Effect of the Symbiosis with Mycoplasma hominis and Candidatus Mycoplasma Girerdii on Trichomonas vaginalis Metronidazole Susceptibility
Abstract
:1. Introduction
2. Results
2.1. Mycoplasma Hominis and Candidatus Mycoplasma Girerdii Detection in T. vaginalis Isolates
2.2. Metronidazole Susceptibility of T. vaginalis Isolates
2.3. Association between Symbionts Presence and Metronidazole Sensitivity in T. vaginalis
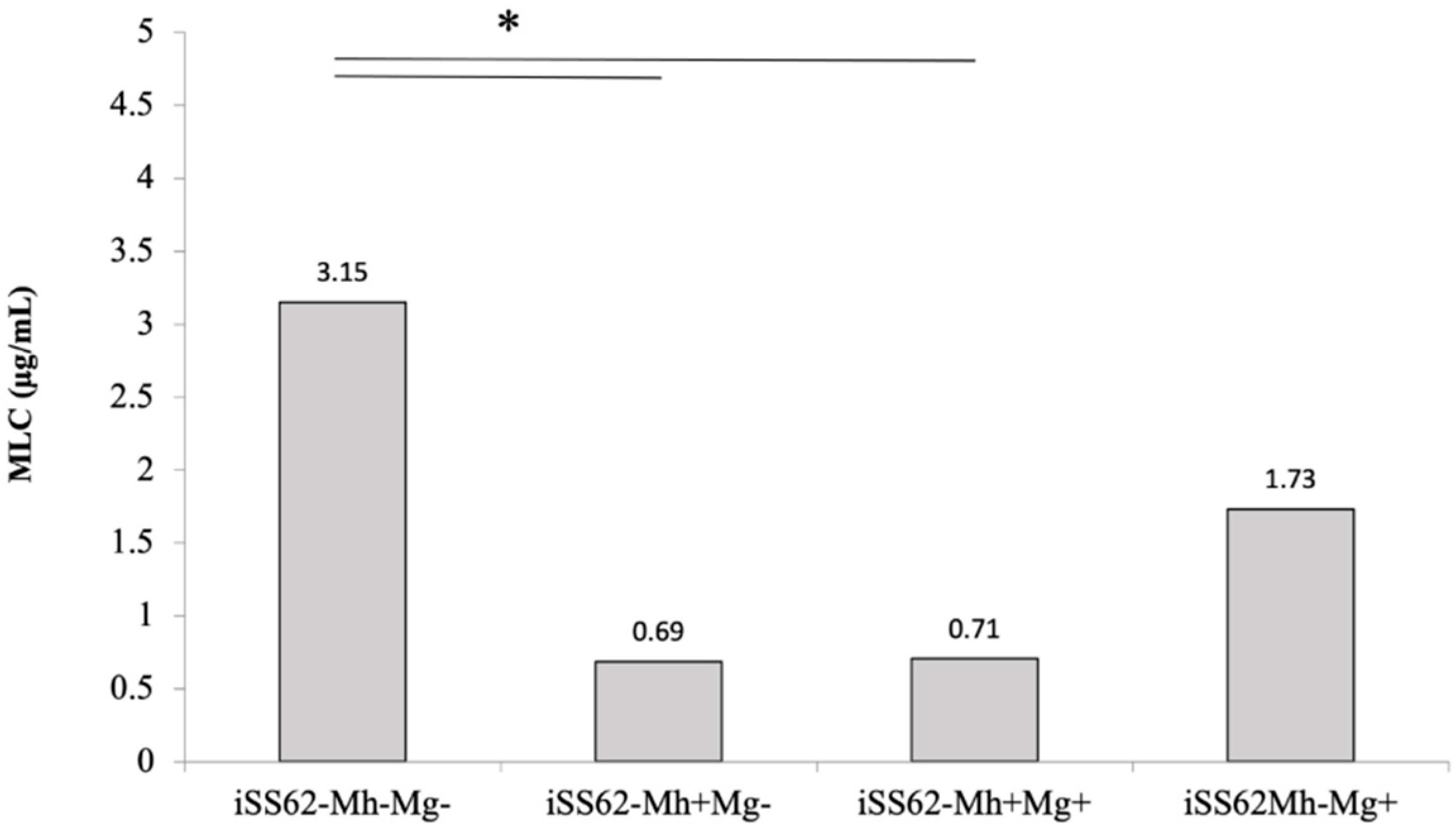
2.4. Sensitivity of Isogenic T. vaginalis Strains to Metronidazole in Aerobic Conditions
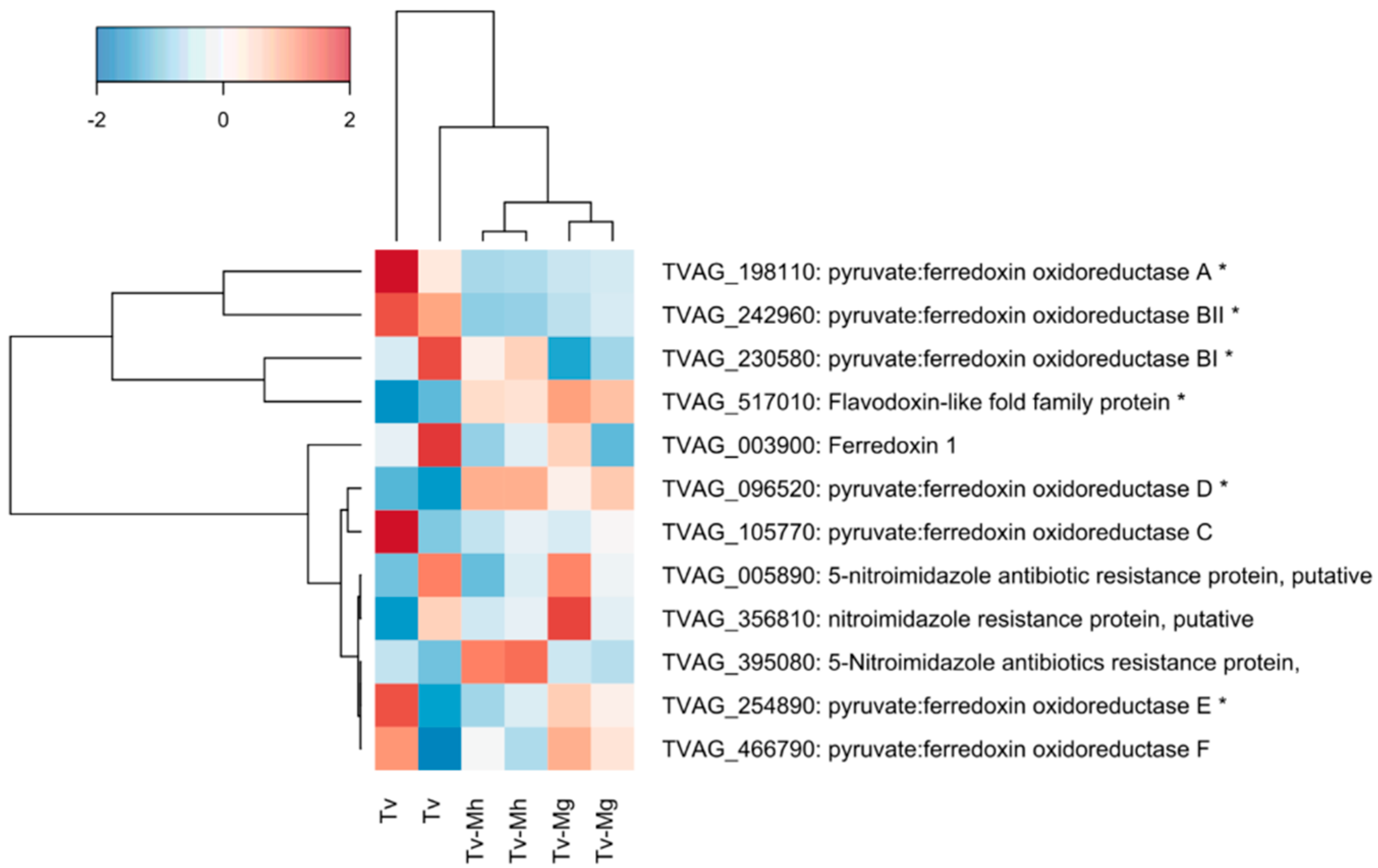
2.5. Expression of Genes Associated with Drug Resistance in T. vaginalis Isogenic Strains
3. Discussion
4. Materials and Methods
4.1. Culture Conditions of Trichomonas vaginalis Isolates
4.2. Screening for Mycoplasma Species in T. vaginalis Isolates
4.3. Generation of Isogenic T. vaginalis Strains
4.4. Metronidazole Susceptibility Assay in Aerobic Conditions
4.5. RNA Preparation
4.6. RNA Sequencing and Data Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, L.; Rowley, J.; Vander, H.S.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.; Kiarie, J.; Temmerman, M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [Green Version]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and Microbiological Aspects of Trichomonas vaginalis. 1998, 11, 300–317. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef] [Green Version]
- Hirt, R.P.; Sherrard, J. Trichomonas vaginalis Origins, Molecular Pathobiology and Clinical Considerations. Curr. Opin. Infect. Dis. 2015, 28, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.R.; Judson, G.; Alderete, J.F.; Mundodi, V.; Kucknoor, A.S.; Giovannucci, E.L.; Platz, E.A.; Sutcliffe, S.; Fall, K.; Kurth, T.; et al. Prospective Study of Trichomonas vaginalis Infection and Prostate Cancer Incidence and Mortality: Physicians’ Health Study. J. Natl. Cancer Inst. 2009, 101, 1406–1411. [Google Scholar] [CrossRef]
- Yang, M.; Li, L.; Jiang, C.; Qin, X.; Zhou, M.; Mao, X.; Xing, H. Co-Infection with Trichomonas vaginalis Increases the Risk of Cervical Intraepithelial Neoplasia Grade 2-3 among HPV16 Positive Female: A Large Population-Based Study. BMC Infect. Dis. 2020, 20, 642. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D. A Review on Metronidazole: An Old Warhorse in Antimicrobial Chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef]
