The Class A β-Lactamase Produced by Burkholderia Species Compromises the Potency of Tebipenem against a Panel of Isolates from the United States
Abstract
1. Introduction
2. Results
2.1. Tebipenem Does Not Demonstrate Clinically Relevant Antimicrobial Activity against Bcc and B. gladioli
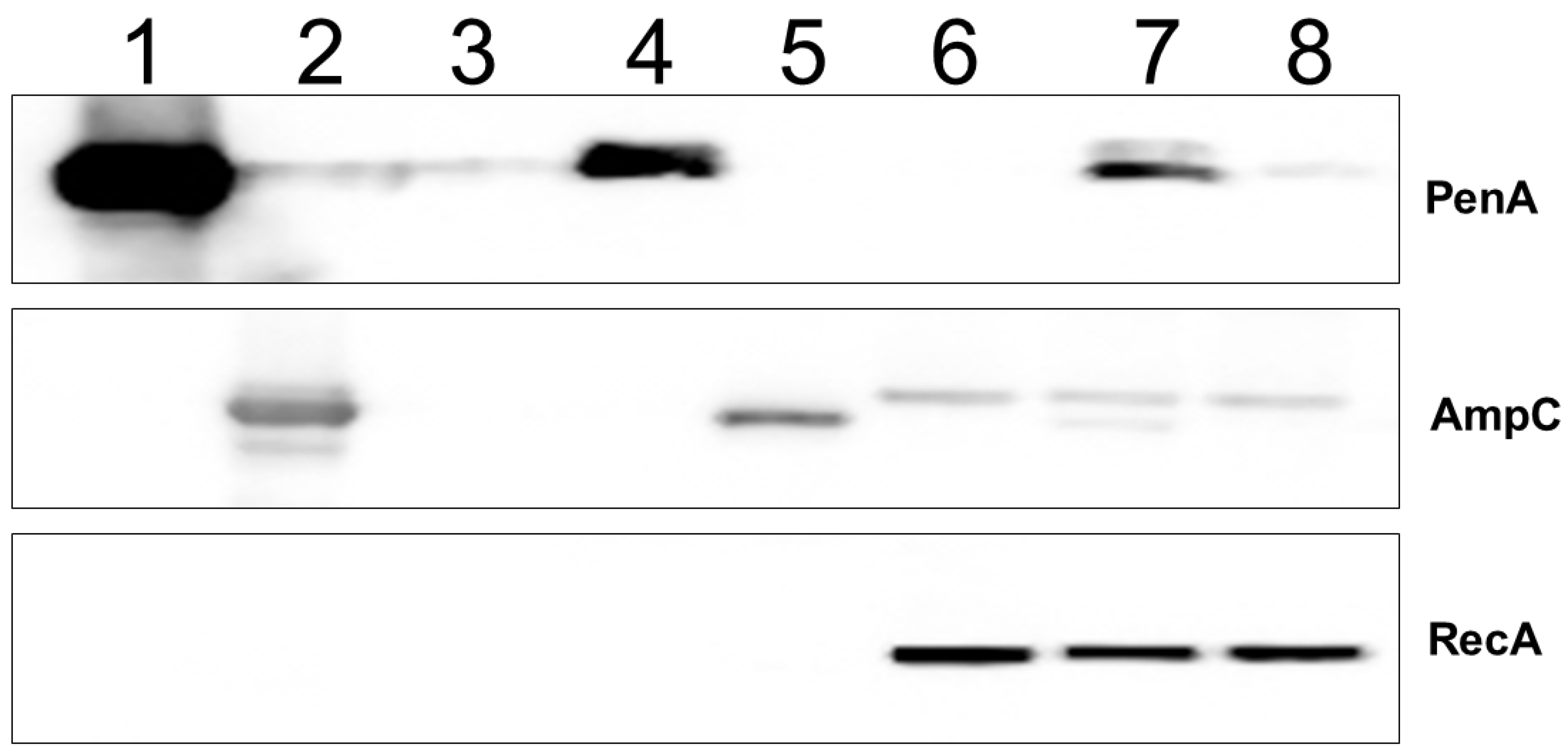
2.2. Tebipenem Is Slowly Hydrolyzed by PenA1 but Inhibits AmpC1, but Eventually Forms a Stable Complex with Both β-lactamases
2.3. Tebipenem Is a Minor Inducer of blaPenA1 Expression
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hikida, M.; Itahashi, K.; Igarashi, A.; Shiba, T.; Kitamura, M. In vitro antibacterial activity of LJC 11,036, an active metabolite of L-084, a new oral carbapenem antibiotic with potent antipneumococcal activity. Antimicrob. Agents Chemother. 1999, 43, 2010–2016. [Google Scholar] [CrossRef]
- Kobayashi, R.; Konomi, M.; Hasegawa, K.; Morozumi, M.; Sunakawa, K.; Ubukata, K. In vitro activity of tebipenem, a new oral carbapenem antibiotic, against penicillin-nonsusceptible Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2005, 49, 889–894. [Google Scholar] [CrossRef][Green Version]
- Jain, A.; Utley, L.; Parr, T.R.; Zabawa, T.; Pucci, M.J. Tebipenem, the first oral carbapenem antibiotic. Expert Rev. Anti Infect. Ther. 2018, 16, 513–522. [Google Scholar] [CrossRef]
- Hasegawa, K.; Chiba, N.; Kobayashi, R.; Murayama, S.Y.; Iwata, S.; Sunakawa, K.; Ubukata, K. Rapidly increasing prevalence of β-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob. Agents Chemother. 2004, 48, 1509–1514. [Google Scholar] [CrossRef][Green Version]
- Kuroki, H.; Tateno, N.; Ikeda, H.; Saito, N. Investigation of pneumonia-causing pathogenic organisms in children and the usefulness of tebipenem pivoxil for their treatment. J. Infect. Chemother. 2010, 16, 280–287. [Google Scholar] [CrossRef]
- McEntee, L.; Johnson, A.; Farrington, N.; Unsworth, J.; Dane, A.; Jain, A.; Cotroneo, N.; Critchley, I.; Melnick, D.; Parr, T.; et al. Pharmacodynamics of tebipenem: New options for oral treatment of multidrug-resistant Gram-negative infections. Antimicrob. Agents Chemother. 2019, 63, e00603-19. [Google Scholar] [CrossRef]
- Thamlikitkul, V.; Lorchirachoonkul, N.; Tiengrim, S. In vitro and in vivo activity of tebipenem against ESBL-producing E. coli. J. Med. Assoc. Thail. 2014, 97, 1259–1268. [Google Scholar]
