Capsaicin Potently Blocks Salmonella typhimurium Invasion of Vero Cells
Abstract
1. Introduction
2. Results and Discussion
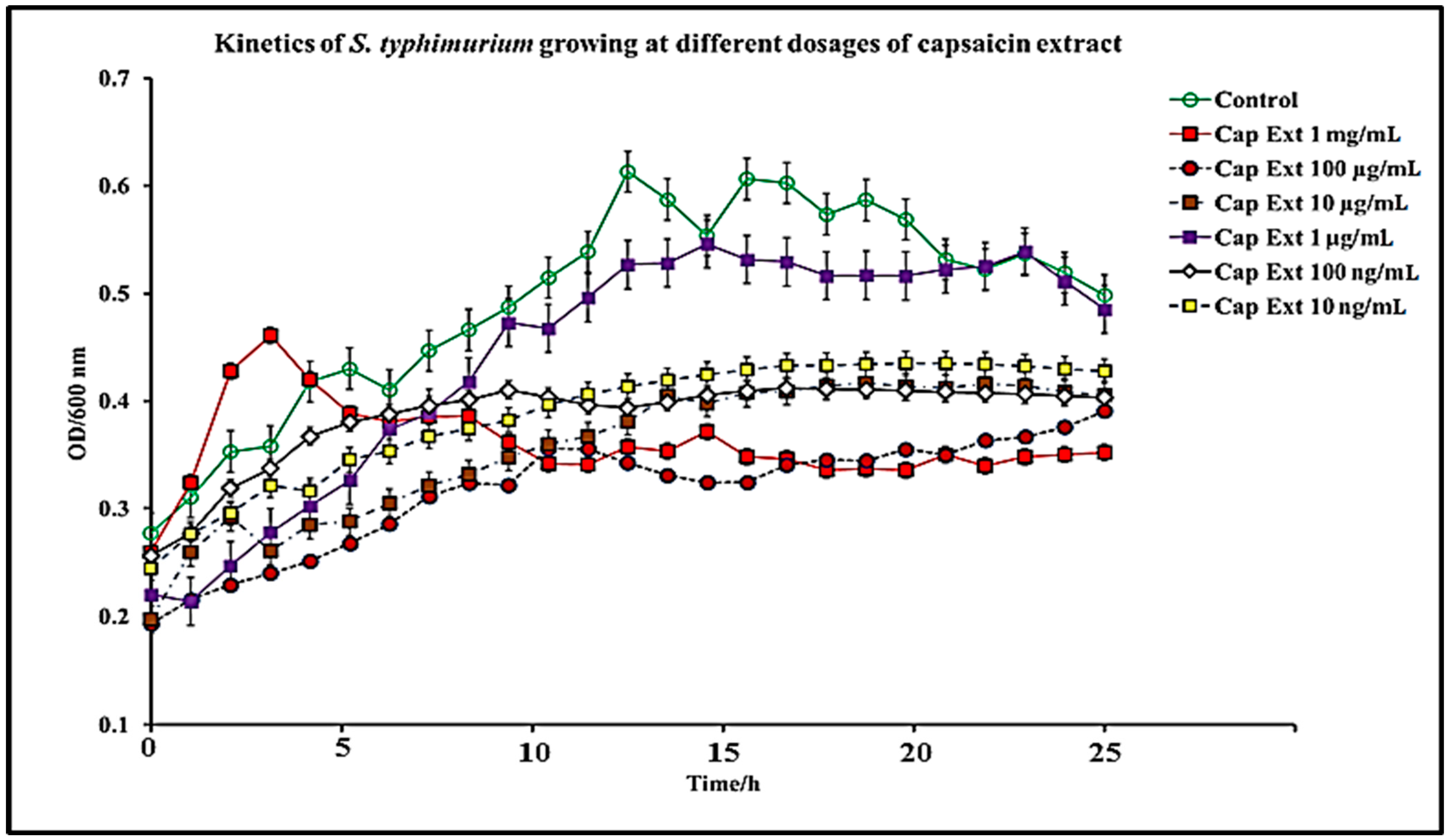
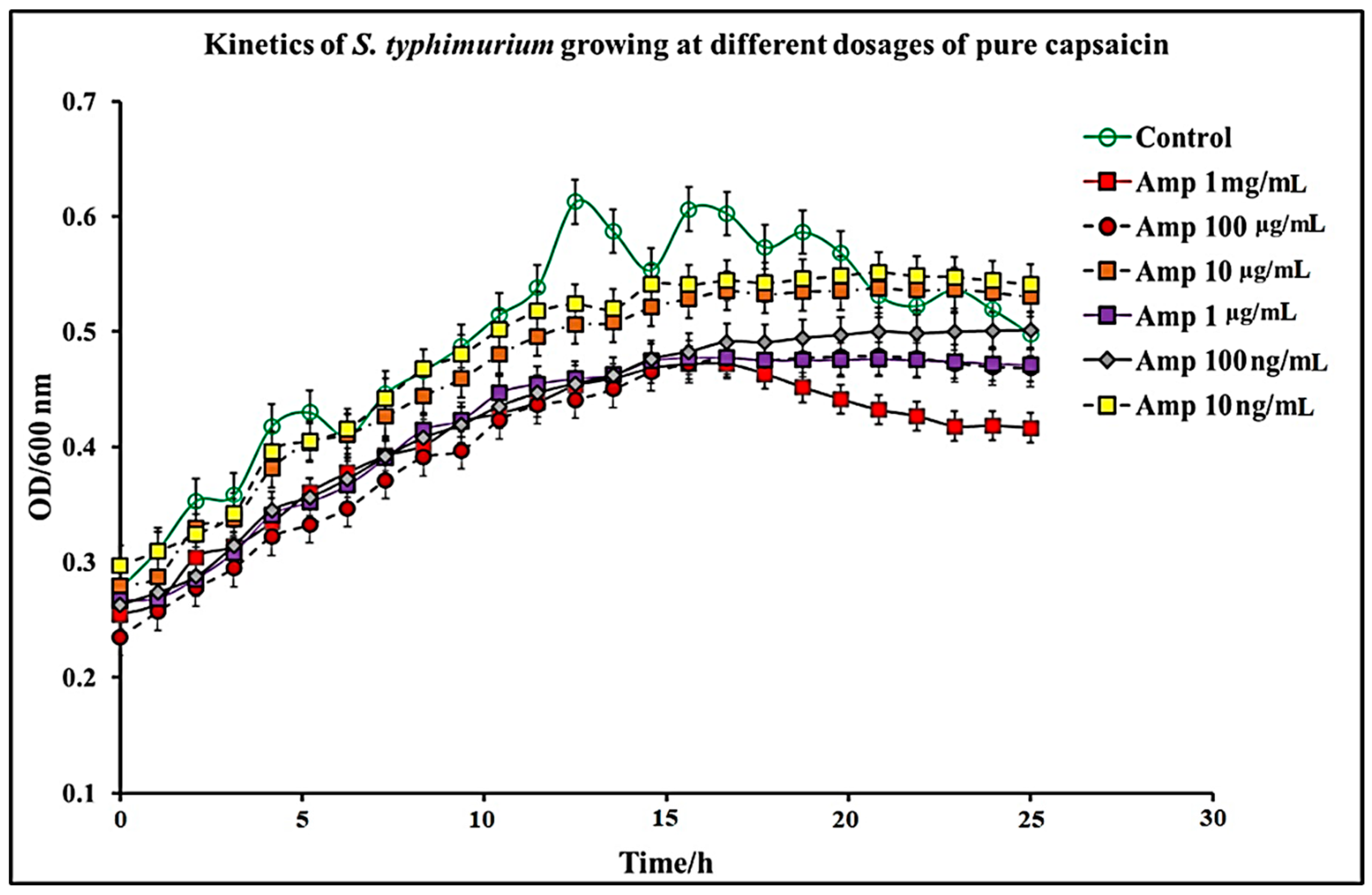
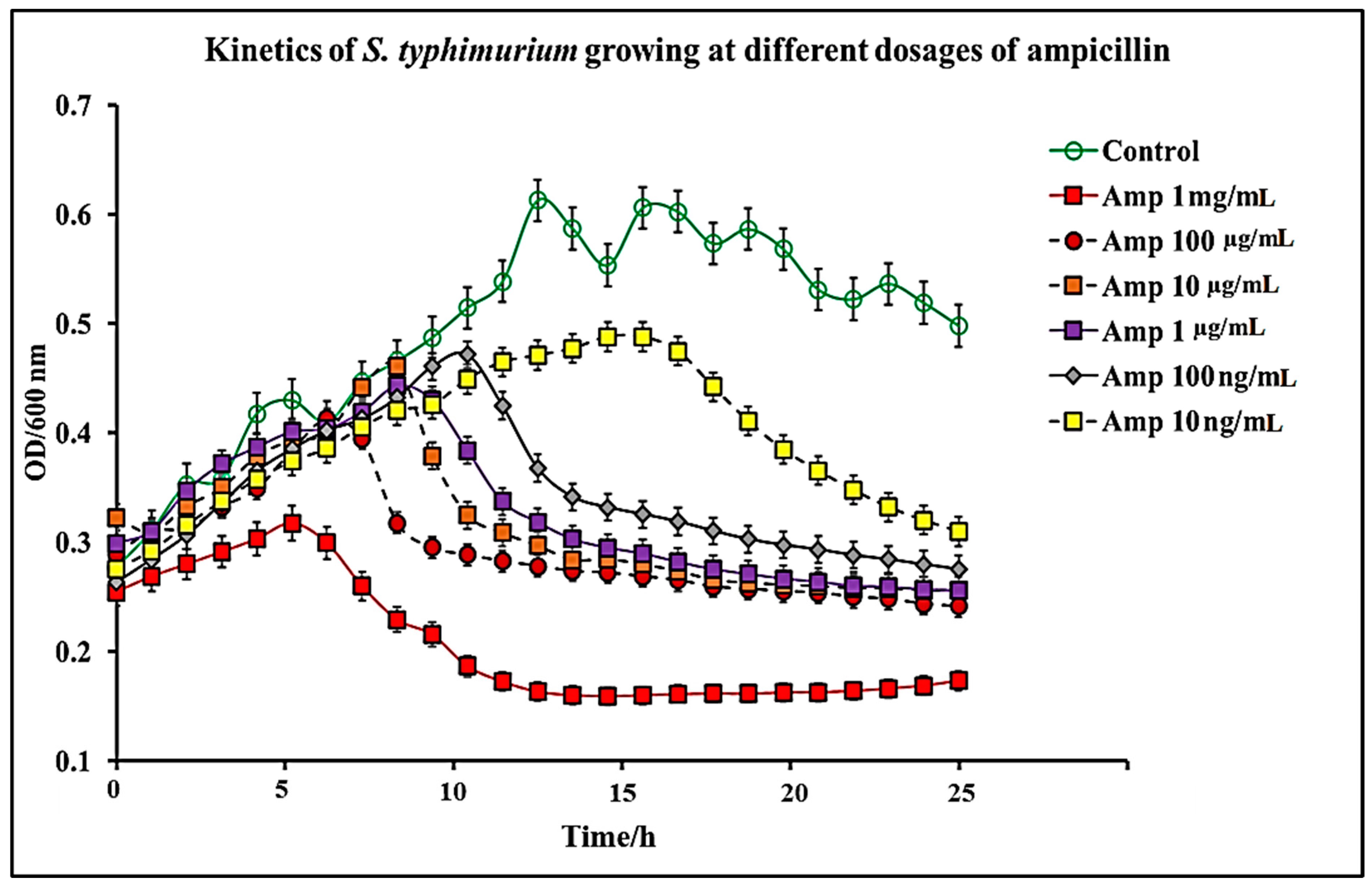
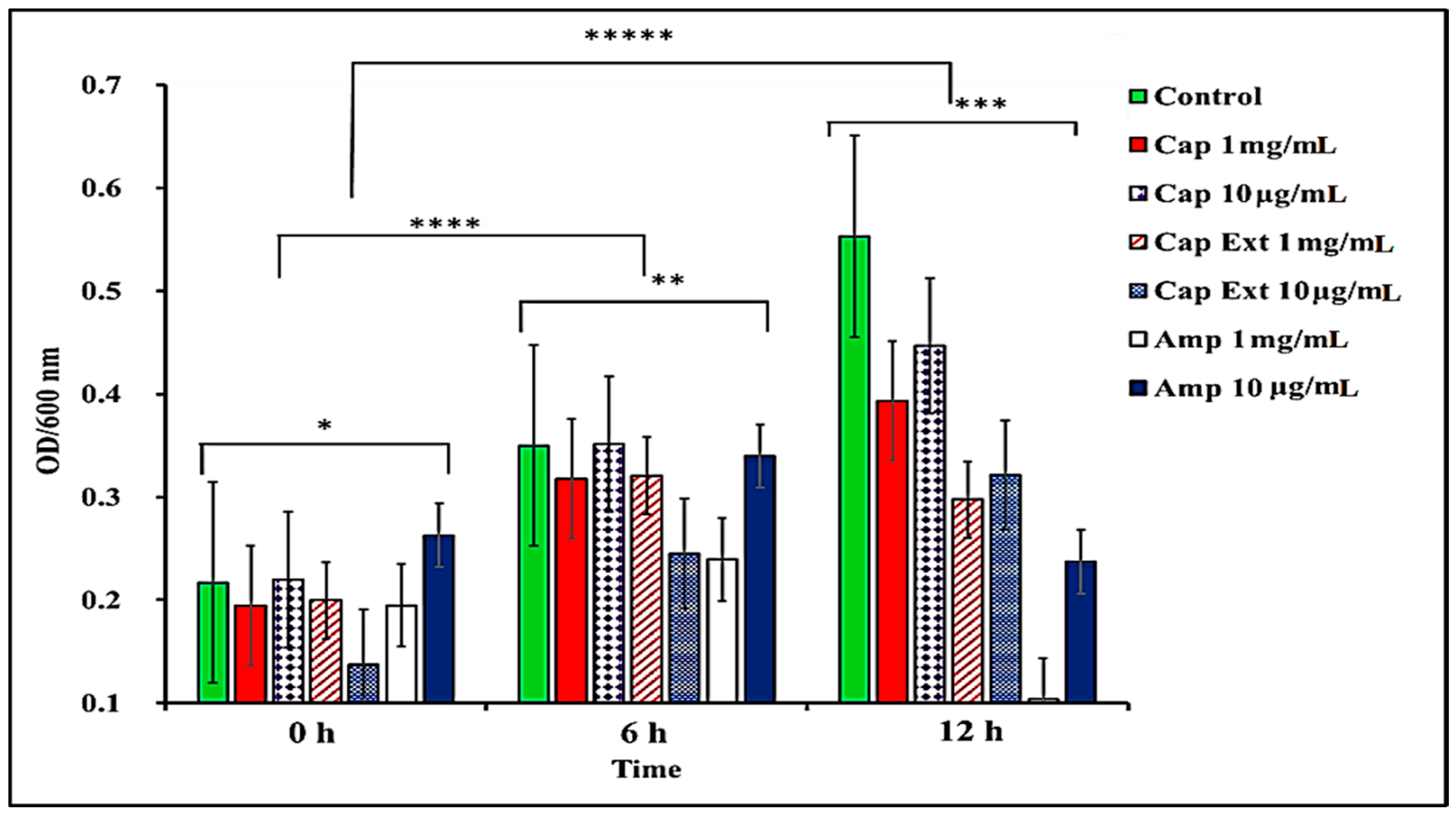
2.1. Effect of C. chinense Extract and Pure Capsaicin on S. typhimurium Growth
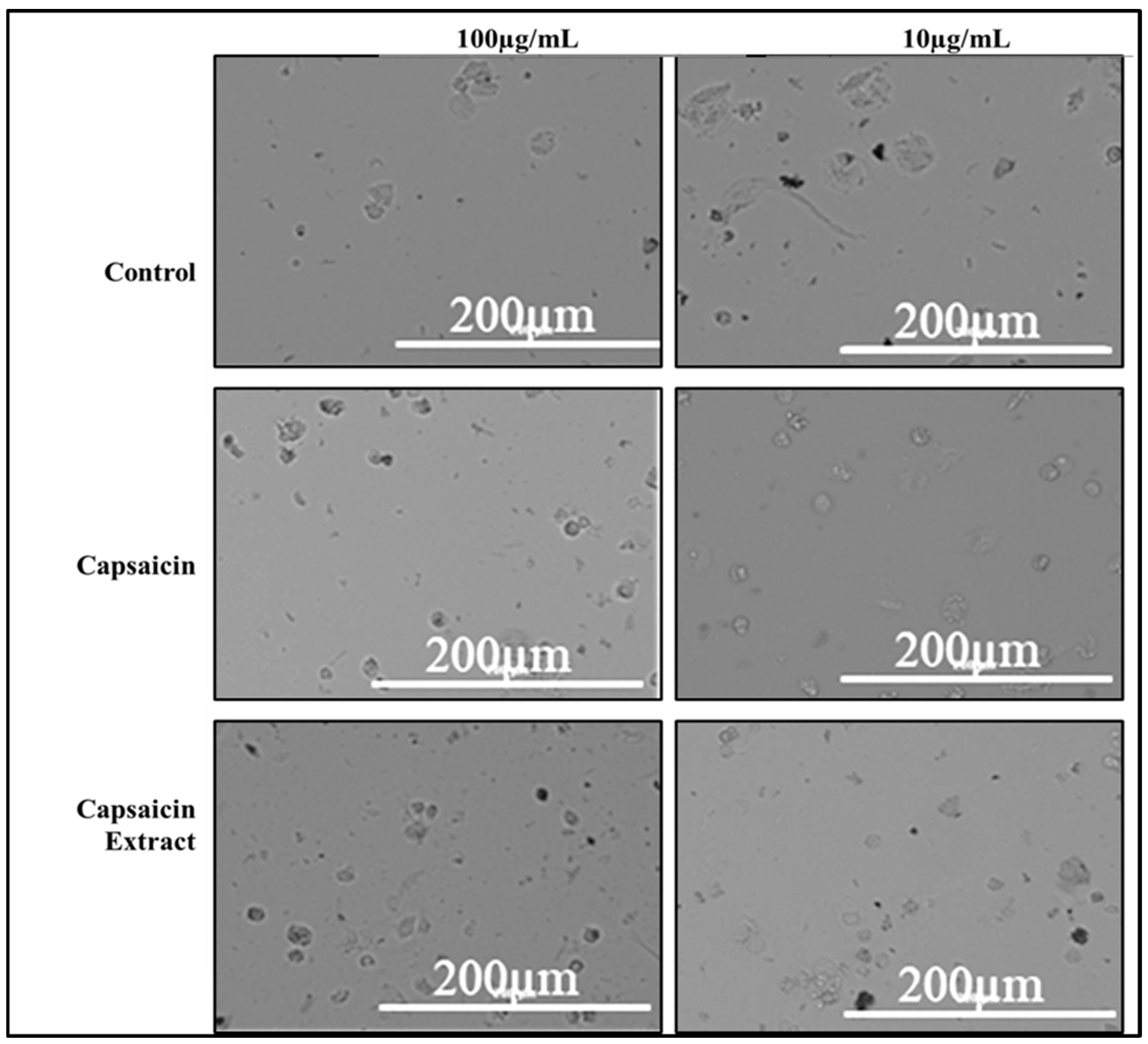
2.2. Cytotoxicity of Capsaicin
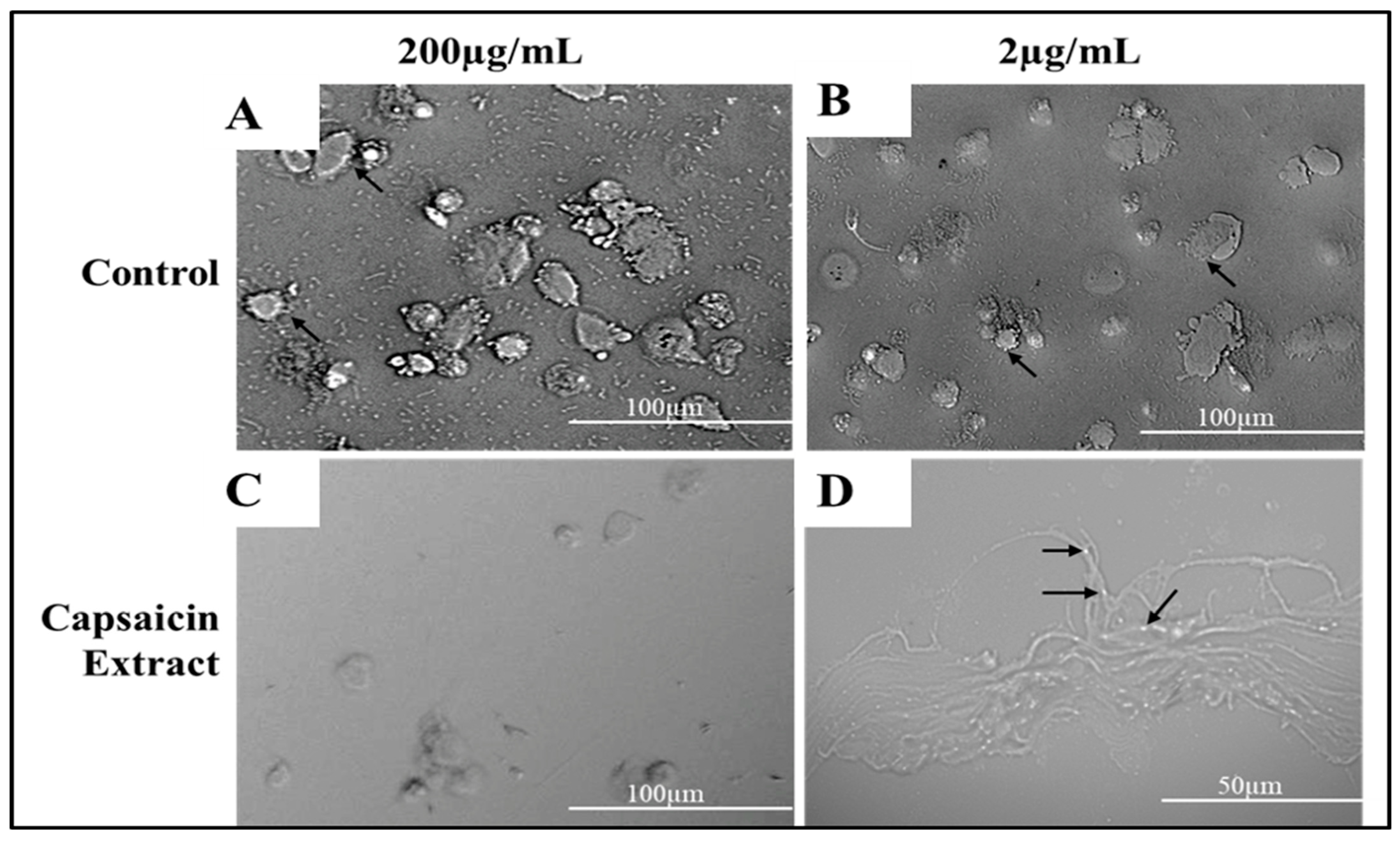
2.3. Adherence of S. typhimurium to Vero Cells
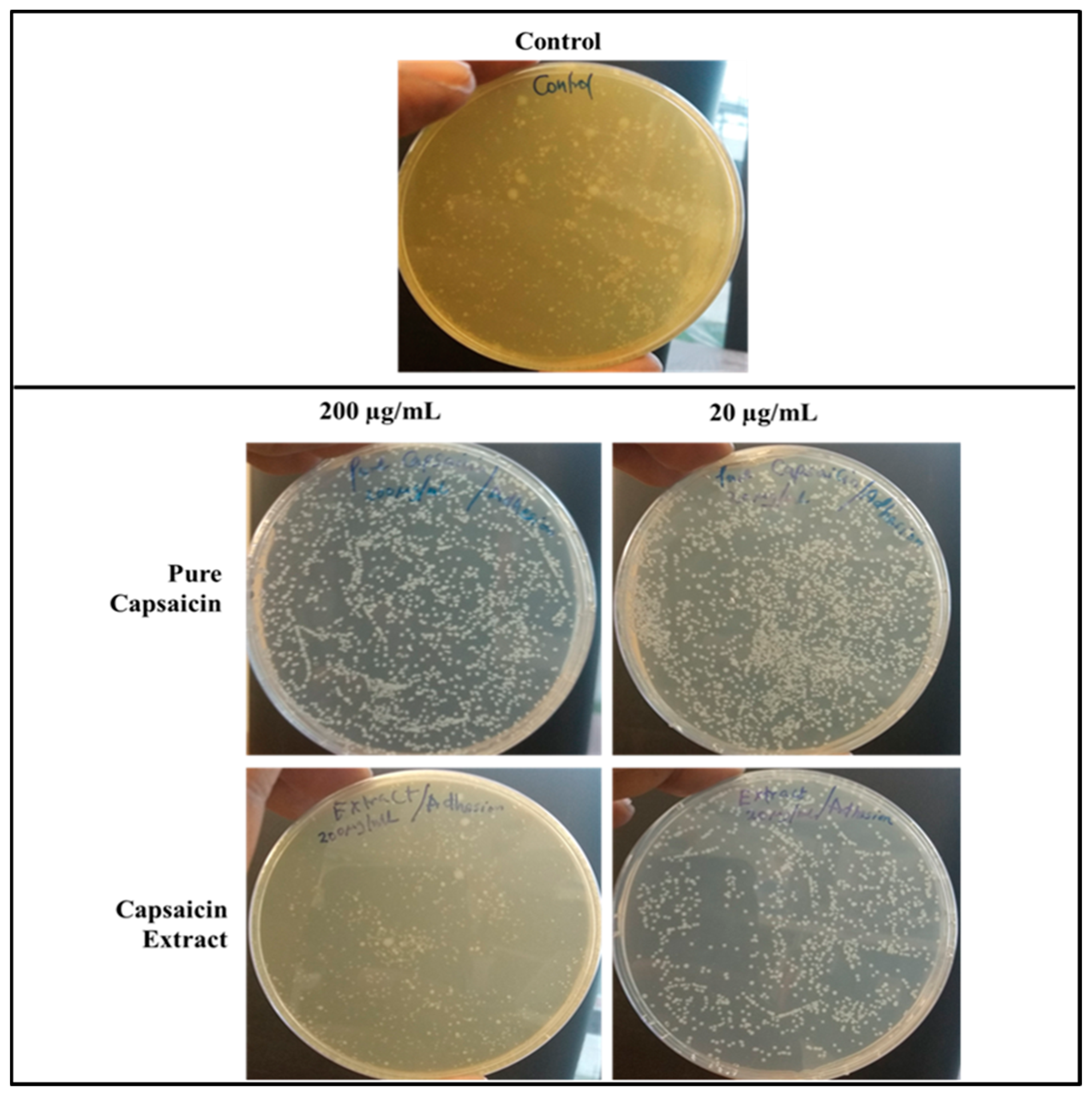
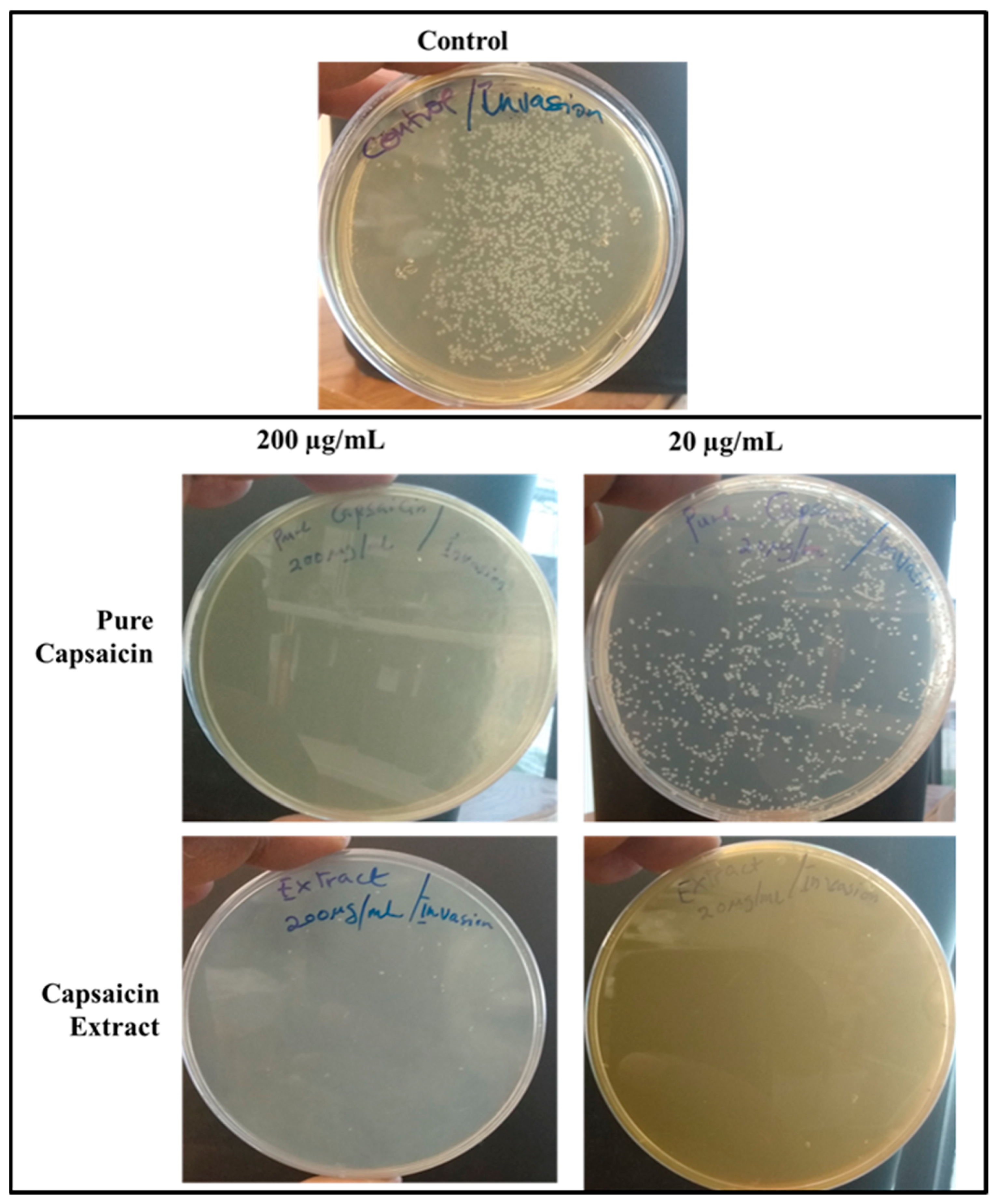
2.4. Invasion of S. typhimurium into Vero Cells
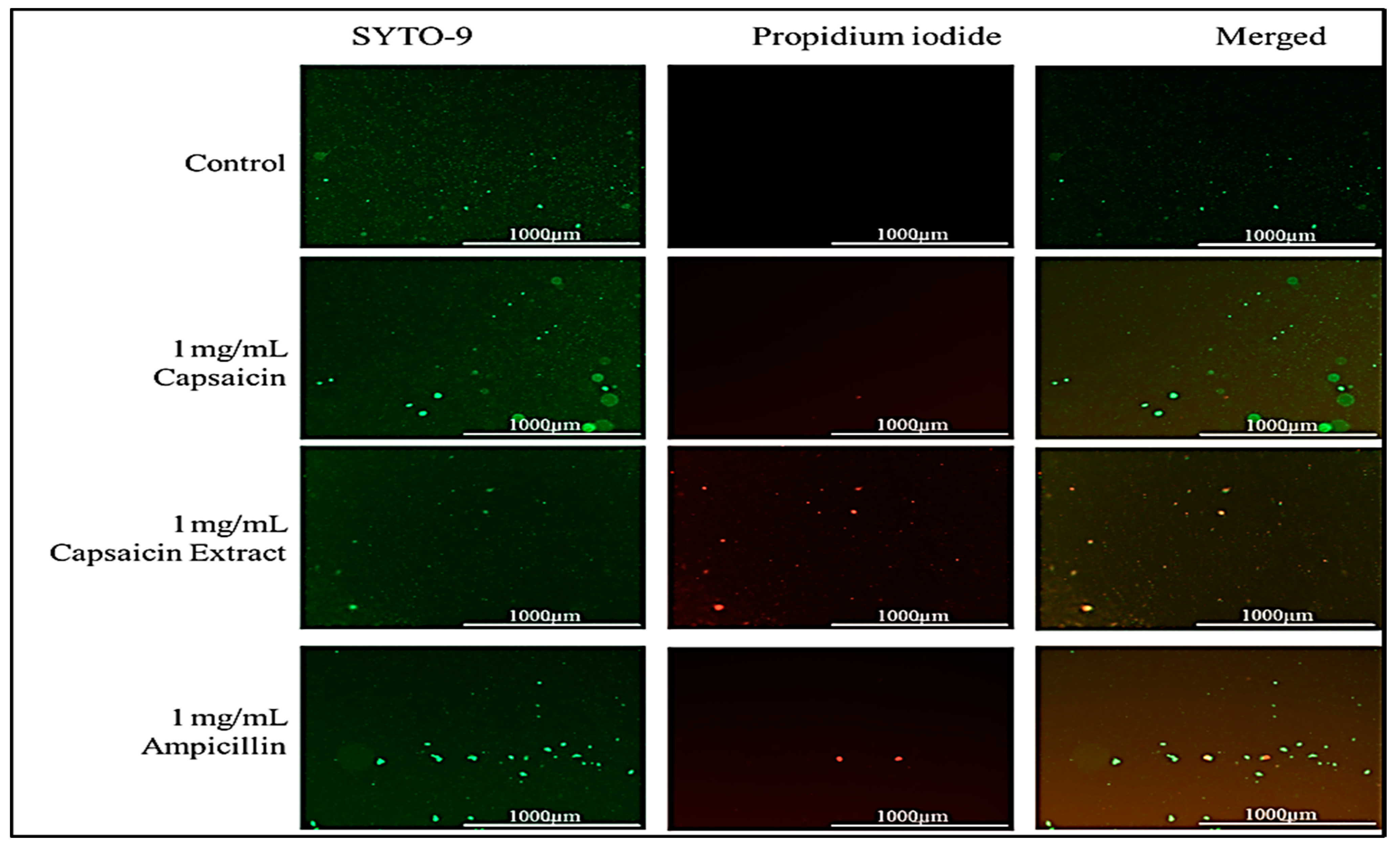
2.5. Effect of Capsaicin or Capsaicin Extract on S. typhimurium Membrane Integrity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal Cells and Bacterial Strain
4.3. Description of Plant and Collection of Plant Material
4.3.1. S. typhimurium Live/Dead Assay
4.3.2. S. typhimurium Membrane Integrity Test
4.4. Vero Cell Viability
4.4.1. Anti-Adhesion Assay
4.4.2. Anti-Invasion Assay
4.4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voetsch, A.C.; Van Gilder, T.J.; Angulo, F.J.; Farley, M.M.; Shallow, S.; Marcus, R.; Cieslak, P.R.; Deneen, V.C.; Tauxe, R.V.; Emerging Infections Program FoodNet Working Group. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin. Infect. Dis. 2004, 38 (Suppl. 3), S127–S134. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, B.; Barrett, T.J.; Fields, P. Surveillance for human Salmonella infections in the United States. J. AOAC Int. 2006, 89, 553–559. [Google Scholar] [PubMed]
