Genetic Resistance Determinants in Clinical Acinetobacter pittii Genomes
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Assays
2.2. Genomic Assembly and Pan-Genome Analysis
2.3. Genomic Annotation and Point Mutations
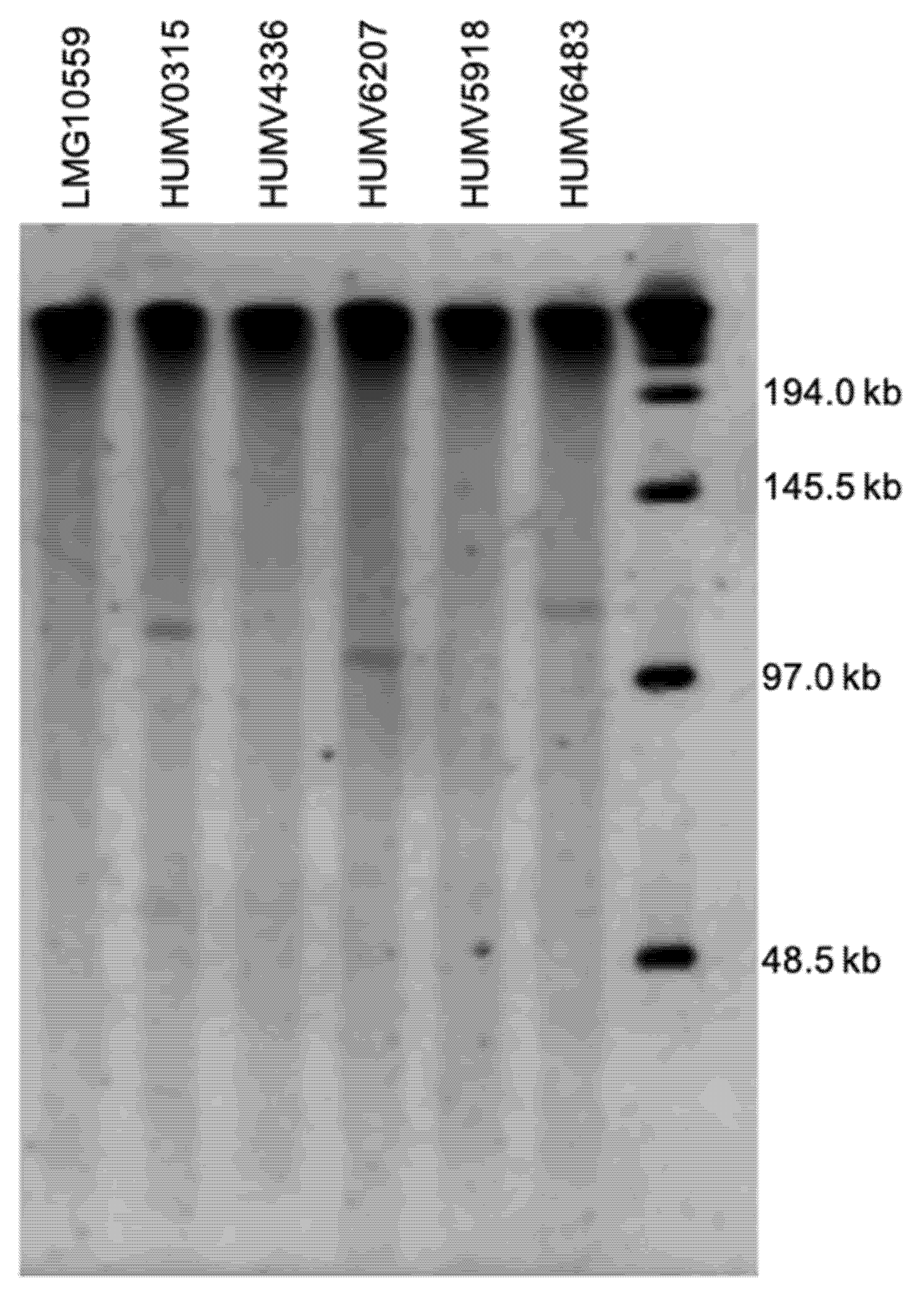
2.4. Plasmid Prediction and Characterization
2.5. PubMLST
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antimicrobial Susceptibility Assays
4.3. DNA Isolation, Sequencing, and Assembly
4.4. Genomic Annotation and Plasmid Prediction
4.5. Pan-Genome Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Peleg, A.Y.; de Breij, A.; Adams, M.D.; Cerqueira, G.M.; Mocali, S.; Galardini, M.; Nibbering, P.H.; Earl, A.M.; Ward, D.V.; Paterson, D.L.; et al. The Success of Acinetobacter Species; Genetic, Metabolic and Virulence Attributes. PLoS ONE 2012, 7, e46984. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Shin, J.H.; Lee, S.Y.; Kim, S.H.; Jang, M.O.; Kang, S.J.; Jung, S.I.; Chung, E.K.; Ko, K.S.; Jang, H.C. The Clinical Characteristics, Carbapenem Resistance, and Outcome of Acinetobacter Bacteremia According to Genospecies. PLoS ONE 2013, 8, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Pailhoriès, H.; Hadjadj, L.; Mahieu, R.; Crochette, N.; Rolain, J.M.; Kempf, M. Fortuitous diagnosis of NDM-1-producing Acinetobacter pittii carriage in a patient from France with no recent history of travel. J. Antimicrob. Chemother. 2016, 72, 942–944. [Google Scholar] [CrossRef][Green Version]
- Horrevorts, A.; Bergman, K.; Kollee, L.; Breuker, I.; Tjernberg, I.; Dijkshoorn, L. Clinical and epidemiological investigations of Acinetobacter genomospecies 3 in a neonatal intensive care unit. J. Clin. Microbiol. 1995, 33, 1567–1572. [Google Scholar] [CrossRef]
- Molina, J.; Cisneros, J.M.; Fernández-Cuenca, F.; Rodríguez-Baño, J.; Ribera, A.; Beceiro, A.; Martínez-Martínez, L.; Pascual, A.; Bou, G.; Vila, J.; et al. Clinical Features of Infections and Colonization by Acinetobacter Genospecies 3. J. Clin. Microbiol. 2010, 48, 4623–4626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, L.; Yuan, J.; Xu, Y.; Zhang, F.; Chen, Z. Comparison of clinical manifestations and antibiotic resistances among three genospecies of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. PLoS ONE 2018, 13, e0191748. [Google Scholar] [CrossRef]
- Bogaerts, P.; Huang, T.D.; De Castro, R.R.; Bouchahrouf, W.; Glupczynski, Y. Could Acinetobacter pittii act as an NDM-1 reservoir for Enterobacteriaceae? J. Antimicrob. Chemother. 2013, 68, 2414–2415. [Google Scholar] [CrossRef][Green Version]
- Chen, F.-J.; Huang, W.-C.; Liao, Y.-C.; Wang, H.-Y.; Lai, J.-F.; Kuo, S.-C.; Lauderdale, T.-L.; Sytwu, H.-K. Molecular Epidemiology of Emerging Carbapenem Resistance in Acinetobacter nosocomialis and Acinetobacter pittii in Taiwan, 2010 to 2014. Antimicrob. Agents Chemother. 2019, 63, e02007–18. [Google Scholar] [CrossRef]
