Association between Augmented Renal Clearance and Inadequate Vancomycin Pharmacokinetic/Pharmacodynamic Targets in Chinese Adult Patients: A Prospective Observational Study
Abstract
:1. Introduction
2. Results
2.1. Patient Enrollment and Characteristics
2.2. ARC Patients and Risk Factors for ARC
2.3. Treatment Outcomes and Microbiological Analysis
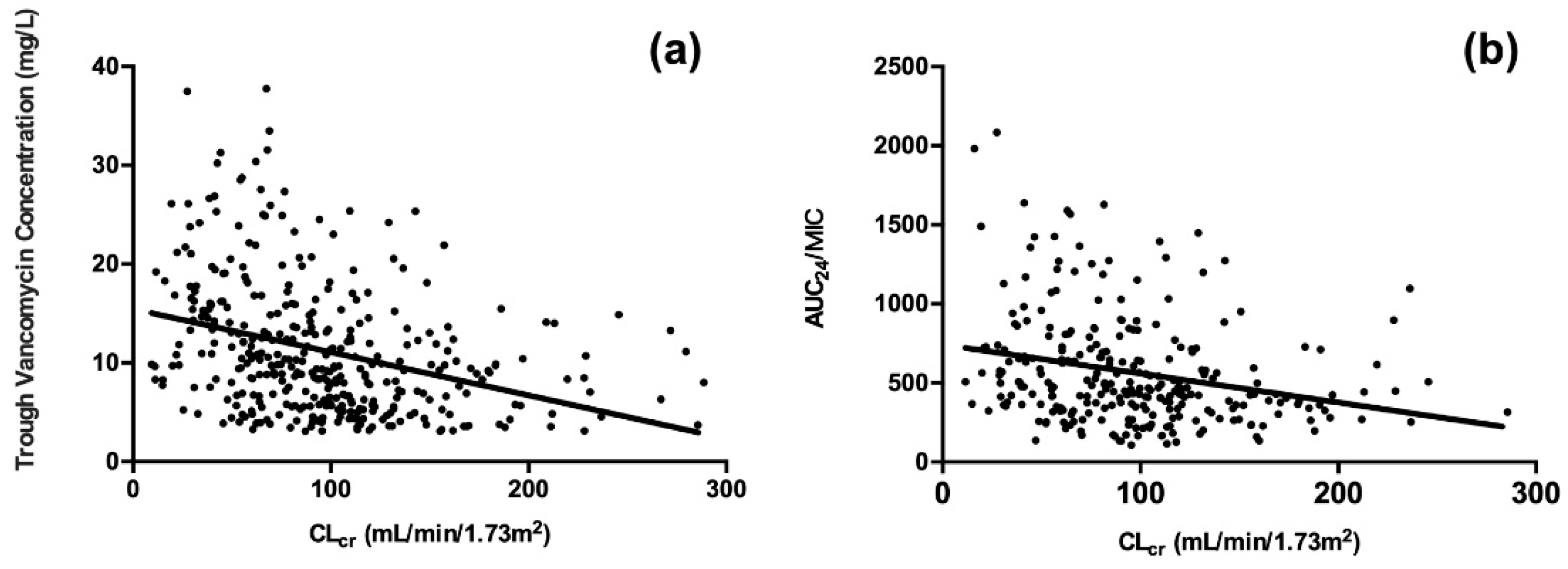
2.4. Associations between ARC and Vancomycin PK/PD Indices
2.5. Evaluation of ARC Scoring Systems
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Population and Data Collection
4.3. ARC Evaluation
4.4. Vancomycin Administration and Sampling
4.5. Clinical Outcome Definition
4.6. Microbiological Data and PK/PD Analysis
4.7. Statistical Analysis
4.7.1. Risk Factors for ARC
4.7.2. Association between ARC and Vancomycin PK/PD Indices
4.7.3. Evaluation of ARC Scoring Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moellering, R.C., Jr. Vancomycin: A 50-year reassessment. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S3–S4. [Google Scholar] [CrossRef] [PubMed]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Song, K.H.; Cho, J.; Kim, H.S.; Kim, N.H.; Kim, T.S.; Choe, P.G.; Chung, J.Y.; Park, W.B.; Bang, J.H.; et al. Area under the concentration-time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant staphylococcus aureus bacteraemia. Int. J. Antimicrob. Agents 2014, 43, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, M.A.; Tacconelli, E.; Grilli, E.; Pea, F.; Petrosillo, N. Continuous versus intermittent infusion of vancomycin for the treatment of gram-positive infections: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2012, 67, 17–24. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases society of America, the American society of health-system pharmacists, and the society of infectious diseases pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef] [Green Version]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American society of health-system pharmacists, the infectious diseases society of america, the pediatric infectious diseases society, and the society of infectious diseases pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar]
- He, N.; Su, S.; Ye, Z.; Du, G.; He, B.; Li, D.; Liu, Y.; Yang, K.; Zhang, X.; Zhang, Y.; et al. Evidence-based guideline for therapeutic drug monitoring of vancomycin: 2020 update by the division of therapeutic drug monitoring, Chinese pharmacological society. Clin. Infect. Dis. 2020, 71, S363–S371. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin. Pharmacokinet. 2010, 49, 539–543. [Google Scholar] [CrossRef]
- Bilbao-Meseguer, I.; Rodriguez-Gascon, A.; Barrasa, H.; Isla, A.; Solinis, M.A. Augmented renal clearance in critically ill patients: A systematic review. Clin. Pharmacokinet. 2018, 57, 1107–1121. [Google Scholar] [CrossRef]