- Leekitcharoenphon, P.; Hendriksen, R.S.; Le Hello, S.; Weill, F.X.; Baggesen, D.L.; Jun, S.R.; Ussery, D.W.; Lund, O.; Crook, D.W.; Wilson, D.J.; et al. Global genomic epidemiology of Salmonella enterica serovar Typhimurium DT104. Appl. Environ. Microbiol. 2016, 82, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Finstad, S.; O’Bryan, C.A.; Marcy, J.A.; Crandall, P.G.; Ricke, S.C. Salmonella and broiler processing in the United States: Relationship to foodborne salmonellosis. Food Res. Int. 2012, 45, 789–794. [Google Scholar] [CrossRef]
- Olsen, S.J.; DeBess, E.E.; McGivern, T.E.; Marano, N.; Eby, T.; Mauvais, S.; Balan, V.K.; Zirnstein, G.; Cieslak, P.R.; Angulo, F.J. A nosocomial outbreak of fluoroquinolone-resistant Salmonella infection. N. Engl. J. Med. 2001, 344, 1572–1579. [Google Scholar] [CrossRef]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef]
- Maurelli, A.T. Black holes, antivirulence genes, and gene inactivation in the evolution of bacterial pathogens. FEMS Microbiol. Lett. 2007, 267, 1–8. [Google Scholar] [CrossRef]
- Butaye, P.; Michael, G.B.; Schwarz, S.; Barrett, T.J.; Brisabois, A.; White, D.G. The clonal spread of multidrug-resistant non-typhi Salmonella serotypes. Microbes Infect. 2006, 8, 1891–1897. [Google Scholar] [CrossRef]