- Turton, J.F.; Shah, J.; Ozongwu, C.; Pike, R. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: Evidence for emerging species. J. Clin. Microbiol. 2010, 48, 1445–1449. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Qiao, L. The Acinetobacter baumannii group: A systemic review. World J. Emerg. Med. 2013, 4, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Paulus, T.; Lugenheim, M.; Stefanik, D.; Higgins, P.G.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J. Infect. 2012, 64, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Bravo, Z.; Chapartegui-Gonzalez, I.; Lazaro-Diez, M.; Ramos-Vivas, J. Acinetobacter pittii biofilm formation on inanimate surfaces after long-term desiccation. J. Hosp. Infect. 2018, 98, 74–82. [Google Scholar] [CrossRef]
- Bravo, Z.; Orruño, M.; Navascues, T.; Ogayar, E.; Ramos-Vivas, J.; Kaberdin, V.R.; Arana, I. Analysis of Acinetobacter baumannii survival in liquid media and on solid matrices as well as effect of disinfectants. J. Hosp. Infect. 2019, 103, e42–e52. [Google Scholar] [CrossRef]
- Karah, N.; Haldorsen, B.; Hegstad, K.; Simonsen, G.S.; Sundsfjord, A.; Samuelsen, Ø. Species identification and molecular characterization of Acinetobacter spp. blood culture isolates from Norway. J. Antimicrob. Chemother. 2011, 66, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Chapartegui-González, I.; Fernández-Martínez, M.; Rodríguez-Fernández, A.; Rocha, D.J.P.; Aguiar, E.R.G.R.; Pacheco, L.G.C.; Ramos-Vivas, J.; Calvo, J.; Martínez-Martínez, L.; Navas, J. Antimicrobial Susceptibility and Characterization of Resistance Mechanisms of Corynebacterium urealyticum Clinical Isolates. Antibiotics 2020, 9, 404. [Google Scholar] [CrossRef]
- Iimura, M.; Hayashi, W.; Arai, E.; Natori, T.; Horiuchi, K.; Matsumoto, G.; Tanaka, H.; Soga, E.; Nagano, Y.; Nagano, N. Detection of Acinetobacter pittii ST220 co-producing NDM-1 and OXA-820 carbapenemases from a hospital sink in a non-endemic country of NDM. J. Glob. Antimicrob. Resist. 2020, 21, 353–356. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Sheng, W.H.; Li, S.Y.; Lin, Y.C.; Wang, J.T.; Chen, Y.C.; Chang, S.C. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with acinetobacter bacteremia. Clin. Infect. Dis. 2011, 52, 352–360. [Google Scholar] [CrossRef]
- Lee, Y.C.; Huang, Y.T.; Tan, C.K.; Kuo, Y.W.; Liao, C.H.; Lee, P.I.; Hsueh, P.R. Acinetobacter baumannii and Acinetobacter genospecies 13tu and 3 bacteraemia: Comparison of clinical features, prognostic factors and outcomes. J. Antimicrob. Chemother. 2011, 66, 1839–1846. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Xie, J.; Liu, N.; Wang, H.; Zhang, L.; Wang, X.; Wang, Y.; Qiu, S.; Song, H. Acinetobacter calcoaceticus from a fatal case of pneumonia harboring bla(NDM-1) on a widely distributed plasmid. BMC Infect. Dis. 2015, 15, 131. [Google Scholar] [CrossRef]