- Hobbs, A.L.; Shea, K.M.; Roberts, K.M.; Daley, M.J. Implications of augmented renal clearance on drug dosing in critically ill patients: A focus on antibiotics. Pharmacotherapy 2015, 35, 1063–1075. [Google Scholar] [CrossRef]
- Claus, B.O.; Hoste, E.A.; Colpaert, K.; Robays, H.; Decruyenaere, J.; De Waele, J.J. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J. Crit. Care 2013, 28, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Russo, A.; Venditti, M.; Novelli, A.; Pai, M.P. Considerations for higher doses of daptomycin in critically ill patients with methicillin-resistant staphylococcus aureus bacteremia. Clin. Infect. Dis. 2013, 57, 1568–1576. [Google Scholar] [CrossRef] [Green Version]
- Giannella, M.; Bartoletti, M.; Gatti, M.; Viale, P. Advances in the therapy of bacterial bloodstream infections. Clin. Microbiol. Infect. 2020, 26, 158–167. [Google Scholar] [CrossRef]
- Carrie, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: A prospective observational study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.T.; Lam, W.M.; Coronado Munoz, A.J.; Modem, V.M. Augmented renal clearance of vancomycin in suspected sepsis: Single-center, retrospective pediatric cohort. Pediatr. Crit. Care Med. 2022, 23, 444–452. [Google Scholar] [CrossRef]
- Campassi, M.L.; Gonzalez, M.C.; Masevicius, F.D.; Vazquez, A.R.; Moseinco, M.; Navarro, N.C.; Previgliano, L.; Rubatto, N.P.; Benites, M.H.; Estenssoro, E.; et al. Augmented renal clearance in critically ill patients: Incidence, associated factors and effects on vancomycin treatment. Rev. Bras. Ter. Intensiva 2014, 26, 13–20. [Google Scholar] [CrossRef]
- Baptista, J.P.; Sousa, E.; Martins, P.J.; Pimentel, J.M. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int. J. Antimicrob. Agents 2012, 39, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Putt, M.T.; Boots, R.J.; Lipman, J. Arc—Augmented renal clearance. Curr. Pharm. Biotechnol. 2011, 12, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; et al. Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: A nested cohort study of the bling-ii randomized, placebo-controlled, clinical trial. Int. J. Antimicrob. Agents 2017, 49, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Minville, V.; Asehnoune, K.; Virtos, M.; Georges, B.; Fourcade, O.; Conil, J.M. Screening of patients with augmented renal clearance in icu: Taking into account the ckd-epi equation, the age, and the cause of admission. Ann. Intensive Care 2015, 5, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Shorr, A.F.; Boots, R.J.; Lipman, J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: Identifying at-risk patients. Crit. Care 2013, 17, R35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udy, A.A.; Baptista, J.P.; Lim, N.L.; Joynt, G.M.; Jarrett, P.; Wockner, L.; Boots, R.J.; Lipman, J. Augmented renal clearance in the icu: Results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit. Care Med. 2014, 42, 520–527. [Google Scholar] [CrossRef]