- Robinson, S.C. Trichomonal Vaginitis Resistant to Metranidazole. Can. Med. Assoc. J. 1962, 86, 665. [Google Scholar] [PubMed]
- Snipes, L.J.; Gamard, P.M.; Narcisi, E.M.; Beard, C.B.; Lehmann, T.; Secor, W.E. Molecular Epidemiology of Metronidazole Resistance in a Population of Trichomonas vaginalis Clinical Isolates. J. Clin. Microbiol. 2000, 38, 3004–3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.J. Metronidazole and Drug Resistance. Parasitol. Today 1993, 9, 183–186. [Google Scholar] [CrossRef]
- Leitsch, D.; Janssen, B.D.; Kolarich, D.; Johnson, P.J.; Duchêne, M. Trichomonas vaginalis Flavin Reductase 1 and Its Role in Metronidazole Resistance. Mol. Microbiol. 2014, 91, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Graves, K.J.; Novak, J.; Secor, W.E.; Kissinger, P.J.; Schwebke, J.R. A Systematic Review of the Literature on Mechanisms of 5- Nitroimidazole Resistance in Trichomonas vaginalis Keonte. Parasitology 2020, 147, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Seña, A.C.; Bachmann, L.H.; Hobbs, M.M. Persistent and Recurrent Trichomonas vaginalis Infections: Epidemiology, Treatment and Management Considerations. Expert Rev. Anti. Infect. Ther. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Kirkcaldy, R.D.; Augostini, P.; Asbel, L.E.; Bernstein, K.T.; Kerani, R.P.; Mettenbrink, C.J.; Pathela, P.; Schwebke, J.R.; Evan Secor, W.; Workowski, K.A.; et al. Trichomonas vaginalis Antimicrobial Drug Resistance in 6 US Cities, STD Surveillance Network, 2009–2010. Emerg. Infect. Dis. 2012, 18, 939–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabaso, N.; Abbai, N. Distribution of Genotypes in Relation to Metronidazole Susceptibility Patterns in Trichomonas vaginalis Isolated from South African Pregnant Women. Parasitol. Res. 2021, 120, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Rappelli, P.; Addis, M.F.; Carta, F.; Fiori, P.L. Mycoplasma hominis Parasitism of Trichomonas vaginalis. Lancet 1998, 352, 1286. [Google Scholar] [CrossRef]
