Māori Experiences and Beliefs about Antibiotics and Antimicrobial Resistance for Acute Upper Respiratory Tract Symptoms: A Qualitative Study
Abstract
:1. Introduction
2. Results
2.1. Participant Characteristics
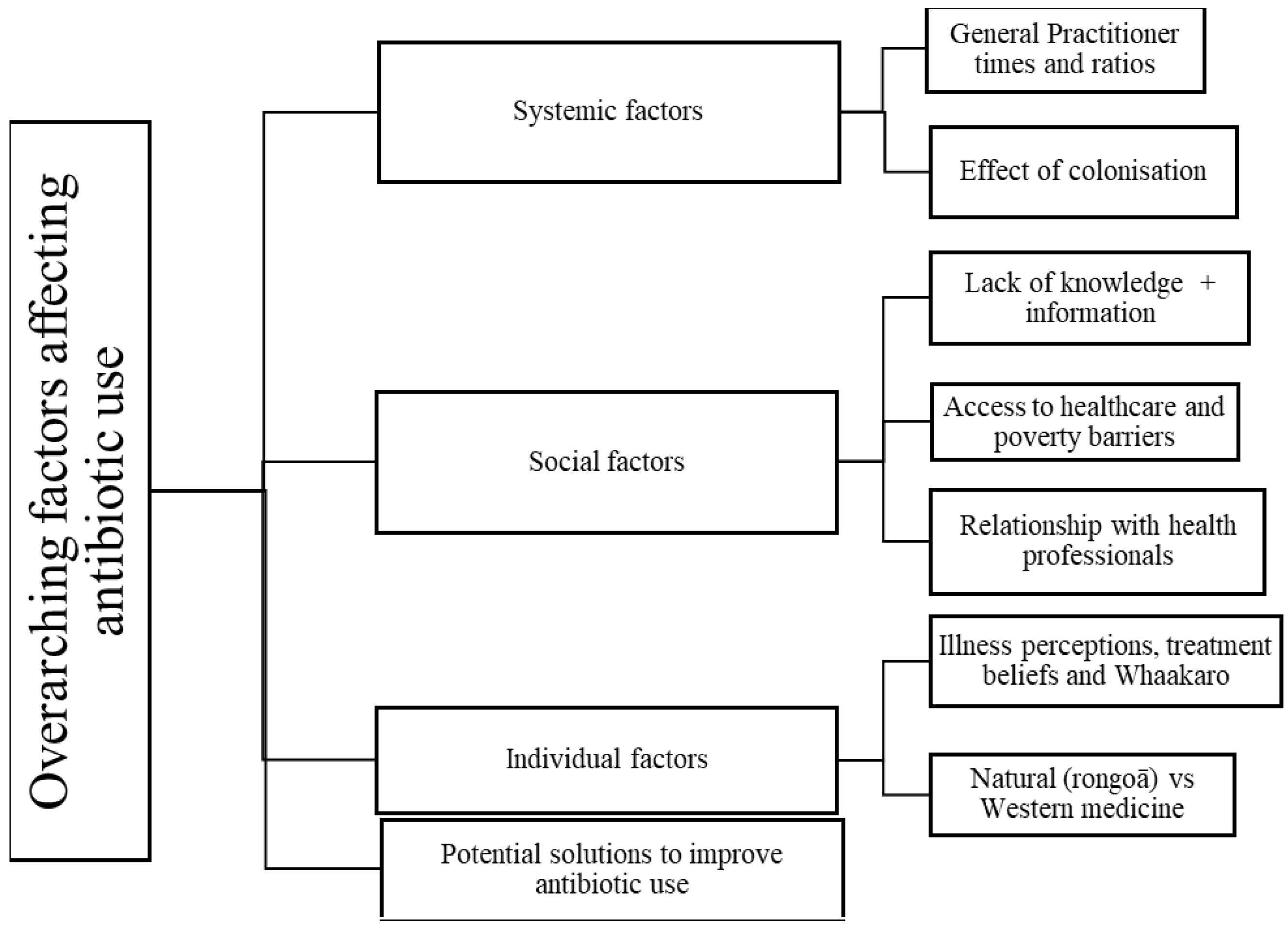
2.2. Key Themes
2.2.1. Theme 1: Systemic Factors Influencing Antibiotic Use
General Practitioner (GP) Times and Ratios
Effects of Colonisation—An Underlying Contributor to Misuse and Underuse of Antibiotics
2.2.2. Theme 2: Social Factors
Lack of Knowledge and Information concerning Antibiotics for Patients
Access to Healthcare and Poverty Barriers to Appropriate Antibiotic Use
Relationship with Health Professionals
2.2.3. Individual Factors
Illness Perceptions, Treatment Beliefs and Whaakaro [Thoughts]
Natural (Rongoā) vs. Western Medicine
2.2.4. Potential Solutions to Improve Antibiotic Use
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. Interview Process
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.G.; Barnard, L.T.; Kvalsvig, A.; Verrall, A.; Zhang, J.; Keall, M.; Wilson, N.; Wall, T.; Chapman, P.H. Increasing incidence of serious infectious diseases and inequalities in New Zealand: A national epidemiological study. Lancet 2012, 379, 1112–1119. [Google Scholar] [CrossRef]
- Came, H.; O’Sullivan, D.; Kidd, J.; McCreanor, T.J.H.; Rights, H. The Waitangi Tribunal’s WAI 2575 report: Implications for decolonizing health systems. Health Hum. Rights 2020, 22, 209. [Google Scholar] [PubMed]
- Whyler, N.; Tomlin, A.; Tilyard, M.; Thomas, M. Ethnic disparities in community antibacterial dispensing in New Zealand, 2015. N. Z. Med. J. 2018, 131, 50–60. [Google Scholar] [PubMed]
- Thomas, M.G.; Smith, A.J.; Tilyard, M. Rising antimicrobial resistance: A strong reason to reduce excessive antimicrobial consumption in New Zealand. N. Z. Med. J. 2014, 127, 72–84. [Google Scholar] [PubMed]
- Adam, R.D. Antimicrobial resistance at a community level. Lancet Planet. Health 2018, 2, e473–e474. [Google Scholar] [CrossRef] [Green Version]
- Selak, V.; Poppe, K.; Grey, C.; Mehta, S.; Winter-Smith, J.; Jackson, R.; Wells, S.; Exeter, D.; Kerr, A.; Riddell, T.; et al. Ethnic differences in cardiovascular risk profiles among 475,241 adults in primary care in Aotearoa, New Zealand. N. Z. Med. J. 2020, 133, 14. [Google Scholar]
- Selak, V.; Rahiri, J.-L.; Jackson, R.; Harwood, M. Acknowledging and acting on racism in the health sector in Aotearoa New Zealand. N. Z. Med. J. 2020, 133, 7–13. [Google Scholar]
- Simpson, J.; Adams, J.; Oben, G.; Wicken, A.; Duncanson, M. Te Ohonga Ake the Determinants of Health for Māori Children and Young People in New Zealand Series Two; Ministry of Health: Wellington, New Zealand, 2016. [Google Scholar]
- Metcalfe, S.; Beyene, K.; Urlich, J.; Jones, R.; Proffitt, C.; Harrison, J.; Andrews, A. Te Wero tonu—the challenge continues: Māori access to medicines 2006/07–2012/13 update. N. Z. Med. J. 2018, 131, 27–47. [Google Scholar]