- Minkute, R.; Briedis, V.; Steponaviciute, R.; Vitkauskiene, A.; Maciulaitis, R. Augmented renal clearance—An evolving risk factor to consider during the treatment with vancomycin. J. Clin. Pharm. Ther. 2013, 38, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Lluch, O.; Geronimo-Pardo, M.; Peyro-Garcia, R.; Lizan-Garcia, M. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth. Intensive Care 2008, 36, 674–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, K.; Ihara, S.; Kinae, A.; Ikegaya, K.; Suzuki, M.; Hirano, K.; Itoh, K. Augmented renal clearance in pediatric patients with febrile neutropenia associated with vancomycin clearance. Ther. Drug Monit. 2016, 38, 393–397. [Google Scholar] [CrossRef]
- Barletta, J.F.; Mangram, A.J.; Byrne, M.; Sucher, J.F.; Hollingworth, A.K.; Ali-Osman, F.R.; Shirah, G.R.; Haley, M.; Dzandu, J.K. Identifying augmented renal clearance in trauma patients: Validation of the augmented renal clearance in trauma intensive care scoring system. J. Trauma Acute Care Surg. 2017, 82, 665–671. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Bu, S.; Chen, X.; Zhou, J.; Liu, X.; Guo, X.; Li, L.; Zhang, J. The relationship between vancomycin auc/mic and trough concentration, age, dose, renal function in Chinese critically ill pediatric patients. Pharmacol. Res. Perspect. 2021, 9, e00885. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Shen, C. Augmented renal clearance in critical illness: An important consideration in drug dosing. Pharmaceutics 2017, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Declercq, P.; Nijs, S.; D’Hoore, A.; Van Wijngaerden, E.; Wolthuis, A.; de Buck van Overstraeten, A.; Wauters, J.; Spriet, I. Augmented renal clearance in non-critically ill abdominal and trauma surgery patients is an underestimated phenomenon: A point prevalence study. J. Trauma Acute Care Surg. 2016, 81, 468–477. [Google Scholar] [CrossRef]
- Smit, C.; De Hoogd, S.; Bruggemann, R.J.M.; Knibbe, C.A.J. Obesity and drug pharmacology: A review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin. Drug Metab. Toxicol. 2018, 14, 275–285. [Google Scholar] [CrossRef]
- Barletta, J.F.; Mangram, A.J.; Byrne, M.; Hollingworth, A.K.; Sucher, J.F.; Ali-Osman, F.R.; Shirah, G.R.; Dzandu, J.K. The importance of empiric antibiotic dosing in critically ill trauma patients: Are we under-dosing based on augmented renal clearance and inaccurate renal clearance estimates? J. Trauma Acute Care Surg. 2016, 81, 1115–1121. [Google Scholar] [CrossRef] [Green Version]