- Fichorova, R.N.; Fraga, J.; Rappelli, P.; Fiori, P.L. Trichomonas vaginalis Infection in Symbiosis with Trichomonasvirus and Mycoplasma. Res. Microbiol. 2017, 168, 882–891. [Google Scholar] [CrossRef]
- Taylor-Robinson, D. Mollicutes in Vaginal Microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Res. Microbiol. 2017, 168, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Margarita, V.; Rappelli, P.; DessÌ, D.; Pintus, G.; Hirt, R.P.; Fiori, P.L. Symbiotic Association with Mycoplasma hominis Can Influence Growth Rate, ATP Production, Cytolysis and Inflammatory Response of Trichomonas vaginalis. Front. Microbiol. 2016, 7, 953. [Google Scholar] [CrossRef] [Green Version]
- Henriquez, F.L.; Mooney, R.; Bandel, T.; Giammarini, E.; Zeroual, M.; Fiori, P.L.; Margarita, V.; Rappelli, P.; Dessì, D. Paradigms of Protist/Bacteria Symbioses Affecting Human Health: Acanthamoeba Species and Trichomonas vaginalis. Front. Microbiol. 2021, 11, 3380. [Google Scholar] [CrossRef]
- Margarita, V.; Fiori, P.L.; Rappelli, P. Impact of Symbiosis Between Trichomonas vaginalis and Mycoplasma hominis on Vaginal Dysbiosis: A Mini Review. Front. Cell. Infect. Microbiol. 2020, 10, 179. [Google Scholar] [CrossRef]
- Fichorova, R.N. Impact of T. vaginalis Infection on Innate Immune Responses and Reproductive Outcome. J. Reprod. Immunol. 2009, 83, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessì, D.; Margarita, V.; Cocco, A.R.; Marongiu, A.; Fiori, P.L.; Rappelli, P. Trichomonas vaginalis and Mycoplasma hominis: New Tales of Two Old Friends. Parasitology 2019, 146, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Thu, T.T.T.; Margarita, V.; Cocco, A.R.; Marongiu, A.; Dessì, D.; Rappelli, P.; Fiori, P.L. Trichomonas vaginalis Transports Virulent Mycoplasma hominis and Transmits the Infection to Human Cells after Metronidazole Treatment: A Potential Role in Bacterial Invasion of Fetal Membranes and Amniotic Fluid. J. Pregnancy 2018, 2018, 5037181. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.H.; Zozaya, M.; Lillis, R.A.; Myers, L.; Nsuami, M.J.; Ferris, M.J. Unique Vaginal Microbiota That Includes an Unknown Mycoplasma -Like Organism Is Associated With Trichomonas vaginalis Infection. J. Infect. Dis. 2013, 207, 1922–1931. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Huang, B.; Brooks, J.P.; Glascock, A.L.; Sheth, N.U.; Strauss, J.F.; Jefferson, K.K.; Buck, G.A. An Emerging Mycoplasma Associated with Trichomoniasis, Vaginal Infection and Disease. PLoS ONE 2014, 9, e110943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.C.; Xie, L.F.; Fang, S.L.; Gao, M.Y.; Zhu, Y.; Song, L.Y.; Zhong, H.M.; Lun, Z.R. Symbiosis of Mycoplasma hominis in Trichomonas vaginalis May Link Metronidazole Resistance in Vitro. Parasitol. Res. 2006, 100, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Fürnkranz, U.; Henrich, B.; Walochnik, J. Mycoplasma hominis Impacts Gene Expression in Trichomonas vaginalis. Parasitol. Res. 2018, 117, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Butler, S.E.; Augostini, P.; Secor, W.E. Mycoplasma hominis Infection of Trichomonas vaginalis Is Not Associated with Metronidazole-Resistant Trichomoniasis in Clinical Isolates from the United States. Parasitol. Res. 2010, 107, 1023–1027. [Google Scholar] [CrossRef]
- Mabaso, N.; Tinarwo, P.; Abbai, N. Lack of Association between Mycoplasma hominis and Trichomonas vaginalis Symbiosis in Relation to Metronidazole Resistance. Parasitol. Res. 2020, 119, 4197–4204. [Google Scholar] [CrossRef]
- da Luz Becker, D.; dos Santos, O.; Frasson, A.P.; de Vargas Rigo, G.; Macedo, A.J.; Tasca, T. High Rates of Double-Stranded RNA Viruses and Mycoplasma hominis in Trichomonas vaginalis Clinical Isolates in South Brazil. Infect. Genet. Evol. 2015, 34, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Margarita, V.; Bailey, N.P.; Rappelli, P.; Diaz, N.; Dessì, D.; Fettweis, J.M.; Hirt, R.P.; Fiori, P.L. Two Different Species of Mycoplasma Endosymbionts Can Influence Trichomonas vaginalis Pathophysiology. mBio 2022, 24, e0091822. [Google Scholar] [CrossRef]
- Fraga, J.; Rodríguez, N.; Fernández, C.; Mondeja, B.; Sariego, I.; Fernández-Calienes, A.; Rojas, L. Mycoplasma hominis in Cuban Trichomonas vaginalis Isolates: Association with Parasite Genetic Polymorphism. Exp. Parasitol. 2012, 131, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, A.; Papaioannou, P.; Magiorkinis, E.; Magana, M.; Ioannidou, V.; Tzanetou, K.; Burriel, A.R.; Tsironi, M.; Chatzipanagiotou, S. Detecting the Diversity of Mycoplasma and Ureaplasma Endosymbionts Hosted by Trichomonas vaginalis Isolates. Front. Microbiol. 2017, 8, 1188. [Google Scholar] [CrossRef]