- Bertini, A.; Poirel, L.; Mugnier, P.D.; Villa, L.; Nordmann, P.; Carattoli, A. Characterization and PCR-based replicon typing of resistance plasmids in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4168–4177. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Goglin, K.; Molyneaux, N.; Hujer, K.M.; Lavender, H.; Jamison, J.J.; MacDonald, I.J.; Martin, K.M.; Russo, T.; Campagnari, A.A.; et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 2008, 190, 8053–8064. [Google Scholar] [CrossRef] [PubMed]
- Touchon, M.; Cury, J.; Yoon, E.-J.; Krizova, L.; Cerqueira, G.C.; Murphy, C.; Feldgarden, M.; Wortman, J.; Clermont, D.; Lambert, T.; et al. The Genomic Diversification of the Whole Acinetobacter Genus: Origins, Mechanisms, and Consequences. Genome Biol. Evol. 2014, 6, 2866–2882. [Google Scholar] [CrossRef] [PubMed]
- Chopjitt, P.; Putthanachote, N.; Ungcharoen, R.; Hatrongjit, R.; Boueroy, P.; Akeda, Y.; Tomono, K.; Hamada, S.; Kerdsin, A. Genomic Characterization of Clinical Extensively Drug-Resistant Acinetobacter pittii Isolates. Microorganisms 2021, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Di Nocera, P.P.; Rocco, F.; Giannouli, M.; Triassi, M.; Zarrilli, R. Genome organization of epidemic Acinetobacter baumannii strains. BMC Microbiol. 2011, 11, 224. [Google Scholar] [CrossRef]
- Sahl, J.W.; Johnson, J.K.; Harris, A.D.; Phillippy, A.M.; Hsiao, W.W.; Thom, K.A.; Rasko, D.A. Genomic comparison of multi-drug resistant invasive and colonizing Acinetobacter baumannii isolated from diverse human body sites reveals genomic plasticity. BMC Genomics 2011, 12, 291. [Google Scholar] [CrossRef]
- Wright, M.S.; Haft, D.H.; Harkins, D.M.; Perez, F.; Hujer, K.M.; Bajaksouzian, S.; Benard, M.F.; Jacobs, M.R.; Bonomo, R.A.; Adams, M.D. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. MBio 2014, 5, e00963–13. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Zhang, Y.; Wang, X.; Zhao, C.; Chen, H.; Zhang, F.; Zhu, B.; Hu, Y.; Wang, H. Evolution of carbapenem-resistant Acinetobacter baumannii revealed through whole-genome sequencing and comparative genomic analysis. Antimicrob. Agents Chemother. 2015, 59, 1168–1176. [Google Scholar] [CrossRef]
- Chapartegui-González, I.; Lázaro-Díez, M.; Redondo-Salvo, S.; Navas, J.; Ramos-Vivas, J. Antimicrobial resistance determinants in genomes and plasmids from Acinetobacter baumannii clinical isolates. Antibiotics 2021, 10, 753. [Google Scholar] [CrossRef]
- Lázaro-Díez, M.; Navascués-Lejarza, T.; Remuzgo-Martínez, S.; Navas, J.; Icardo, J.M.; Acosta, F.; Martínez-Martínez, L.; Ramos-Vivas, J. Acinetobacter baumannii and A. pittii clinical isolates lack adherence and cytotoxicity to lung epithelial cells in vitro. Microbes Infect. 2016, 18, 559–564. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genomics 2008, 9, 75. [Google Scholar] [CrossRef]
- Vallenet, D.; Nordmann, P.; Barbe, V.; Poirel, L.; Mangenot, S.; Bataille, E.; Dossat, C.; Gas, S.; Kreimeyer, A.; Lenoble, P.; et al. Comparative analysis of acinetobacters: Three genomes for three lifestyles. PLoS ONE 2008, 3, e1805. [Google Scholar] [CrossRef] [PubMed]