- Baptista, J.P.; Udy, A.A.; Sousa, E.; Pimentel, J.; Wang, L.; Roberts, J.A.; Lipman, J. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit. Care 2011, 15, R139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uster, D.W.; Wicha, S.G. Optimized sampling to estimate vancomycin drug exposure: Comparison of pharmacometric and equation-based approaches in a simulation-estimation study. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.F.; Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar] [PubMed]
- Pan, X.F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Shen, K.; Yang, M.; Fan, Y.; Liang, X.; Chen, Y.; Wu, J.; Yu, J.; Zhang, H.; Wang, R.; Zhang, F.; et al. Model-based evaluation of the clinical and microbiological efficacy of vancomycin: A prospective study of Chinese adult in-house patients. Clin. Infect. Dis. 2018, 67, S256–S262. [Google Scholar] [CrossRef]
| Characteristics | Total Patients (n = 414) | ARC (n = 88) | Non-ARC (n = 326) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Male sex | 277 (66.9) | 66 (75.0) | 211 (64.7) | 0.069 |
| Age (years) | 61 (49–74) | 50 (33–60) | 64 (53–76) | <0.001 * |
| Age < 50 | 109 (26.3) | 40 (45.5) | 69 (21.2) | <0.001 * |
| BSA (m2) | 1.78 (1.67–1.91) | 1.80 (1.66–1.93) | 1.78 (1.67–1.90) | 0.202 |
| BMI (kg/m2) | 22.0 (19.8–24.2) | 23.2 (20.3–25.6) | 21.6 (19.6–24.1) | 0.025 * |
| Overweight a | 122 (29.5) | 36 (40.9) | 86 (26.4) | 0.008 * |
| Baseline renal function | ||||
| SCr (μmol/L) | 62 (46–85) | 39 (31–46) | 69 (55–94) | <0.001 * |
| CLcr (mL/min/1.73m2) | 92 (61–121) | 159 (144–193) | 78 (55–101) | <0.001 * |
| eGFR (mL/min/1.73m2) | 114 (80–153) | 200 (170–244) | 103 (70–128) | <0.001 * |
| Comorbidities | ||||
| Charlson comorbidity index | 2 (1–3) | 2 (0–2) | 2 (1–3) | 0.050 |
| Cardiovascular disease | 156 (37.7) | 13 (14.8) | 143 (43.9) | <0.001 * |
| Diabetes mellitus | 65 (15.7) | 7 (8.0) | 58 (17.8) | 0.024 * |
| Stroke | 97 (23.4) | 13 (14.8) | 84 (25.8) | 0.031 * |
| Trauma | 30 (7.2) | 9 (10.2) | 21 (6.4) | 0.224 |
| Malignancy | 110 (26.6) | 31 (35.2) | 79 (24.2) | 0.038 * |
| Exposures | ||||
| Vascular catheter | 295 (71.3) | 66 (75.0) | 229 (70.2) | 0.382 |
| Urinary catheter | 280 (67.6) | 63 (71.6) | 217 (66.6) | 0.371 |
| Mechanical ventilation | 129 (31.2) | 34 (38.6) | 95 (29.1) | 0.088 |
| Enteral nutrition | 78 (18.8) | 24 (27.3) | 54 (16.6) | 0.023 * |
| ICU admission | 252 (60.9) | 56 (63.6) | 196 (60.1) | 0.549 |
| ICU duration | 21 (12–36) | 26 (16–2) | 21 (10–34) | 0.057 |
| Primary infection site | ||||
| BSI | 147 (35.5) | 34 (38.6) | 113 (34.7) | 0.489 |
| IE | 8 (1.9) | 1 (1.1) | 7 (2.1) | 1.000 |
| Pneumonia | 127 (30.7) | 26 (29.5) | 101 (31.0) | 0.795 |
| SSTI | 29 (7.0) | 4 (4.5) | 25 (7.7) | 0.479 |
| UTI | 22 (5.3) | 0 | 22 (6.7) | 0.006 * |
| CNS infection | 18 (4.3) | 7 (8.0) | 11 (3.4) | 0.076 |
| IAI | 31 (7.5) | 10 (11.4) | 21 (6.4) | 0.120 |