- Margarita, V.; Marongiu, A.; Diaz, N.; Dessì, D.; Fiori, P.L.; Rappelli, P. Prevalence of Double-Stranded RNA Virus in Trichomonas vaginalis Isolated in Italy and Association with the Symbiont Mycoplasma hominis. Parasitol. Res. 2019, 118, 3565–3570. [Google Scholar] [CrossRef]
- Land, K.M.; Delgadillo-Correa, M.G.; Tachezy, J.; Vanacova, S.; Hsieh, C.L.; Sutak, R.; Johnson, P.J. Targeted Gene Replacement of a Ferredoxin Gene in Trichomonas vaginalis Does Not Lead to Metronidazole Resistance. Mol. Microbiol. 2004, 51, 115–122. [Google Scholar] [CrossRef]
- Dessì, D.; Delogu, G.; Emonte, E.; Catania, R.; Fiori, P.L.; Rappelli, P.; Dessı, D.; Catania, M.R. Long-Term Survival and Intracellular Replication of Mycoplasma hominis in Trichomonas vaginalis cells: Potential Role of the protozoan in transmetting bacterial infection. Infect. Immun. 2005, 73, 1180–1186. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, A.; Yanez, A.; Dybvig, K.; Watson, H.L.; Griffiths, G.; Cassell, G.H. Evaluation of Intraspecies Genetic Variation within the 16S RRNA Gene of Mycoplasma hominis and Detection by Polymerase Chain Reaction. J. Clin. Microbiol. 1993, 31, 1358–1361. [Google Scholar] [CrossRef] [Green Version]
- Rappelli, P.; Carta, F.; Delogu, G.; Addis, M.F.; Dessì, D.; Cappuccinelli, P.; Fiori, P.L. Mycoplasma hominis and Trichomonas vaginalis Symbiosis: Multiplicity of Infection and Transmissibility of M. hominis to Human Cells. Arch. Microbiol. 2001, 175, 70–74. [Google Scholar] [CrossRef]
- Morada, M.; Manzur, M.; Lam, B.; Tan, C.; Tachezy, J.; Rappelli, P.; Dessì, D.; Fiori, P.L.; Yarlett, N. Arginine Metabolism in Trichomonas vaginalis Infected with Mycoplasma hominis. Microbiology 2010, 156, 3734–3743. [Google Scholar] [CrossRef] [Green Version]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher, A.L.; Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, U.C.M.; Besteiro, S.; et al. Draft Genome Sequence of the Sexually Transmitted Pathogen Trichomonas vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; Oshlack, A. A Scaling Normalization Method for Differential Expression Analysis of RNA-Seq Data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER: A Library of Protein Families and Subfamilies Indexed by Function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Microbial Association | T. vaginalis (Vietnam) N (%) | T. vaginalis (Italy) N (%) |