- Giles, W.P.; Benson, A.K.; Olson, M.E.; Hutkins, R.W.; Whichard, J.M.; Winokur, P.L.; Fey, P.D. DNA sequence analysis of regions surrounding bla CMY-2 from multiple Salmonella plasmid backbones. Antimicrob. Agents Chemother. 2004, 48, 2845–2852. [Google Scholar] [CrossRef][Green Version]
- Majid, R.; Demla, V.; Mohammed, A.O.M.; Friedman, E.R.; Kee, P.; Schmitt, K. Salmonella enteritidis concurrent spinal epidural abscess, urinary tract infection and endocarditis in an immunocompetent host: Case report and a review of the literature. J. Trop. Dis. 2018, 6, 2. [Google Scholar] [CrossRef]
- Hakim, S.; Davila, F.; Amin, M.; Hader, I.; Cappell, M.S. Infectious aortitis: A life-threatening endovascular complication of nontyphoidal salmonella bacteremia. Case Rep. Med. 2018, 2018, 6845617. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States—Unspecified agents. Emerg. Infect. Dis. 2011, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Fyfe, M.; Doré, K.; Buxton, J.A.; Pollari, F.; Henry, B.; Middleton, D.; Ahmed, R.; Jamieson, F.; Ciebin, B.; et al. Increased burden of illness associated with antimicrobial-resistant Salmonella enterica serotype Typhimurium infections. J. Infect. Dis. 2004, 189, 377–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Helms, M.; Simonsen, J.; Mølbak, K. Quinolone resistance is associated with increased risk of invasive illness and death in Salmonella Typhimurium infection. J. Infect. Dis. 2004, 190, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Satti, L.; Hanif, F.; Zehra, N.M.; Nadeem, S.; Bangash, T.M.; Peter, A. Typhoidal Salmonella strains in Pakistan: An impending threat of extensively drug-resistant Salmonella Typhi. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2145–2149. [Google Scholar] [CrossRef]
