neut, D.; Jutte, P.C. Successful treatment of Candida albicans-infected total hip prosthesis with staged procedure using an antifungal-loaded cement spacer. J. Arthroplast. 2013, 28, 374.e5–374.e8. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.H.; Hsu, K.Y. Candidal arthritis in revision knee arthroplasty successfully treated with sequential parenteral-oral fluconazole and amphotericin B-loaded cement spacer. Knee. Surg. Sports. Traumatol. Arthrosc. 2011, 19, 273–276. [Google Scholar] [CrossRef]
- Gaston, G.; Ogden, J. Candida glabrata periprosthetic infection: A case report and literature review. J. Arthroplast. 2004, 19, 927–930. [Google Scholar] [CrossRef]
- Marra, F.; Robbins, G.M.; Masri, B.A.; Duncan, C.; Wasan, K.M.; Kwong, E.H.; Jewesson, P.J. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can. J Surg. 2001, 44, 383–386. [Google Scholar]
- Burgo, F.J.; Mengelle, D.E.; Abraham, A.; Kremer, G.; Autorino, C.M. Periprosthetic fungal infection of a hip caused by Trichosporon inkin. Arthroplast. Today 2017, 4, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Denes, E.; Fiorenza, F.; Saint-Marcoux, F.; Megherbi, M.; Dupon, M.; Weinbreck, P. Voriconazole stability in cement spacers. Med. Mal. Infect. 2012, 42, 567–568. [Google Scholar] [CrossRef]
- Reddy, K.J.; Shah, J.D.; Kale, R.V.; Reddy, T.J. Fungal prosthetic joint infection after total knee arthroplasty. Indian. J. Orthop. 2013, 47, 526–529. [Google Scholar] [CrossRef]
- Bruce, A.S.; Kerry, R.M.; Norman, P.; Stockley, I. Fluconazole-impregnated beads in the management of fungal infection of prosthetic joints. J. Bone Joint Surg. Br. 2001, 83, 183–184. [Google Scholar] [CrossRef]
- Phelan, D.M.; Osmon, D.R.; Keating, M.R.; Hanssen, A.D. Delayed reimplantation arthroplasty for Candida prosthetic joint infection: A report of 4 cases and review of the literature. Clin. Infect. Dis. 2002, 34, 930–938. [Google Scholar] [CrossRef] [Green Version]
- Selmon, G.P.; Slater, R.N.; Shepperd, J.A.; Wright, E.P. Successful 1-stage exchange total knee arthroplasty for fungal infection. J. Arthroplast. 1998, 13, 114–115. [Google Scholar] [CrossRef]
- McGregor, R.R.; Schimmer, B.M.; Steinberg, M.E. Results of combined amphotericin B-5 fluorcytosine therapy for prosthetic knee joint infected with Candida parapsilosis. J. Rheumatol. 1979, 6, 451–455. [Google Scholar]
- Belden, K.; Cao, L.; Chen, J.; Deng, T.; Fu, J.; Guan, H.; Jia, C.; Kong, X.; Kuo, F.C.; Li, R.; et al. Hip and knee section, fungal periprosthetic joint infection, diagnosis and treatment: Proceeding of International Consensus On Orthopedic Infections. J. Arthroplast. 2019, 34, S387–S391. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K. Therapeutic use of antibiotic-loaded bone cement in the treatment of hip and knee joint infections. J. Bone Jt. Infect. 2017, 2, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouse, M.S.; Heijnk, A.; Steckelberg, J.M.; Patel, R. Are anidulafungin or voriconazole released from polymethylmethacrylate in vitro? Clin. Orthop. Relat. Res. 2011, 469, 1466–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anagnostakos, K.; Meyer, C. Antibiotic elution from hip and knee acrylic bone cement spacers: A systematic review. Biomed. Res. Int. 2017, 2017, 4657874. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, D.M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Comparison of biofilms formed by Candida albicans and Candida parapsilossis on bioprosthetic surfaces. Infect. Immun. 2002, 70, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Anagnostakos, K.; Kelm, J.; Schmitt, E.; Jung, J. Fungal periprosthetic hip and knee joint infections: Clinical experience with a 2-stage treatment protocol. J. Arthroplast. 2012, 27, 293–298. [Google Scholar] [CrossRef]
- Cushing, R.D.; Fulgenzi, W.R. Synovial fluid levels of fluconazole in a patient with Candida parapsillosis prosthetic joint infection who had an excellent clinical response. J. Arthroplast. 1997, 12, 950. [Google Scholar] [CrossRef]
- Lerch, K.; Kalteis, T.; Schubert, T.; Lehn, N.; Grifka, J. Prosthetic joint infections with osteomyelitis due to Candida albicans. Mycoses 2003, 46, 462–466. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Fink, B. Antibiotic-loaded cement spacers–lessons learned from the past 20 years. Expert. Rev. Med. Devices. 2018, 15, 231–245. [Google Scholar] [CrossRef]
- Pocket Guide to Diagnosis & Treatment of Periprosthetic Joint Infection (PJI). Available online: https://pro-implant.org/tools/pocket-guide/1 (accessed on 13 June 2022).

