The In-Vitro Activity of a Cold Atmospheric Plasma Device Utilizing Ambient Air against Bacteria and Biofilms Associated with Periodontal or Peri-Implant Diseases
Abstract
:1. Introduction
2. Materials and Methods
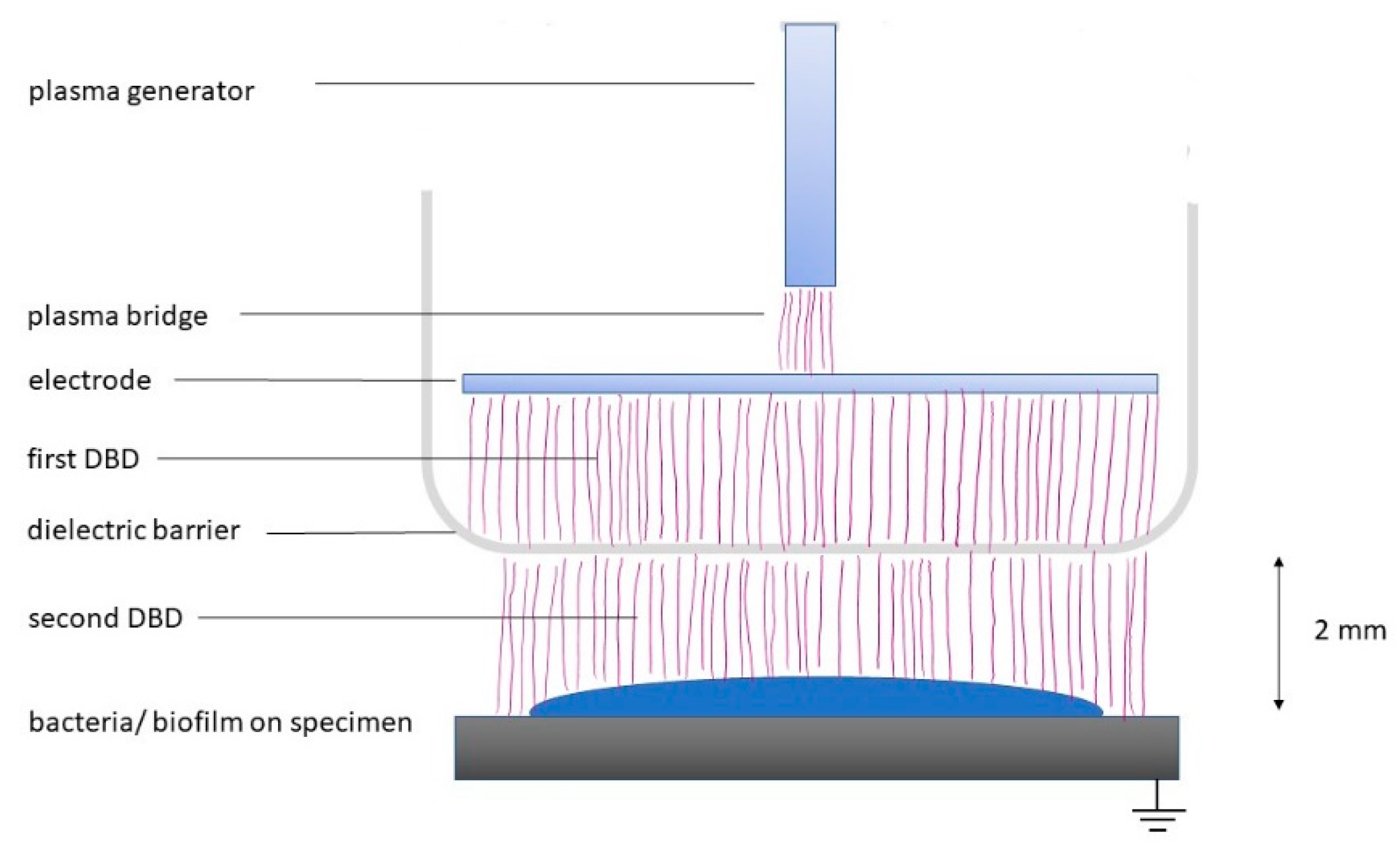
2.1. Plasma Device
2.2. Bacteria
2.3. Activity on Planktonic Bacteria
2.4. Dentin and Titanium Specimen Preparation
2.5. Multi-Species Biofilm
- (a)
- The total numbers of CFU on TSA plates were counted after an incubation time of 8 d.