- Brown, A.C.; Grass, J.E.; Richardson, L.C.; Nisler, A.L.; Bicknese, A.S.; Gould, L.H. Antimicrobial resistance in Salmonella that caused foodborne disease outbreaks: United States, 2003–2012. Epidemiol. Infect. 2017, 145, 766–774. [Google Scholar] [CrossRef]
- Chatham-Stephens, K.; Medalla, F.; Hughes, M.; Appiah, G.D.; Aubert, R.D.; Caidi, H.; Angelo, K.M.; Walker, A.T.; Hatley, N.; Masani, S.; et al. Emergence of extensively drug-resistant Salmonella Typhi infections among travelers to or from Pakistan—United States, 2016–2018. Morb. Mortal. Wkly. Rep. 2019, 68, 11. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. Characterization of integrons and resistance genes in multidrug-resistant Salmonella enterica isolated from meat and dairy products in Egypt. Int. J. Food Microbiol. 2014, 189, 39–44. [Google Scholar] [CrossRef]
- Varma, J.K.; Mølbak, K.; Barrett, T.J.; Beebe, J.L.; Jones, T.F.; Rabatsky-Ehr, T.; Smith, K.E.; Vugia, D.J.; Chang, H.G.H.; Angulo, F.J. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J. Infect. Dis. 2005, 191, 554–561. [Google Scholar] [CrossRef]
- Gildea, L.; Ayariga, J.A.; Ajayi, O.S.; Xu, J.; Villafane, R.; Samuel-Foo, M. Cannabis sativa CBD Extract Shows Promising Antibacterial Activity against Salmonella typhimurium and S. newington. Molecules 2022, 27, 2669. [Google Scholar] [CrossRef]