- Padigos, J.; Ritchie, S.; Lim, A.G. Nurses have a major role in antimicrobial stewardship. Kai Tiaki Nurs. New Zealand 2017, 23, 16–45. [Google Scholar]
- O’Doherty, J.; Leader, L.F.W.; O’Regan, A.; Dunne, C.; Puthoopparambil, S.J.; O’Connor, R. Over prescribing of antibiotics for acute respiratory tract infections; a qualitative study to explore Irish general practitioners’ perspectives. BMC Fam. Pract. 2019, 20, 27. [Google Scholar] [CrossRef]
- Curry, M.; Sung, L.; Arroll, B.; Felicity, G.-S.; Kerse, N.; Norris, P. Public views and use of antibiotics for the common cold before and after an education campaign in New Zealand. N. Z. Med. J. 2006, 119, 1233. [Google Scholar]
- Saliba-Gustafsson, E.A.; Nyberg, A.; Borg, M.A.; Senia, R.-K.; Lundborg, C.S. Barriers and facilitators to prudent antibiotic prescribing for acute respiratory tract infections: A qualitative study with general practitioners in Malta. PLoS ONE 2021, 16, e0246782. [Google Scholar] [CrossRef]
- Norris, P.; Chamberlain, K.; Dew, K.; Gabe, J.; Hodgetts, D.; Madden, H. Public Beliefs about Antibiotics, Infection and Resistance: A Qualitative Study. Antibiotics 2013, 2, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.; Churchward, M.; Fa’alau, F.; Va’ai, C. Understanding and use of antibiotics amongst Samoan people in New Zealand. J. Prim. Health Care 2009, 1, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, A.I.; Thomas, M.G.; Petrie, K.J.; Frater, J.; Dang, D.; Schache, K.R.; Akroyd, A.F.; Ritchie, S.R. Reducing expectations for antibiotics in patients with upper respiratory tract infections: A primary care randomized controlled trial. Ann. Fam. Med. 2021, 19, 232–239. [Google Scholar] [CrossRef]
- Reid, P.; Paine, S.-J.; Curtis, E.; Jones, R.; Anderson, A.; Willing, E.; Harwood, M. Achieving health equity in Aotearoa: Strengthening responsiveness to Māori in health research. N. Z. Med. J. 2017, 130, 96–103. [Google Scholar]
- Gelo, O.; Braakmann, D.; Benetka, G. Quantitative and Qualitative Research: Beyond the Debate. Integr. Psychol. Behav. Sci. 2008, 42, 266–290. [Google Scholar] [CrossRef]
- Onwuegbuzie, A.J.; Leech, N.L. On Becoming a Pragmatic Researcher: The Importance of Combining Quantitative and Qualitative Research Methodologies. Int. J. Soc. Res. Methodol. 2005, 8, 375–387. [Google Scholar] [CrossRef]
- Metcalfe, S.; Vallabh, M.; Murray, P.; Proffitt, C.; Williams, G. Over and under? Ethnic inequities in community antibacterial prescribing. N. Z. Med. J. 2019, 132, 65–68. [Google Scholar]
- Anderson, A.; Spray, J. Beyond awareness: Towards a critically conscious health promotion for rheumatic fever in Aotearoa, New Zealand. Soc. Sci. Med. 2020, 247, 112798. [Google Scholar] [CrossRef]
- Sánchez, X.; Orrico, M.; Morillo, T.; Manzano, A.; Jimbo, R.; Armijos, L. Reducing unnecessary antibiotic prescription through implementation of a clinical guideline on self-limiting respiratory tract infections. PLoS ONE 2021, 16, e0249475. [Google Scholar] [CrossRef] [PubMed]