- Boo, T.W.; Walsh, F.; Crowley, B. Molecular characterization of carbapenem-resistant Acinetobacter species in an Irish university hospital: Predominance of Acinetobacter genomic species 3. J. Med. Microbiol. 2009, 58, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhang, X.; Hou, M.; Han, D.; Li, Y.; Xiong, W. Draft genome sequence of an OXA-23, OXA-66, ADC-25 and TEM-1D co-producing Acinetobacter baumannii ST195 isolated from a patient with neonatal pneumonia in China. J. Glob. Antimicrob. Resist. 2019, 16, 1–3. [Google Scholar] [CrossRef]
- Kamolvit, W.; Higgins, P.G.; Paterson, D.L.; Seifert, H. Multiplex PCR to detect the genes encoding naturally occurring oxacillinases in Acinetobacter spp. J. Antimicrob. Chemother. 2014, 69, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Kamolvit, W.; Derrington, P.; Paterson, D.L.; Sidjabat, H.E. A case of IMP-4-, OXA-421-, OXA-96-, and CARB-2-producing Acinetobacter pittii sequence type 119 in Australia. J. Clin. Microbiol. 2015, 53, 727–730. [Google Scholar] [CrossRef]
- Evans, B.A.; Amyes, S.G.B. OXA β-lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef]
- Gordon, N.C.; Wareham, D.W. Multidrug-resistant Acinetobacter baumannii: Mechanisms of virulence and resistance. Int. J. Antimicrob. Agents 2010, 35, 219–226. [Google Scholar] [CrossRef]
- Coyne, S.; Courvalin, P.; Périchon, B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011, 55, 947–953. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, G.W.; Pos, K.M.; Piddock, L.J.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Mussi, M.A.; Limansky, A.S.; Viale, A.M. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: Natural insertional inactivation of a gene encoding a member of a novel family of beta-barrel outer membrane proteins. Antimicrob. Agents Chemother. 2005, 49, 1432–1440. [Google Scholar] [CrossRef]
- Evans, B.; Hamouda, A.; Towner, K.; Amyes, S. Novel genetic context of multiple blaOXA-58 genes in Acinetobacter genospecies 3. J. Antimicrob. Chemother. 2010, 65, 1586–1588. [Google Scholar] [CrossRef]
- Holt, K.; Kenyon, J.J.; Hamidian, M.; Schultz, M.B.; Pickard, D.J.; Dougan, G.; Hall, R. Five decades of genome evolution in the globally distributed, extensively antibiotic-resistant Acinetobacter baumannii global clone 1. Microb. genomics 2016, 2, e000052. [Google Scholar] [CrossRef]
- Pagano, M.; Martins, A.F.; Barth, A.L. Mobile genetic elements related to carbapenem resistance in Acinetobacter baumannii. Brazilian J. Microbiol. 2016, 47, 785–792. [Google Scholar] [CrossRef]
- Yakkala, H.; Samantarrai, D.; Siddavattam, D. Direct GenBank Submission. 2018. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 31 March 2022).