- Haruna, A.; Yahaya, S.M. Recent Advances in the Chemistry of Bioactive Compounds from Plants and Soil Microbes: A Review. Chem. Afr. 2021, 4, 231–248. [Google Scholar] [CrossRef]
- Abugri, D.A.; Ayariga, J.A.; Tiimob, B.J.; Yedjou, C.G.; Mrema, F.; Witola, W.H. Medicinal mushrooms as novel sources for new antiparasitic drug development. In Medicinal Mushrooms; Springer: Singapore, 2019; pp. 251–273. [Google Scholar]
- Papoiu, A.D.; Yosipovitch, G. Topical capsaicin. The fire of a ‘hot’medicine is reignited. Expert Opin. Pharmacother. 2010, 11, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Escogido, M.D.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Haanpää, M.; Treede, R.D. Capsaicin for neuropathic pain: Linking traditional medicine and molecular biology. Eur. Neurol. 2012, 68, 264–275. [Google Scholar] [CrossRef]
- Narang, N.; Jiraungkoorskul, W.; Jamrus, P. Current understanding of antiobesity property of capsaicin. Pharmacogn. Rev. 2017, 11, 23. [Google Scholar]
- Omolo, M.A.; Wong, Z.Z.; Borh, W.G.; Hedblom, G.A.; Dev, K.; Baumler, D.J. Comparative analysis of capsaicin in twenty-nine varieties of unexplored Capsicum and its antimicrobial activity against bacterial and fungal pathogens. J. Med. Plants Res. 2018, 12, 544–556. [Google Scholar]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and anti-virulence activity of capsaicin against erythromycin-resistant, cell-invasive group A streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef]