- Yao, Q.; Wang, J.; Cui, T.; Yang, Z.; Su, M.; Zhao, P.; Yan, H.; Zhan, Y.; Yang, H. Antibacterial properties of tebipenem pivoxil tablet, a new rral carbapenem preparation against a variety of pathogenic bacteria in vitro and in vivo. Molecules 2016, 21, 62. [Google Scholar] [CrossRef]
- Rubio, A.; Pucci, M.J.; Jain, A. Characterization of SPR994, an orally available carbapenem, with activity comparable to intravenously administered carbapenems. ACS Infect. Dis. 2018, 4, 1436–1438. [Google Scholar] [CrossRef]
- Arends, S.J.R.; Rhomberg, P.R.; Cotroneo, N.; Rubio, A.; Flamm, R.K.; Mendes, R.E. Antimicrobial activity evaluation of tebipenem (SPR859), an orally available carbapenem, against a global set of Enterobacteriaceae isolates, including a challenge set of organisms. Antimicrob. Agents Chemother. 2019, 63, e02618-18. [Google Scholar] [CrossRef]
- Cotroneo, N.; Rubio, A.; Critchley, I.A.; Pillar, C.; Pucci, M.J. In vitro and in vivo characterization of tebipenem, an oral carbapenem. Antimicrob. Agents Chemother. 2020, 64, e02240-19. [Google Scholar] [CrossRef]
- Lacasse, E.; Brouillette, E.; Larose, A.; Parr, T.R.; Rubio, A., Jr.; Malouin, F. In vitro activity of tebipenem (SPR859) against penicillin-binding proteins of Gram-negative and Gram-positive bacteria. Antimicrob. Agents Chemother. 2019, 63, e02181-18. [Google Scholar] [CrossRef]
- Sodhi, V.; Kronsberg, K.A.; Clark, M.; Cho, J.C. Tebipenem pivoxil hydrobromide-No PICC, no problem! Pharmacotherapy 2021, 41, 748–761. [Google Scholar] [CrossRef]
- Seenama, C.; Tiengrim, S.; Thamlikitkul, V. In vitro activity of tebipenem against Burkholderia pseudomallei. Int. J. Antimicrob. Agents 2013, 42, 375. [Google Scholar] [CrossRef]
- Clayton, N.P.; Jain, A.; Halasohoris, S.A.; Pysz, L.M.; Lembirik, S.; Zumbrun, S.D.; Kane, C.D.; Hackett, M.J.; Pfefferle, D.; Smiley, M.A.; et al. In vitro and in vivo characterization of tebipenem (TBP), an orally active carbapenem, against biothreat pathogens. Antimicrob. Agents Chemother. 2021, 65, e02385-20. [Google Scholar] [CrossRef]
- Marson, F.A.; Hortencio, T.D.; Aguiar, K.C.; Ribeiro, J.D. Demographic, clinical, and laboratory parameters of cystic fibrosis during the last two decades: A comparative analysis. BMC Pulm. Med. 2015, 15, 3. [Google Scholar] [CrossRef]