- Repizo, G.D.; Viale, A.M.; Borges, V.; Cameranesi, M.M.; Taib, N.; Espariz, M.; Brochier-Armanet, C.; Gomes, J.P.; Salcedo, S.P. The Environmental Acinetobacter baumannii Isolate DSM30011 Reveals Clues into the Preantibiotic Era Genome Diversity, Virulence Potential, and Niche Range of a Predominant Nosocomial Pathogen. Genome Biol. Evol. 2017, 9, 2292–2307. [Google Scholar] [CrossRef]
- Hamidian, M.; Ambrose, S.J.; Hall, R.M. A large conjugative Acinetobacter baumannii plasmid carrying the sul2 sulphonamide and strAB streptomycin resistance genes. Plasmid 2016, 87–88, 43–50. [Google Scholar] [CrossRef]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S.L. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef]
- Liu, Y.H.; Kuo, S.C.; Lee, Y.T.; Chang, I.C.Y.; Yang, S.P.; Chen, T.L.; Fung, C.P. Amino acid substitutions of quinolone resistance determining regions in GyrA and ParC associated with quinolone resistance in Acinetobacter baumannii and Acinetobacter genomic species 13TU. J. Microbiol. Immunol. Infect. 2012, 45, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Ruiz, J.; Goni, P.; Marcos, A.; De Anta, T.J. Mutation in the gyrA gene of quinolone-resistant clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 1995, 39, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Ruiz, J.; Goni, P.; Jimenez de Anta, T. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J. Antimicrob. Chemother. 1997, 39, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Nhu, N.T.K.; Riordan, D.W.; Nhu, T.D.H.; Thanh, D.P.; Thwaites, G.; Lan, N.P.H.; Wren, B.W.; Baker, S.; Stabler, R.A. The induction and identification of novel Colistin resistance mutations in Acinetobacter baumannii and their implications. Sci. Rep. 2016, 6, 28291. [Google Scholar] [CrossRef]
- Lean, S.S.; Yeo, C.C.; Suhaili, Z.; Thong, K.L. Comparative genomics of two ST 195 carbapenem-resistant Acinetobacter baumannii with different susceptibility to polymyxin revealed underlying resistance mechanism. Front. Microbiol. 2016, 6, 1445. [Google Scholar] [CrossRef]
- Karaiskos, I.; Souli, M.; Galani, I.; Giamarellou, H. Colistin: Still a lifesaver for the 21st century? Expert Opin. Drug Metab. Toxicol. 2017, 13, 59–71. [Google Scholar] [CrossRef]
- Abbo, A.; Navon-Venezia, S.; Hammer-Muntz, O.; Krichali, T.; Siegman-Igra, Y.; Carmeli, Y. Multidrug-resistant Acinetobacter baumannii. Emerg. Infect. Dis. 2005, 11, 22–29. [Google Scholar] [CrossRef]
- Mancilla-Rojano, J.; Castro-Jaimes, S.; Ochoa, S.A.; Bobadilla del Valle, M.; Luna-Pineda, V.M.; Bustos, P.; Laris-González, A.; Arellano-Galindo, J.; Parra-Ortega, I.; Hernández-Castro, R.; et al. Whole-Genome Sequences of Five Acinetobacter baumannii Strains From a Child With Leukemia M2. Front. Microbiol. 2019, 10, 132. [Google Scholar] [CrossRef]