- Anogianaki, A.; Negrev, N.N.; Shaik, Y.B.; Castellani, M.L.; Frydas, S.; Vecchiet, J.; Tete, S.; Salini, V.; De Amicis, D.; De Lutiis, M.A.; et al. Capsaicin: An irritant anti-inflammatory compound. J. Biol. Regul. Homeost. Agents 2007, 21, 1. [Google Scholar]
- Cao, S.; Chen, H.; Xiang, S.; Hong, J.; Weng, L.; Zhu, H.; Liu, Q. Anti-cancer effects and mechanisms of capsaicin in chili peppers. Am. J. Plant Sci. 2015, 6, 3075. [Google Scholar] [CrossRef]
- Ayariga, J.; Abugri, D.A.; Griffin, G.D. Capsaicin and Dihydrocapsaicin Extracted from Capsicum chinense Decrease Cell Viability of Neuroblastoma SH-SY5Y Cells in Vitro. Preprints 2021. [Google Scholar] [CrossRef]
- Sun, F.; Xiong, S.; Zhu, Z. Dietary capsaicin protects cardiometabolic organs from dysfunction. Nutrients 2016, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.P.; Kovacs, E.M.; Westerterp-Plantenga, M.S. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br. J. Nutr. 2003, 90, 651–659. [Google Scholar] [CrossRef]
- Di Cara, F.; Andreoletti, P.; Trompier, D.; Vejux, A.; Bülow, M.H.; Sellin, J.; Lizard, G.; Cherkaoui-Malki, M.; Savary, S. Peroxisomes in immune response and inflammation. Int. J. Mol. Sci. 2019, 20, 3877. [Google Scholar] [CrossRef]
- Qiu, J.; Niu, X.; Wang, J.; Xing, Y.; Leng, B.; Dong, J.; Li, H.; Luo, M.; Zhang, Y.; Dai, X.; et al. Capsaicin protects mice from community-associated methicillin-resistant Staphylococcus aureus pneumonia. PLoS ONE 2012, 7, e33032. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Asakura, M.; Chowdhury, N.; Neogi, S.B.; Sugimoto, N.; Haldar, S.; Awasthi, S.P.; Hinenoya, A.; Aoki, S.; Yamasaki, S. Capsaicin, a potential inhibitor of cholera toxin production in Vibrio cholerae. FEMS Microbiol. Lett. 2010, 306, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Deka, M.; Gogoi, K. Antimicrobial Activity of Chilli Extracts (Capsicum chinense) Against Food Borne Pathogens Escherichia coli and Staphylococcus aureus. Int. J. Res. Anal. Rev. (IJRAR) 2018, 5, 717–720. [Google Scholar]
- Zeyrek, F.Y.; Oguz, E. In vitro activity of capsaicin against Helicobacter pylori. Ann. Microbiol. 2005, 55, 125–127. [Google Scholar]
- Kar, D.; Bandyopadhyay, S.; Dimri, U.; Mondal, D.B.; Nanda, P.K.; Das, A.K.; Batabyal, S.; Dandapat, P.; Bandyopadhyay, S. Antibacterial effect of silver nanoparticles and capsaicin against MDR-ESBL producing Escherichia coli: An in vitro study. Asian Pac. J. Trop. Dis. 2016, 6, 807–810. [Google Scholar] [CrossRef]
- Helms, M.; Ethelberg, S.; Mølbak, K. DT104 Study Group International Salmonella typhimurium DT104 infections, 1992–2001. Emerg. Infect. Dis. 2005, 11, 859. [Google Scholar] [CrossRef]
- Herikstad, H.; Motarjemi, Y.; Tauxe, R. Salmonella surveillance: A global survey of public health serotyping. Epidemiol. Infect. 2002, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, B.O.; Kotsia, E.M.; Stapleton, P.D.; Gibbons, S. Capsaicin and gingerol analogues inhibit the growth of efflux-multidrug resistant bacteria and R-plasmids conjugal transfer. J. Ethnopharmacol. 2019, 245, 111871. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.N.; Chaudhuri, K. Gram-negative bacteria: The cell membranes. In Outer Membrane Vesicles of Bacteria; Springer: Heidelberg/Berlin, Germany, 2012; pp. 15–34. [Google Scholar]