- Abbott, I.J.; Peleg, A.Y. Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: Antimicrobial resistance and therapeutic strategies. Semin. Respir. Crit. Care Med. 2015, 36, 99–110. [Google Scholar] [CrossRef]
- Hanulik, V.; Webber, M.A.; Chroma, M.; Uvizl, R.; Holy, O.; Whitehead, R.N.; Baugh, S.; Matouskova, I.; Kolar, M. An outbreak of Burkholderia multivorans beyond cystic fibrosis patients. J. Hosp. Infect. 2013, 84, 248–251. [Google Scholar] [CrossRef]
- Chiappini, E.; Taccetti, G.; de Martino, M. Bacterial lung infections in cystic fibrosis patients: An update. Pediatr. Infect. Dis. J. 2014, 33, 653–654. [Google Scholar] [CrossRef]
- Gautam, V.; Singhal, L.; Ray, P. Burkholderia cepacia complex: Beyond Pseudomonas and Acinetobacter. Indian J. Med. Microbiol. 2011, 29, 4–12. [Google Scholar] [CrossRef]
- Avgeri, S.G.; Matthaiou, D.K.; Dimopoulos, G.; Grammatikos, A.P.; Falagas, M.E. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: A systematic review of the clinical evidence. Int. J. Antimicrob. Agents 2009, 33, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Wuthiekanun, V.; Peacock, S.J. Management of melioidosis. Expert Rev. Anti Infect. Ther. 2006, 4, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Becka, S.A.; Zeiser, E.T.; Ohuchi, N.; Mojica, M.F.; Gatta, J.A.; Falleni, M.; Tosi, D.; Borghi, E.; Winkler, M.L.; et al. Overcoming an extremely drug resistant (XDR) pathogen: Avibactam restores susceptibility to ceftazidime for Burkholderia cepacia complex isolates from Cystic Fibrosis patients. ACS Infect. Dis. 2017, 3, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Van Dalem, A.; Herpol, M.; Echahidi, F.; Peeters, C.; Wybo, I.; De Wachter, E.; Vandamme, P.; Pierard, D. In vitro susceptibility of Burkholderia cepacia complex isolated from Cystic Fibrosis patients to ceftazidime-avibactam and ceftolozane-tazobactam. Antimicrob. Agents Chemother. 2018, 62, e00590-18. [Google Scholar] [CrossRef]
- Cheung, T.K.; Ho, P.L.; Woo, P.C.; Yuen, K.Y.; Chau, P.Y. Cloning and expression of class A β-lactamase gene blaA(BPS) in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 2002, 46, 1132–1135. [Google Scholar] [CrossRef][Green Version]
- Godfrey, A.J.; Wong, S.; Dance, D.A.; Chaowagul, W.; Bryan, L.E. Pseudomonas pseudomallei resistance to β-lactam antibiotics due to alterations in the chromosomally encoded β-lactamase. Antimicrob. Agents Chemother. 1991, 35, 1635–1640. [Google Scholar] [CrossRef][Green Version]