- Lean, S.S.; Yeo, C.C. Small, enigmatic plasmids of the nosocomial pathogen, Acinetobacter baumannii: Good, bad, who knows? Front. Microbiol. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Chapartegui-Gonzalez, I.; Lazaro-Diez, M.; Redondo-Salvo, S.; Alted-Perez, L.; Ocejo-Vinyals, J.G.; Navas, J.; Ramos-Vivas, J. Whole-Genome Sequence of Acinetobacter pittii HUMV-6483 Isolated from Human Urine. Genome Announc. 2017, 5, e00658–17. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.P.; Sutton, G.; DePew, J.; Krishnakumar, R.; Choi, Y.; Huang, X.-Z.; Beck, E.; Harkins, D.M.; Kim, M.; Lesho, E.P.; et al. A novel method of consensus pan-chromosome assembly and large-scale comparative analysis reveal the highly flexible pan-genome of Acinetobacter baumannii. Genome Biol. 2015, 16, 143. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, J.; Zhou, H.; Jiang, Y.; Fu, Y.; Yu, Y.; Zhou, J. Characterization of a novel plasmid type and various genetic contexts of bla OXA-58 in Acinetobacter spp. from multiple cities in China. PLoS ONE 2014, 9, e84680. [Google Scholar] [CrossRef]
- Janssen, P.; Maquelin, K.; Coopman, R.; Tjernberg, I.; Bouvet, P.; Kersters, K.; Dijkshoorn, L. Discrimination of Acinetobacter Genomic Species by AFLP Fingerprinting. Int. J. Syst. Bacteriol. 1997, 47, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yan, Y.; Zhang, W.; Yu, H.; Chen, M.; Lu, W.; Ping, S.; Peng, Z.; Yuan, M.; Zhou, Z.; et al. Genome sequence of Acinetobacter calcoaceticus PHEA-2, isolated from industry wastewater. J. Bacteriol. 2011, 193, 2672–2673. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standars Institute: Wayne, AR, USA, 2018; ISBN 1-56238-838. [Google Scholar]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 8.1. Available online: http://www.eucast.org (accessed on 31 March 2022).
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Chin, C.S.; Alexander, D.H.; Marks, P.; Klammer, A.A.; Drake, J.; Heiner, C.; Clum, A.; Copeland, A.; Huddleston, J.; Eichler, E.E.; et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 2013, 10, 563–569. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, 206–214. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Larsen, M.V.; Salvatore, C.; Simon, R.; Carsten, F.; Henrik, H.; Lykke, M.R.; Lars, J.; Thomas, S.-P.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus Sequence Typing of Total-Genome-Sequenced Bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- McArthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L.; et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 2013, 57, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Vielva, L.; de Toro, M.; Lanza, V.F.; de la Cruz, F. PLACNETw: A web-based tool for plasmid reconstruction from bacterial genomes. Bioinformatics 2017, 33, 3796–3798. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Rodriguez, R.L.M.; Konstantinidis, K.T. The enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Prepr. 2016, 4, e1900v1. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Fouts, D.E.; Brinkac, L.; Beck, E.; Inman, J.; Sutton, G. PanOCT: Automated clustering of orthologs using conserved gene neighborhood for pan-genomic analysis of bacterial strains and closely related species. Nucleic Acids Res. 2012, 40, e172. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, V.G.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]