- (b)
- Quantification of the biofilm was made after staining with crystal violet according to a recently published protocol [33,34]. After CAP treatment, the 100 µL of the biofilm suspension was transferred to a 96-well plate and fixed for 1 hour at 60 °C. For staining, 50 μL of 0.06% (w/v) crystal violet (Merck KGaA, Darmstadt, Germany) was added per well. The biofilm mixture was incubated for 10 min at room temperature and dissolved with 200 μL of 30% acetic acid. The plate was read at 600 nm by a microplate reader (ELx808, BioTek Instruments, Winooski, VT, USA).
- (c)
- The metabolic activity of the biofilm was assessed using resazurin as a redox indicator. After transferring 100 µL of the biofilm suspension to another 96-well-plate, 100 μL of nutrient broth containing 0.06 µL resazurin solution (alamarBlue® reagent, Thermo Fisher Scientific Inc., Waltham, MA, USA) was added per well. After 1 h of incubation, the 96-well plate was measured at 570 nm against 600 nm using (ELx808, BioTek Instruments, Winooski, VT, USA).
2.6. Adhesion of Oral Fibroblasts
2.7. Experiments on Toxicity
2.8. Statistical Analysis
3. Results
3.1. Bactericidal Effect on Oral Bacteria
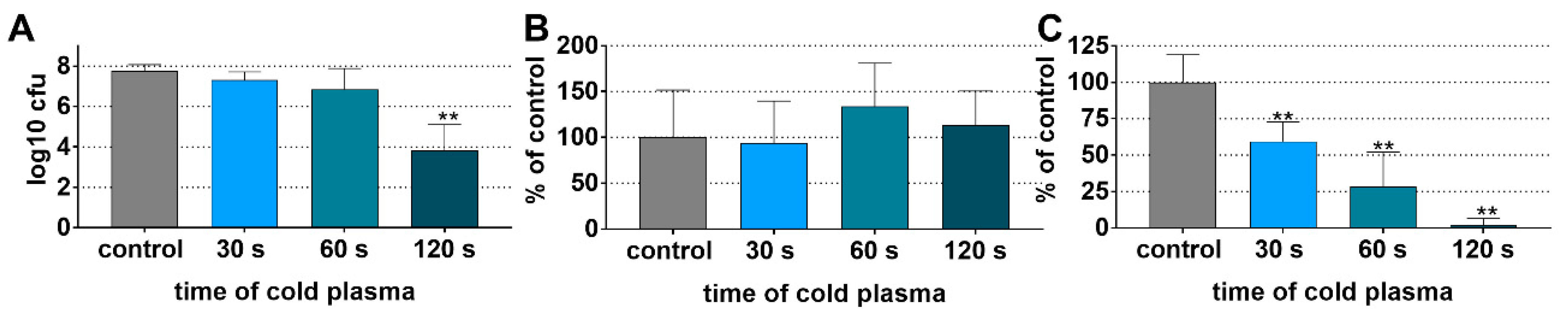
3.2. Preformed Multi-Species Biofilm on Dentin and Titanium Specimens
3.2.1. Reduced Biofilm Viability on Dentin Specimens after CAP Application
3.2.2. Reduced Biofilm Viability on Titanium Specimens after CAP Application
3.3. No Effect of CAP on De Novo Biofilm Formation
3.4. Increased Adhesion of Gingival Fibroblasts to Titanium Surfaces after CAP Pretreatment
3.5. No Adverse Effect of Cap on Host Tissue Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Fragkioudakis, I.; Riggio, M.P.; Apatzidou, D.A. Understanding the microbial components of periodontal diseases and periodontal treatment-induced microbiological shifts. J. Med. Microbiol. 2021, 70, 001247. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Curtis, M.A. Oral biofilms revisited: A novel host tissue of bacteriological origin. Periodontol. 2000 2021, 86, 8–13. [Google Scholar] [CrossRef]
- Joseph, S.; Curtis, M.A. Microbial transitions from health to disease. Periodontol. 2000 2021, 86, 201–209. [Google Scholar] [CrossRef]
- Fu, J.-H.; Wang, H.-L. Breaking the wave of peri-implantitis. Periodontol. 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- Retamal-Valdes, B.; Formiga, M.d.C.; Almeida, M.L.; Fritoli, A.; Figueiredo, K.A.; Westphal, M.; Gomes, P.; Feres, M. Does subgingival bacterial colonization differ between implants and teeth? A systematic review. Braz. Oral Res. 2019, 33, e064. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol. 2000 2021, 86, 231–240. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I. Treatment of pathologic peri-implant pockets. Periodontol. 2000 2018, 76, 180–190. [Google Scholar] [CrossRef]
- Ong, A.; Kim, J.; Loo, S.; Quaranta, A.; Rincon, A.J.C. Prescribing trends of systemic antibiotics by periodontists in Australia. J. Periodontol. 2019, 90, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Feres, M.; Oud, V.; Martín, C.; Matesanz, P.; Herrera, D. Adjunctive effect of systemic antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 257–281. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; von Woedtke, T.; Weltmann, K.-D. (Eds.) Plasmamedizin: Kaltplasma in der medizinischen Anwendung; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-52644-6. [Google Scholar]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [Green Version]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Cold atmospheric plasma as an interface biotechnology for enhancing surgical implants. Crit. Rev. Biotechnol. 2021, 41, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10, 6475. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio 2022, 13, 100200. [Google Scholar] [CrossRef]