- Tribuddharat, C.; Moore, R.A.; Baker, P.; Woods, D.E. Burkholderia pseudomallei class A β-lactamase mutations that confer selective resistance against ceftazidime or clavulanic acid inhibition. Antimicrob. Agents Chemother. 2003, 47, 2082–2087. [Google Scholar] [CrossRef]
- Trepanier, S.; Prince, A.; Huletsky, A. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrob. Agents Chemother. 1997, 41, 2399–2405. [Google Scholar] [CrossRef]
- Poirel, L.; Rodriguez-Martinez, J.M.; Plesiat, P.; Nordmann, P. Naturally occurring class A β-lactamases from the Burkholderia cepacia complex. Antimicrob. Agents Chemother. 2009, 53, 876–882. [Google Scholar] [CrossRef]
- Becka, S.A.; Zeiser, E.T.; Marshall, S.H.; Gatta, J.A.; Nguyen, K.; Singh, I.; Greco, C.; Sutton, G.G.; Fouts, D.E.; LiPuma, J.J.; et al. Sequence heterogeneity of the PenA carbapenemase in clinical isolates of Burkholderia multivorans. Diagn. Microbiol. Infect. Dis. 2018, 92, 253–258. [Google Scholar] [CrossRef]
- Becka, S.A.; Zeiser, E.T.; Barnes, M.D.; Taracila, M.A.; Nguyen, K.; Singh, I.; Sutton, G.G.; LiPuma, J.J.; Fouts, D.E.; Papp-Wallace, K.M. Characterization of the AmpC β-lactamase from Burkholderia multivorans. Antimicrob. Agents Chemother. 2018, 62, e01140-18. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Taracila, M.A.; Gatta, J.A.; Ohuchi, N.; Bonomo, R.A.; Nukaga, M. Insights into β-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J. Biol. Chem. 2013, 288, 19090–19102. [Google Scholar] [CrossRef]
- Dhar, S.; Kumari, H.; Balasubramanian, D.; Mathee, K. Cell-wall recycling and synthesis in Escherichia coli and Pseudomonas aeruginosa-their role in the development of resistance. J. Med. Microbiol. 2018, 67, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Shapiro, A.B.; Becka, S.A.; Zeiser, E.T.; LiPuma, J.J.; Lane, D.J.; Panchal, R.G.; Mueller, J.P.; O’Donnell, J.P.; Miller, A.A. In vitro antibacterial activity and in vivo efficacy of sulbactam-durlobactam against pathogenic Burkholderia species. Antimicrob. Agents Chemother. 2021, 65, e01930-20. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, E.T.; Becka, S.A.; Barnes, M.D.; Taracila, M.A.; LiPuma, J.J.; Papp-Wallace, K.M. Resurrecting old β-lactams: Potent inhibitory activity of temocillin against multidrug-resistant Burkholderia species isolates from the United States. Antimicrob. Agents Chemother. 2019, 63, e02315-18. [Google Scholar] [CrossRef]