|---|---|---|
| T.vaginalis Mhneg Mgneg | 29 (62%) | 2 (12%) |
| T.vaginalis Mhpos Mgneg | 9 (19%) | 5 (29%) |
| T.vaginalis Mhneg Mgpos | 3 (6%) | 2 (12%) |
| T.vaginalis Mhpos Mgpos | 6 (13%) | 8 (47%) |
| MLC (µg/mL) | T. vaginalis Total N (%) | T. vaginalis (Vietnam) N (%) | T. vaginalis (Italy) N (%) |
|---|---|---|---|
| ≤4.3 µg/mL | 48 (75 %) | 34 (72%) | 14 (82%) |
| 8.6 µg/mL | 11 (17%) | 9 (19%) | 2 (12%) |
| ≥17.1 µg/mL | 5 (8%) | 4 (9%) | 1 (6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margarita, V.; Cao, L.C.; Bailey, N.P.; Ngoc, T.H.T.; Ngo, T.M.C.; Nu, P.A.T.; Diaz, N.; Dessì, D.; Hirt, R.P.; Fiori, P.L.; et al. Effect of the Symbiosis with Mycoplasma hominis and Candidatus Mycoplasma Girerdii on Trichomonas vaginalis Metronidazole Susceptibility. Antibiotics 2022, 11, 812. https://doi.org/10.3390/antibiotics11060812
Margarita V, Cao LC, Bailey NP, Ngoc THT, Ngo TMC, Nu PAT, Diaz N, Dessì D, Hirt RP, Fiori PL, et al. Effect of the Symbiosis with Mycoplasma hominis and Candidatus Mycoplasma Girerdii on Trichomonas vaginalis Metronidazole Susceptibility. Antibiotics. 2022; 11(6):812. https://doi.org/10.3390/antibiotics11060812
Chicago/Turabian StyleMargarita, Valentina, Le Chi Cao, Nicholas P. Bailey, Thuy Ha Thi Ngoc, Thi Minh Chau Ngo, Phuong Anh Ton Nu, Nicia Diaz, Daniele Dessì, Robert P. Hirt, Pier Luigi Fiori, and et al. 2022. "Effect of the Symbiosis with Mycoplasma hominis and Candidatus Mycoplasma Girerdii on Trichomonas vaginalis Metronidazole Susceptibility" Antibiotics 11, no. 6: 812. https://doi.org/10.3390/antibiotics11060812
APA StyleMargarita, V., Cao, L. C., Bailey, N. P., Ngoc, T. H. T., Ngo, T. M. C., Nu, P. A. T., Diaz, N., Dessì, D., Hirt, R. P., Fiori, P. L., & Rappelli, P. (2022). Effect of the Symbiosis with Mycoplasma hominis and Candidatus Mycoplasma Girerdii on Trichomonas vaginalis Metronidazole Susceptibility. Antibiotics, 11(6), 812. https://doi.org/10.3390/antibiotics11060812