- Yao, Y.; Song, K.; Chen, H.; Ding, X.; Shi, Q.; Lu, X.; Cao, Y. In vitro and in vivo research of atmosphere pressure nonequilibrium plasmas on root canal disinfection: Implication for alternative strategy for irrigation. Clin. Oral Investig. 2021, 25, 5833–5842. [Google Scholar] [CrossRef]
- Küçük, D.; Savran, L.; Ercan, U.K.; Yarali, Z.B.; Karaman, O.; Kantarci, A.; Sağlam, M.; Köseoğlu, S. Evaluation of efficacy of non-thermal atmospheric pressure plasma in treatment of periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2020, 24, 3133–3145. [Google Scholar] [CrossRef]
- Hui, W.L.; Perrotti, V.; Iaculli, F.; Piattelli, A.; Quaranta, A. The Emerging Role of Cold Atmospheric Plasma in Implantology: A Review of the Literature. Nanomaterials 2020, 10, 1505. [Google Scholar] [CrossRef]
- Shi, Q.; Song, K.; Zhou, X.; Xiong, Z.; Du, T.; Lu, X.; Cao, Y. Effects of non-equilibrium plasma in the treatment of ligature-induced peri-implantitis. J. Clin. Periodontol. 2015, 42, 478–487. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Xie, P.; Ao, X.; Zheng, Z.; Dong, X.; Li, H.; Yu, Q.; Zhu, Z.; Chen, M.; et al. Non-thermal plasma reduces periodontitis-induced alveolar bone loss in rats. Biochem. Biophys. Res. Commun. 2018, 503, 2040–2046. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, D.; Liang, D.; Zhang, W.; Shi, Q.; Cao, Y. Evaluation of modified cold-atmospheric pressure plasma (MCAP) for the treatment of peri-implantitis in beagles. Oral Dis. 2020, 28, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lackmann, J.-W.; Bandow, J.E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014, 98, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.A.; Tehon, S.W. Solid State Magnetic and Dielectric Devices; John Wiley & Sons: London, UK, 1959. [Google Scholar]
- Korzec, D.; Hoppenthaler, F.; Nettesheim, S. Piezoelectric Direct Discharge: Devices and Applications. Plasma 2021, 4, 1–41. [Google Scholar] [CrossRef]
- Zeng, Y.; Komasa, S.; Nishida, H.; Agariguchi, A.; Sekino, T.; Okazaki, J. Enhanced Osseointegration and Bio-Decontamination of Nanostructured Titanium Based on Non-Thermal Atmospheric Pressure Plasma. Int. J. Mol. Sci. 2020, 21, 3533. [Google Scholar] [CrossRef]
- Timmermann, E.; Bansemer, R.; Gerling, T.; Hahn, V.; Weltmann, K.-D.; Nettesheim, S.; Puff, M. Piezoelectric-driven plasma pen with multiple nozzles used as a medical device: Risk estimation and antimicrobial efficacy. J. Phys. D Appl. Phys. 2021, 54, 25201. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, C.R.; Hindle, B.J.; Saad, S.; Stratakos, A.C. Inactivation of Listeria monocytogenes and Salmonella on Stainless Steel by a Piezoelectric Cold Atmospheric Plasma Generator. Appl. Sci. 2021, 11, 3567. [Google Scholar] [CrossRef]
- Yan, M.; Hartjen, P.; Gosau, M.; Vollkommer, T.; Grust, A.L.C.; Fuest, S.; Kluwe, L.; Burg, S.; Smeets, R.; Henningsen, A. Effects of a Novel Cold Atmospheric Plasma Treatment of Titanium on the Proliferation and Adhesion Behavior of Fibroblasts. Int. J. Mol. Sci. 2021, 23, 420. [Google Scholar] [CrossRef]