- Zeiser, E.T.; Becka, S.A.; Wilson, B.M.; Barnes, M.D.; LiPuma, J.J.; Papp-Wallace, K.M. “Switching partners”: Piperacillin-avibactam is a highly potent xombination against multidrug-eesistant Burkholderia cepacia complex and Burkholderia gladioli Cystic Fibrosis isolates. J. Clin. Microbiol. 2019, 57, e00181-19. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptiblity Testing. CLSI Supplement M100 , 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Becka, S.A.; Zeiser, E.T.; LiPuma, J.J.; Papp-Wallace, K.M. Activity of imipenem-relebactam against multidrug- and extensively drug-resistant Burkholderia cepacia complex and Burkholderia gladioli. Antimicrob. Agents Chemother. 2021, 65, e0133221. [Google Scholar] [CrossRef]
- Tremblay, L.W.; Fan, F.; Blanchard, J.S. Biochemical and structural characterization of Mycobacterium tuberculosis beta-lactamase with the carbapenems ertapenem and doripenem. Biochemistry 2010, 49, 3766–3773. [Google Scholar] [CrossRef]
- Endimiani, A.; Doi, Y.; Bethel, C.R.; Taracila, M.; Adams-Haduch, J.M.; O’Keefe, A.; Hujer, A.M.; Paterson, D.L.; Skalweit, M.J.; Page, M.G.; et al. Enhancing resistance to cephalosporins in class C beta-lactamases: Impact of Gly214Glu in CMY-2. Biochemistry 2010, 49, 1014–1023. [Google Scholar] [CrossRef]
- Drawz, S.M.; Babic, M.; Bethel, C.R.; Taracila, M.; Distler, A.M.; Ori, C.; Caselli, E.; Prati, F.; Bonomo, R.A. Inhibition of the class C beta-lactamase from Acinetobacter spp.: Insights into effective inhibitor design. Biochemistry 2010, 49, 329–340. [Google Scholar] [CrossRef]
- Hugonnet, J.E.; Tremblay, L.W.; Boshoff, H.I.; Barry, C.E.; Blanchard, J.S., 3rd. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 2009, 323, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Spilker, T.; Martin, A.; LiPuma, J.J. Comparative assessment of genotyping methods for epidemiologic study of Burkholderia cepacia genomovar III. J. Clin. Microbiol. 2002, 40, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, D.E.; Jahic, H.; Ross, P.L.; Gu, R.F.; Hu, J.; Durand-Reville, T.F.; Lahiri, S.; Thresher, J.; Livchak, S.; Gao, N.; et al. Kinetics of avibactam inhibition against Class A, C, and D β-lactamases. J. Biol. Chem. 2013, 288, 27960–27971. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Winkler, M.L.; Gatta, J.A.; Taracila, M.A.; Chilakala, S.; Xu, Y.; Johnson, J.K.; Bonomo, R.A. Reclaiming the efficacy of β-lactam-β-lactamase inhibitor combinations: Avibactam restores the susceptibility of CMY-2-producing Escherichia coli to ceftazidime. Antimicrob. Agents Chemother. 2014, 58, 4290–4297. [Google Scholar] [CrossRef][Green Version]
- Papp-Wallace, K.M.; Becka, S.A.; Taracila, M.A.; Winkler, M.L.; Gatta, J.A.; Rholl, D.A.; Schweizer, H.P.; Bonomo, R.A. Exposing a β-lactamase “twist”: The mechanistic basis for the high level of ceftazidime resistance in the C69F variant of the Burkholderia pseudomallei PenI β-lactamase. Antimicrob. Agents Chemother. 2016, 60, 777–788. [Google Scholar] [CrossRef]
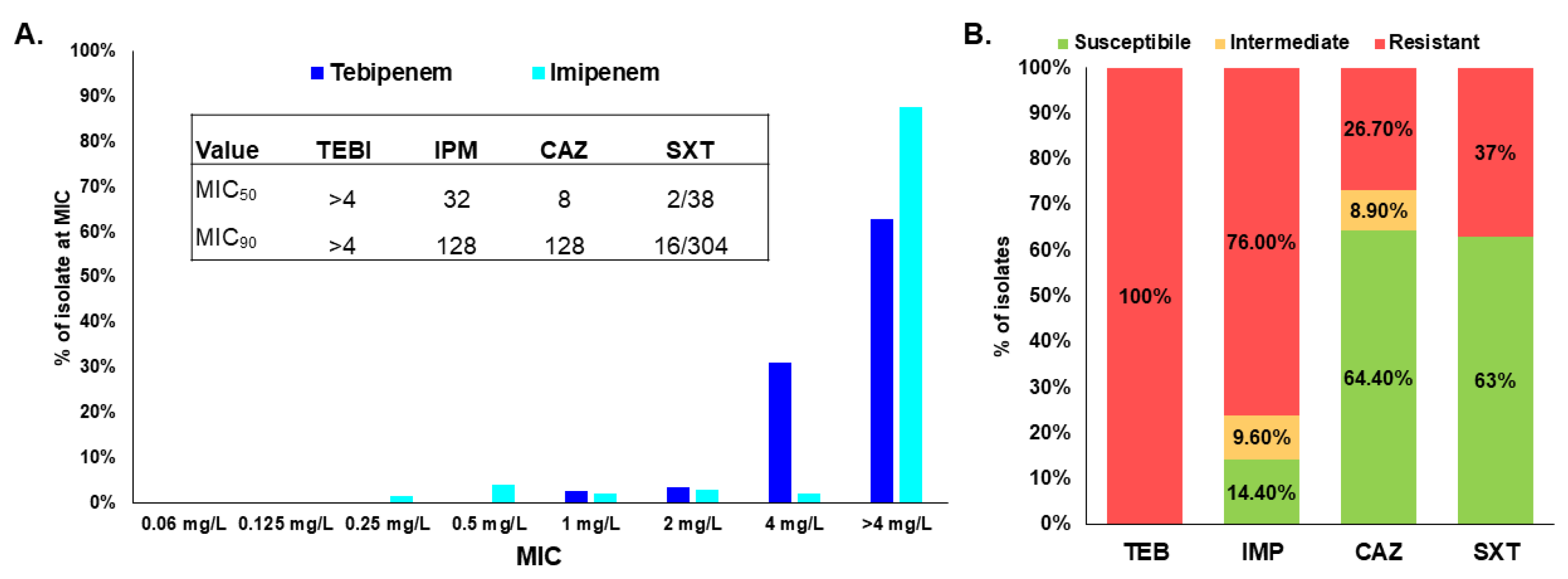


| Control Strain | Tebipenem |
|---|---|
| B. multivorans ATCC 17616 | >4 |
| E. coli ATCC 25922 | ≤0.06 |
| P. aeruginosa ATCC 27853 | >4 |
| Parameter | PenA1 | AmpC1 |
|---|---|---|
| Ki app (µM) | 4.7 ± 0.5 | 22 ± 2 |
| k2/K (M−1s−1) | N/D | 1.9 ± 0.1 × 103 |
| koff (s−1) | N/D | 3 ± 1 × 10−4 |
| tn at 15 min | 4000 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becka, S.A.; Zeiser, E.T.; LiPuma, J.J.; Papp-Wallace, K.M. The Class A β-Lactamase Produced by Burkholderia Species Compromises the Potency of Tebipenem against a Panel of Isolates from the United States. Antibiotics 2022, 11, 674. https://doi.org/10.3390/antibiotics11050674
Becka SA, Zeiser ET, LiPuma JJ, Papp-Wallace KM. The Class A β-Lactamase Produced by Burkholderia Species Compromises the Potency of Tebipenem against a Panel of Isolates from the United States. Antibiotics. 2022; 11(5):674. https://doi.org/10.3390/antibiotics11050674
Chicago/Turabian StyleBecka, Scott A., Elise T. Zeiser, John J. LiPuma, and Krisztina M. Papp-Wallace. 2022. "The Class A β-Lactamase Produced by Burkholderia Species Compromises the Potency of Tebipenem against a Panel of Isolates from the United States" Antibiotics 11, no. 5: 674. https://doi.org/10.3390/antibiotics11050674
APA StyleBecka, S. A., Zeiser, E. T., LiPuma, J. J., & Papp-Wallace, K. M. (2022). The Class A β-Lactamase Produced by Burkholderia Species Compromises the Potency of Tebipenem against a Panel of Isolates from the United States. Antibiotics, 11(5), 674. https://doi.org/10.3390/antibiotics11050674