| COL | MER | AMP * | GEN | CIP | TET ** | ERY | Resistance Profile | ||
|---|---|---|---|---|---|---|---|---|---|
| MIC | LMG10559 | 8 | 2 | >64 | 4 | >0.25 | 1 | >128 | CAE |
| HUMV0315 | 4 | >16 | >64 | 4 | >4 | 1 | 64 | CMAPE | |
| HUMV4336 | 8 | 2 | >64 | 4 | >0.125 | 1 | >128 | CAE | |
| HUMV6207 | 4 | 2 | >64 | 16 | >0.25 | <0.5 | 64 | CAGE | |
| HUMV5918 | 8 | 2 | >64 | 1 | <0.06 | 1 | 64 | CAE | |
| HUMV6483 | 8 | 2 | >64 | 2 | <0.06 | 1 | 64 | CAE | |
| MIC50 | >8 | 2 | >64 | 2 | 0.125 | 1 | 64 | ||
| MIC90 | >8 | >16 | >64 | 16 | >4 | 1 | >128 | ||
| Range (mg/L) | 0.125–8 | 0.25–16 | 1–64 | 0.5–32 | 0.06–4 | 0.5–32 | 2–128 | ||
| HUMV0315 | HUMV4336 | HUMV6207 | HUMV5918 * | HUMV6483 | ||
|---|---|---|---|---|---|---|
| Size (bp) | 4,040,319 | 3,936,103 | 3,970,495 | 3,990,911 | 4,008,138 | |
| % GC | 38.8 | 38.8 | 38.8 | 38.9 | 38.9 | |
| Subsystems | 448 | 454 | 459 | 308 | 452 | |
| CDS | RAST | 3791 | 3664 | 3725 | 3827 | 3777 |
| Prokka | 3777 | 3651 | 3697 | 3678 | 3770 | |
| tRNA | 63 | 63 | 63 | 74 | 64 | |
| mRNA | 1 | 1 | 1 | 1 | 1 | |
| rRNA | 3 | 3 | 3 | 18 | 3 | |
| Strains | Antimicrobial Classes Resistance Genes | Efflux Pumps | ||||
|---|---|---|---|---|---|---|
| AME | β-LACTAM | RND | SMR | MATE | ||
| ampC | OXA | |||||
| HUMV0315 | ADC-18 | OXA-58 OXA-58 (97) OXA-213 (325) | adeAB adeFGH adeIJK | abeS | abeM | |
| HUMV4336 | ADC-25 | OXA-213 (325) OXA-213 (421) | adeAB adeFGH adeIJK | abeS | abeM | |
| HUMV6207 | ADC-43 | OXA-213 (325) OXA-213 (421) | adeFGH adeIJK | abeS | abeM | |
| HUMV5918 | ADC-25 | OXA-213 (325) OXA-213 (421) | adeAB adeFGH adeIJK | abeS | abeM | |
| HUMV6483 | ant(3″)-IIa | ADC-19 | OXA-213 (325) OXA-213 (421) | adeAB adeFGH adeIJK | abeS | abeM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapartegui-González, I.; Lázaro-Díez, M.; Ramos-Vivas, J. Genetic Resistance Determinants in Clinical Acinetobacter pittii Genomes. Antibiotics 2022, 11, 676. https://doi.org/10.3390/antibiotics11050676
Chapartegui-González I, Lázaro-Díez M, Ramos-Vivas J. Genetic Resistance Determinants in Clinical Acinetobacter pittii Genomes. Antibiotics. 2022; 11(5):676. https://doi.org/10.3390/antibiotics11050676
Chicago/Turabian StyleChapartegui-González, Itziar, María Lázaro-Díez, and José Ramos-Vivas. 2022. "Genetic Resistance Determinants in Clinical Acinetobacter pittii Genomes" Antibiotics 11, no. 5: 676. https://doi.org/10.3390/antibiotics11050676
APA StyleChapartegui-González, I., Lázaro-Díez, M., & Ramos-Vivas, J. (2022). Genetic Resistance Determinants in Clinical Acinetobacter pittii Genomes. Antibiotics, 11(5), 676. https://doi.org/10.3390/antibiotics11050676