| Study | Publication Year | Joint | No. of Cases/Joints | Age | Sex | Predisposing Comorbidities | Fungal Organism |
|---|---|---|---|---|---|---|---|
| Deelstra et al. [30] | 2013 | Hip | 1/1 | 73 y. | Female | None | Candida albicans + coagulase-negative staphylococci |
| Wu et al. [31] | 2011 | Knee | 1/1 | 72 y. | Male | None | Candida albicans |
| Gaston and Ogden [32] | 2004 | Knee | 1/1 | 42 y. | Female | Steroids for lupus | Candida glabrata |
| Marra et al. [33] | 2001 | Hip | 1/1 | 59 y. | Male | 2 × aseptic revision arthroplasty surgeries | Candida albicans |
| Burgo et al. [34] | 2017 | Hip | 1/1 | 53 y. | Female | Corticosteroids for myasthenia gravis, chronic hepatitis B, obesity, non-insulin-dependent diabetes mellitus, prior PJI | Trichosporon inkin |
| Denes et al. [35] | 2012 | Hip | 1/2 | 55 y. | Male | n.r. | Candida glabrata |
| Reddy et al. [36] | 2013 | Knee | 1/1 | 62 y. | Female | None | Candida tropicalis |
| Bruce et al. [37] | 2001 | Hip | 2/2 | 51 y./ 68 y. | Female/Female | n.r. | Candida parapsilosis, Candida albicans |
| Phelan et al. [38] | 2002 | Hip | 1/1 | 75 y. | Female | Rheumatoid arthritis, prior PJI | Candida albicans |
| Selmon et al. [39] | 1998 | Knee | 1/1 | 75 y. | Female | Prior abdominal surgery | Candida glabrata |
| Study | Cement Used | Cement Impregnation | Surgical Treatment | 2-Stage Interval | Systemic Therapy | Length of Systemic Therapy | Complications | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Deelstra et al. [30] | Palacos | 0.5 g gentamicin + 1 g vancomycin + 1 g voriconazole + 0.25 g amphotericin B/40 g cement | 2-stage | 3 months | Fluconazole + vancomycin | n.r. | None | 6 years |
| Wu et al. [31] | n.r. | 1.2 g amphotericin B/40 g cement | 2-stage | 6 months | Fluconazole | 6 months | None | 1 year |
| Gaston and Ogden [32] | n.r. | Vancomycin + amphotericin B | 2-stage | 2 months | Voriconazole | 2 months | Culture-negative infection after 2 months, ending in an above-knee amputation | 6 months |
| Marra et al. [33] | Palacos | Total 750 mg amphotericin B | 2-stage | 10 weeks | Fluconazole | 6 weeks | Reinfection with E. coli at reimplantation | n.r. |
| Burgo et al. [34] | n.r. | Voriconazole + vancomycin | 2-stage | n.r. | Voriconazole | 6 months | None | 2 years |
| Denes et al. [35] | Simplex | Total 600 mg voriconazole right hip/total 400 mg voriconazole left hip | 2-stage | n.r. | Caspofungin | n.r. | n.r. | n.r. |
| Reddy et al. [36] | n.r. | Vancomycin + amphotericin B | 2-stage | 20 weeks | Fluconazole | 18 weeks | None | 2 years |
| Bruce et al. [37] | Palacos | 2 g fluconazole/ 40 g cement | 2-stage | 10 months/ 3 months | Fluconazole | n.r. | None | 7 years/ 4 years |
| Phelan et al. [38] | n.r. | Total 200 mg fluconazole | 2-stage | 2.4 months | Fluconazole | 47 days | None | 17 months |
| Selmon et al. [39] | n.r. | Gentamicin + 200 mg amphotericin B | 1-stage | n.r. | Amphotericin B/itraconazole | 1 week/ 8 weeks | None | 4 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anagnostakos, K.; Becker, S.L.; Sahan, I. Antifungal-Loaded Acrylic Bone Cement in the Treatment of Periprosthetic Hip and Knee Joint Infections: A Review. Antibiotics 2022, 11, 879. https://doi.org/10.3390/antibiotics11070879
Anagnostakos K, Becker SL, Sahan I. Antifungal-Loaded Acrylic Bone Cement in the Treatment of Periprosthetic Hip and Knee Joint Infections: A Review. Antibiotics. 2022; 11(7):879. https://doi.org/10.3390/antibiotics11070879
Chicago/Turabian StyleAnagnostakos, Konstantinos, Sören L. Becker, and Ismail Sahan. 2022. "Antifungal-Loaded Acrylic Bone Cement in the Treatment of Periprosthetic Hip and Knee Joint Infections: A Review" Antibiotics 11, no. 7: 879. https://doi.org/10.3390/antibiotics11070879
APA StyleAnagnostakos, K., Becker, S. L., & Sahan, I. (2022). Antifungal-Loaded Acrylic Bone Cement in the Treatment of Periprosthetic Hip and Knee Joint Infections: A Review. Antibiotics, 11(7), 879. https://doi.org/10.3390/antibiotics11070879