- Voyce, M. Maori healers in New Zealand: The Tohunga Suppression Act 1907. J. Oceania. 1989, 60, 99–123. [Google Scholar] [CrossRef]
- Axelsson, P.; Kukutai, T.; Kippen, R. The field of Indigenous health and the role of colonisation and history. J. Popul. Res. 2016, 33, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Robson, B.; Harris, R. Hauora: Màori Standards of Health IV. A Study of the Years 2000–2005; Te Ropu Rangahau Hauora a Eru Pomare: Wellington, New Zealand, 2007. [Google Scholar]
- Office of the Prime Minister’s Chief Science Advisor. Kotahitanga: Uniting Aotearoa against Infectious Diseases and Antimicrobial Resistance; Prime Minister’s Chief Science Advisor: Wellington, New Zealand, 2021. [Google Scholar]
- Mutu, M. Māori Issues. Contemp. Pac. 2020, 32, 240–249. [Google Scholar] [CrossRef]
- Mutu, M. ‘To honour the treaty, we must first settle colonisation’ (Moana Jackson 2015): The long road from colonial devastation to balance, peace and harmony. J. R. Soc. N. Z. 2019, 49 (Suppl. S1), 4–18. [Google Scholar]
- Morse, J.M. Determining Sample Size. Qual. Health Res. 2000, 10, 3–5. [Google Scholar] [CrossRef]
- Walker, S.; Eketone, A.; Gibbs, A. An exploration of kaupapa Maori research, its principles, processes and applications. Int. J. Soc. Res. Methodol. 2006, 9, 331–344. [Google Scholar] [CrossRef]
- Independent Maori Statutory Board. The Rangatiratanga Report for Tāmaki Makaurau 2019; Independent Maori Statutory Board: Auckland, New Zealand, 2019. [Google Scholar]
- Lambert, S.D.; Loiselle, C.G. Combining individual interviews and focus groups to enhance data richness. J. Adv. Nurs. 2008, 62, 228–237. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hika, K.; Harwood, M.; Ritchie, S.; Chan, A.H.Y. Māori Experiences and Beliefs about Antibiotics and Antimicrobial Resistance for Acute Upper Respiratory Tract Symptoms: A Qualitative Study. Antibiotics 2022, 11, 714. https://doi.org/10.3390/antibiotics11060714
Hika K, Harwood M, Ritchie S, Chan AHY. Māori Experiences and Beliefs about Antibiotics and Antimicrobial Resistance for Acute Upper Respiratory Tract Symptoms: A Qualitative Study. Antibiotics. 2022; 11(6):714. https://doi.org/10.3390/antibiotics11060714
Chicago/Turabian StyleHika, Kayla, Matire Harwood, Stephen Ritchie, and Amy Hai Yan Chan. 2022. "Māori Experiences and Beliefs about Antibiotics and Antimicrobial Resistance for Acute Upper Respiratory Tract Symptoms: A Qualitative Study" Antibiotics 11, no. 6: 714. https://doi.org/10.3390/antibiotics11060714
APA StyleHika, K., Harwood, M., Ritchie, S., & Chan, A. H. Y. (2022). Māori Experiences and Beliefs about Antibiotics and Antimicrobial Resistance for Acute Upper Respiratory Tract Symptoms: A Qualitative Study. Antibiotics, 11(6), 714. https://doi.org/10.3390/antibiotics11060714