| Laboratory indicators | ||||
| Neutrophil percentage (%) | 82.4 (73.9–88.0) | 78.7 (72.0–86.9) | 83.2 (74.5–88.6) | 0.013 * |
| Febrile neutropenia | 14 (3.4) | 7 (8.0) | 7 (2.1) | 0.015 * |
| ALB (g/L) | 37 (27–36) | 32 (28–36) | 32 (27–36) | 0.736 |
| ALT (U/L) | 29 (18–54) | 36 (22–85) | 28 (16–49) | 0.002 * |
| AST (U/L) | 31 (20–53) | 32 (22–64) | 30 (19–51) | 0.143 |
| Combination therapy | ||||
| Loop diuretic | 82 (19.8) | 18 (20.5) | 64 (19.6) | 0.864 |
| Dehydrating agent | 28 (6.8) | 10 (11.4) | 18 (5.5) | 0.053 |
| Characteristics | OR | 95% CI for OR | p Value |
|---|---|---|---|
| Male sex | 2.588 | 1.388–4.825 | 0.003 * |
| Age < 50 years | 2.713 | 1.548–4.754 | <0.001 * |
| Overweight a | 2.072 | 1.185–3.625 | 0.011 * |
| Cardiovascular disease | 0.281 | 0.144–0.550 | <0.001 * |
| Mechanical ventilation | 1.785 | 1.002–3.181 | 0.049 * |
| Enteral nutrition | 2.317 | 1.185–4.528 | 0.014 * |
| Neutrophil percentage | 0.975 | 0.959–0.991 | 0.003 * |
| Characteristics | Total Patients (n = 414) | ARC (n = 88) | Non-ARC (n = 326) | OR | 95%CI for OR | p Value |
|---|---|---|---|---|---|---|
| Initial daily dose (g/d) | 2 (1–2) | 2 (2–2) | 2 (1–2) | 3.238 | 1.952–5.369 | <0.001 * |
| PK/PD values | ||||||
| Cmin (mg/L) | 9.0 (5.0–14.1) | 7.1 (3.9–10.6) | 9.6 (5.3–15.3) | - | - | 0.001 * |
| <10 | 238 (57.5) | 63 (71.6) | 175 (53.7) | 2.174 | 1.303–3.628 | 0.003 * |
| 10–20 | 132 (31.0) | 21 (23.9) | 111 (34.0) | 0.607 | 0.353–1.043 | 0.071 |
| >20 | 44 (10.6) | 4 (4.5) | 40 (12.3) | 0.340 | 0.118–0.979 | 0.046 * |
| AUC24 | 409.9 (318.5–558.9) | 357.7 (271.5–419.1) | 423.8 (339.9–546.0) | - | - | <0.001 * |
| AUC24/MIC | 457.4 (322.0–711.8) | 360.5 (253.8–475.0) | 494.7 (357.3–728.2) | - | - | <0.001 * |
| <400 | 164 (39.6) | 56 (63.6) | 108 (33.1) | 3.156 | 1.813–5.495 | <0.001* |
| 400–600 | 110 (26.6) | 17 (19.3) | 93 (28.5) | 0.585 | 0.282–1.214 | 0.083 |
| >600 | 140 (33.8) | 15 (17.0) | 125 (38.3) | 0.392 | 0.190–0.805 | <0.001 * |
| PK/PD values corrected for dose a | ||||||
| Cmin per dose | 5.7 (3.6–10.2) | 4.2 (2.5–5.9) | 6.6 (4.2–11.5) | - | - | <0.001 * |
| AUC24 per dose | 249.3 (185.3–358.1) | 180.3 (161.5–215.0) | 278.9 (208.6–397.1) | - | - | <0.001 * |
| AUC24/MIC per dose | 287.5 (180.0–457.3) | 180.2 (157.9–245.8) | 309.7 (205.9–530.6) | - | - | <0.001 * |
| High-Risk Score | Scoring System | Gold Standard * | Sensitivity | Specificity | PPV | NPV | Consistency Rate | |
|---|---|---|---|---|---|---|---|---|
| ARC | Non-ARC | |||||||
| ARC Score ≥ 7 | Positive | 33 | 42 | 58.9% | 78.6% | 44.0% | 87.0% | 74.2% |
| Negative | 23 | 154 | ||||||
| ARCTIC Score ≥ 6 | Positive | 8 | 1 | 88.9% | 95.2% | 88.9% | 95.2% | 93.3% |
| Negative | 1 | 20 | ||||||
| Critically Ill Patients (n = 252) | ARC Score ≥ 7 (n = 75) | ARC Score < 7 (n = 177) | OR | 95%CI for OR | p Value | |