- Zollinger, L.; Schnyder, S.; Nietzsche, S.; Sculean, A.; Eick, S. In-vitro activity of taurolidine on single species and a multispecies population associated with periodontitis. Anaerobe 2015, 32, 18–23. [Google Scholar] [CrossRef]
- Kwasny, S.M.; Opperman, T.J. Static biofilm cultures of Gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr. Protoc. Pharmacol. 2010, 50, 13A.8.1–13A.8.23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirracchio, L.; Joos, A.; Luder, N.; Sculean, A.; Eick, S. Activity of taurolidine gels on ex vivo periodontal biofilm. Clin. Oral Investig. 2018, 22, 2031–2037. [Google Scholar] [CrossRef]
- Matsuyama, T.; Aoki, A.; Oda, S.; Yoneyama, T.; Ishikawa, I. Effects of the Er:YAG laser irradiation on titanium implant materials and contaminated implant abutment surfaces. J. Clin. Laser Med. Surg. 2003, 21, 7–17. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Jepsen, K.; Falk, W.; Brune, F.; Fimmers, R.; Jepsen, S.; Bekeredjian-Ding, I. Prevalence and antibiotic susceptibility trends of periodontal pathogens in the subgingival microbiota of German periodontitis patients: A retrospective surveillance study. J. Clin. Periodontol. 2021, 48, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Boekema, B.; Stoop, M.; Vlig, M.; van Liempt, J.; Sobota, A.; Ulrich, M.; Middelkoop, E. Antibacterial and safety tests of a flexible cold atmospheric plasma device for the stimulation of wound healing. Appl. Microbiol. Biotechnol. 2021, 105, 2057–2070. [Google Scholar] [CrossRef]
- Liguori, A.; Cochis, A.; Stancampiano, A.; Laurita, R.; Azzimonti, B.; Sorrentino, R.; Varoni, E.M.; Petri, M.; Colombo, V.; Gherardi, M.; et al. Cold atmospheric plasma treatment affects early bacterial adhesion and decontamination of soft reline palatal obturators. Clin. Plasma Med. 2017, 7–8, 36–45. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Guo, J.; Zhou, X.; Liu, Z.; Wang, C.; Wang, K.; Zhang, J.; Wang, Z. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: An in vitro study. Dent. Mater. J. 2018, 37, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Reitberger, H.H.; Czugala, M.; Chow, C.; Mohr, A.; Burkovski, A.; Gruenert, A.K.; Schoenebeck, R.; Fuchsluger, T.A. Argon Cold Plasma-A Novel Tool to Treat Therapy-resistant Corneal Infections. Am. J. Ophthalmol. 2018, 190, 150–163. [Google Scholar] [CrossRef]
- Winter, S.; Meyer-Lindenberg, A.; Wolf, G.; Reese, S.; Nolff, M.C. In vitro evaluation of the decontamination effect of cold atmospheric argon plasma on selected bacteria frequently encountered in small animal bite injuries. J. Microbiol. Methods 2020, 169, 105728. [Google Scholar] [CrossRef] [PubMed]
- Hottmann, I.; Borisova, M.; Schäffer, C.; Mayer, C. Peptidoglycan Salvage Enables the Periodontal Pathogen Tannerella forsythia to Survive within the Oral Microbial Community. Microb. Physiol. 2021, 31, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.K.; Roy, S.; Noirel, J.; Douglas, I.; Wright, P.C.; Stafford, G.P. A quantitative proteomic analysis of biofilm adaptation by the periodontal pathogen Tannerella forsythia. Proteomics 2010, 10, 3130–3141. [Google Scholar] [CrossRef]
- Jaramillo, R.D.; Barraza, B.C.; Polo, A.; Sará, M.; Contreras, M.; Escamilla, J.E. The aerobic electron transport system of Eikenella corrodens. Can. J. Microbiol. 2002, 48, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S152–S157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, M.; Canullo, L.; Donnarumma, G.; Caputo, P.; Nastri, L.; Guida, L. Bacterial inactivation/sterilization by argon plasma treatment on contaminated titanium implant surfaces: In vitro study. Med. Oral Patol. Oral Cir. Bucal. 2015, 21, e118–e121. [Google Scholar] [CrossRef]
- Carreiro, A.F.P.; Delben, J.A.; Guedes, S.; Silveira, E.J.D.; Janal, M.N.; Vergani, C.E.; Pushalkar, S.; Duarte, S. Low-temperature plasma on peri-implant-related biofilm and gingival tissue. J. Periodontol. 2019, 90, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, K.-H.; Park, S.-Y.; Yoon, S.-Y.; Kim, G.-H.; Lee, Y.-M.; Rhyu, I.-C.; Seol, Y.-J. The bactericidal effect of an atmospheric-pressure plasma jet on Porphyromonas gingivalis biofilms on sandblasted and acid-etched titanium discs. J. Periodontal Implant Sci. 2019, 49, 319–329. [Google Scholar] [CrossRef]
- Diaz, P.I.; Valm, A.M. Microbial Interactions in Oral Communities Mediate Emergent Biofilm Properties. J. Dent. Res. 2020, 99, 18–25. [Google Scholar] [CrossRef]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S12–S22. [Google Scholar] [CrossRef]
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms with Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Koban, I.; Holtfreter, B.; Hübner, N.-O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.-D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—Proof of principle experiment. J. Clin. Periodontol. 2011, 38, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Kamionka, J.; Matthes, R.; Holtfreter, B.; Pink, C.; Schlüter, R.; von Woedtke, T.; Kocher, T.; Jablonowski, L. Efficiency of cold atmospheric plasma, cleaning powders and their combination for biofilm removal on two different titanium implant surfaces. Clin. Oral Investig. 2022, 26, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- van den Driessche, F.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J. Microbiol. Methods 2014, 98, 31–34. [Google Scholar] [CrossRef]
- Liu, T.; Wu, L.; Babu, J.P.; Hottel, T.L.; Garcia-Godoy, F.; Hong, L. Effects of atmospheric non-thermal argon/oxygen plasma on biofilm viability and hydrophobicity of oral bacteria. Am. J. Dent. 2017, 30, 52–56. [Google Scholar]
- Jablonowski, L.; Fricke, K.; Matthes, R.; Holtfreter, B.; Schlüter, R.; von Woedtke, T.; Weltmann, K.-D.; Kocher, T. Removal of naturally grown human biofilm with an atmospheric pressure plasma jet: An in-vitro study. J. Biophoton. 2017, 10, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Hui, W.L.; Ipe, D.; Perrotti, V.; Piattelli, A.; Fang, Z.; Ostrikov, K.; Quaranta, A. Novel technique using cold atmospheric plasma coupled with air-polishing for the treatment of titanium discs grown with biofilm: An in-vitro study. Dent. Mater. 2021, 37, 359–369. [Google Scholar] [CrossRef]
- Hui, W.L.; Perrotti, V.; Piattelli, A.; Ostrikov, K.; Fang, Z.; Quaranta, A. Cold atmospheric plasma coupled with air abrasion in liquid medium for the treatment of peri-implantitis model grown with a complex human biofilm: An in vitro study. Clin. Oral Investig. 2021, 25, 6633–6642. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-H.; Choi, E.-H.; Kim, K.-M.; Kim, K.-N. Air atmospheric-pressure plasma-jet treatment enhances the attachment of human gingival fibroblasts for early peri-implant soft tissue seals on titanium dental implant abutments. Acta Odontol. Scand. 2015, 73, 67–75. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kwon, J.-S.; Choi, E.-H.; Kim, K.-M. The Effects of Non-Thermal Atmospheric Pressure Plasma treated Titanium Surface on Behaviors of Oral Soft Tissue Cells. Sci. Rep. 2018, 8, 15963. [Google Scholar] [CrossRef] [Green Version]