|---|---|---|---|---|---|---|
| Cmin (mg/L) | 9.0 (5.0–14.1) | 6.7 (3.8–9.8) | 9.5 (5.3–15.0) | - | - | <0.001 * |
| <10 | 150 (59.5) | 63 (84.0) | 87 (49.2) | 5.431 | 2.740–10.764 | <0.001 * |
| 10–20 | 73 (29.0) | 10 (13.3) | 63 (35.6) | 0.278 | 0.134–0.580 | 0.001 * |
| >20 | 29 (11.5) | 2 (2.7) | 27 (15.3) | 0.152 | 0.035–0.658 | 0.012 * |
| AUC24/MIC | 476.7 (320.1–710.4) | 380.1 (265.3–619.0) | 486.2 (353.3–757.6) | - | - | 0.093 |
| <400 | 100 (39.7) | 39 (52.0) | 61 (34.5) | 1.998 | 1.061–3.766 | 0.009 * |
| 400–600 | 67 (26.6) | 15 (20.0) | 52 (29.4) | 0.693 | 0.317–1.516 | 0.123 |
| >600 | 85 (33.7) | 21 (28.0) | 64 (36.2) | 0.717 | 0.351–1.464 | 0.210 |
| Trauma Patients (n = 30) | ARCTIC Score ≥ 6 (n = 9) | ARCTIC Score < 6 (n = 21) | p Value | |
|---|---|---|---|---|
| Cmin (mg/L) | 8.5 (3.8–13.3) | 5.6 (3.8–7.6) | 9.2 (4.0–14.6) | 0.178 |
| <10 | 20 (66.7) | 8 (88.9) | 12 (57.1) | 0.204 |
| 10–20 | 9 (30.0) | 1 (11.1) | 7 (33.3) | 0.374 |
| >20 | 2 (6.7) | 0 | 2 (9.5) | 1.000 |
| AUC24/MIC | 492.0 (303.1–781.2) | 277.5 (208.2–397.8) | 538.5 (379.8–910.2) | 0.011 * |
| < 400 | 12 (40.0) | 7 (77.8) | 5 (23.8) | 0.013 * |
| 400–600 | 8 (26.7) | 1 (11.1) | 6 (28.6) | 0.393 |
| >600 | 10 (33.3) | 1 (11.1) | 10 (47.6) | 0.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Fan, Y.; Yang, M.; Liang, X.; Wu, J.; Chen, Y.; Guo, B.; Zhang, H.; Wang, R.; Zhang, F.; et al. Association between Augmented Renal Clearance and Inadequate Vancomycin Pharmacokinetic/Pharmacodynamic Targets in Chinese Adult Patients: A Prospective Observational Study. Antibiotics 2022, 11, 837. https://doi.org/10.3390/antibiotics11070837
Zhao J, Fan Y, Yang M, Liang X, Wu J, Chen Y, Guo B, Zhang H, Wang R, Zhang F, et al. Association between Augmented Renal Clearance and Inadequate Vancomycin Pharmacokinetic/Pharmacodynamic Targets in Chinese Adult Patients: A Prospective Observational Study. Antibiotics. 2022; 11(7):837. https://doi.org/10.3390/antibiotics11070837
Chicago/Turabian StyleZhao, Jinjin, Yaxin Fan, Minjie Yang, Xiaoyu Liang, Jufang Wu, Yuancheng Chen, Beining Guo, Huifang Zhang, Ruilan Wang, Fengying Zhang, and et al. 2022. "Association between Augmented Renal Clearance and Inadequate Vancomycin Pharmacokinetic/Pharmacodynamic Targets in Chinese Adult Patients: A Prospective Observational Study" Antibiotics 11, no. 7: 837. https://doi.org/10.3390/antibiotics11070837
APA StyleZhao, J., Fan, Y., Yang, M., Liang, X., Wu, J., Chen, Y., Guo, B., Zhang, H., Wang, R., Zhang, F., Hang, J., Li, H., & Zhang, J. (2022). Association between Augmented Renal Clearance and Inadequate Vancomycin Pharmacokinetic/Pharmacodynamic Targets in Chinese Adult Patients: A Prospective Observational Study. Antibiotics, 11(7), 837. https://doi.org/10.3390/antibiotics11070837