- Timon, C.; Keady, C. Thermal Osteonecrosis Caused by Bone Drilling in Orthopedic Surgery: A Literature Review. Cureus 2014, 11, e5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngson, C.C.; Barclay, C.W. A pilot study of intraoral temperature changes. Clin. Oral Investig. 2000, 4, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Gao, Z.; Shen, J. Emission rates of indoor ozone emission devices: A literature review. Build. Environ. 2019, 158, 302–318. [Google Scholar] [CrossRef]
- Millar, B.J.; Hodson, N. Assessment of the safety of two ozone delivery devices. J. Dent. 2007, 35, 195–200. [Google Scholar] [CrossRef]
- Lang, K.N.; Sculean, A.; Eick, S.; Stähli, A. A novel in vitro periodontal pocket model to evaluate the effect of root surface instrumentation on biofilm-epithelial cell interactions. Clin. Oral Investig. 2022, 26, 4021–4029. [Google Scholar] [CrossRef]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]




| Species | Application Time | ||||
|---|---|---|---|---|---|
| Control | 10 s | 30 s | 60 s | 120 s | |
| A. naeslundii ATCC 12104 | 6.98 ± 0.25 | 6.72 ± 0.14 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| C. gingivalis ATCC 33624 | 7.51 ± 0.04 | 6.93±0.15 | 2.45 ± 2.83 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| C. rectus ATCC 33624 | 7.43 ± 0.04 | 6.65 ± 0.05 | 3.52 ± 2.36 | 0.00 ± 0.00 | 0.75 ± 1.50 |
| E. corrodens ATCC 23834 | 7.46 ± 0.01 | 6.64 ± 0.04 | 1.14 ± 2.28 | 1.25 ± 2.49 | 1.71 ± 1.49 |
| F. alocis ATCC 33238 | 7.50 ± 0.02 | 6.68 ± 0.03 | 2.33 ± 2.69 | 1.02 ± 2.03 | 0.90 ± 1.80 |
| F. nucleatum ATCC 25586 | 6.92 ± 0.24 | 6.64 ± 0.10 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| P. gingivalis ATCC 33277 | 7.75 ± 0.06 | 6.83 ± 0.03 | 2.59 ± 2.40 | 1.39 ± 2.06 | 0.00 ± 0.00 |
| P. intermedia ATCC 25611 | 7.56 ± 0.05 | 6.64 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| P. micra ATCC 33270 | 7.61 ± 0.04 | 6.79 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| S. gordonii ATCC 10558 | 7.54 ± 0.08 | 6.40 ± 0.54 | 3.13 ± 2.02 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| T. forsythia ATCC 43037 | 6.93 ± 0.05 | 6.71 ± 0.03 | 6.73 ± 0.01 | 3.38 ± 3.90 | 0.00 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungbauer, G.; Favaro, L.; Müller, S.; Sculean, A.; Eick, S. The In-Vitro Activity of a Cold Atmospheric Plasma Device Utilizing Ambient Air against Bacteria and Biofilms Associated with Periodontal or Peri-Implant Diseases. Antibiotics 2022, 11, 752. https://doi.org/10.3390/antibiotics11060752
Jungbauer G, Favaro L, Müller S, Sculean A, Eick S. The In-Vitro Activity of a Cold Atmospheric Plasma Device Utilizing Ambient Air against Bacteria and Biofilms Associated with Periodontal or Peri-Implant Diseases. Antibiotics. 2022; 11(6):752. https://doi.org/10.3390/antibiotics11060752
Chicago/Turabian StyleJungbauer, Gert, Leandro Favaro, Steffen Müller, Anton Sculean, and Sigrun Eick. 2022. "The In-Vitro Activity of a Cold Atmospheric Plasma Device Utilizing Ambient Air against Bacteria and Biofilms Associated with Periodontal or Peri-Implant Diseases" Antibiotics 11, no. 6: 752. https://doi.org/10.3390/antibiotics11060752
APA StyleJungbauer, G., Favaro, L., Müller, S., Sculean, A., & Eick, S. (2022). The In-Vitro Activity of a Cold Atmospheric Plasma Device Utilizing Ambient Air against Bacteria and Biofilms Associated with Periodontal or Peri-Implant Diseases. Antibiotics, 11(6), 752. https://doi.org/10.3390/antibiotics11060752