- Sharma, N.; Phan, H.T.; Yoda, T.; Shimokawa, N.; Vestergaard, M.D.C.; Takagi, M. Effects of capsaicin on biomimetic membranes. Biomimetics 2019, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef]
- Omolo, M.A.; Wong, Z.Z.; Mergen, K.; Hastings, J.C.; Le, N.C.; Reil, H.A.; Case, K.A.; Baumler, D.J. Antimicrobial properties of chili peppers. J. Infect. Dis. Ther. 2014, 2, 4. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 2762649. 2014, Ethidium Monoazide Bromide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ethidium-monoazide-bromide (accessed on 1 January 2022).
- Hayman, M.; Kam, P.C. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Lundbaek, J.A.; Birn, P.; Tape, S.E.; Toombes, G.E.; Søgaard, R.; Koeppe, R.E.; Gruner, S.M.; Hansen, A.J.; Andersen, O.S. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol. Pharmacol. 2005, 68, 680–689. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayariga, J.A.; Abugri, D.A.; Amrutha, B.; Villafane, R. Capsaicin Potently Blocks Salmonella typhimurium Invasion of Vero Cells. Antibiotics 2022, 11, 666. https://doi.org/10.3390/antibiotics11050666
Ayariga JA, Abugri DA, Amrutha B, Villafane R. Capsaicin Potently Blocks Salmonella typhimurium Invasion of Vero Cells. Antibiotics. 2022; 11(5):666. https://doi.org/10.3390/antibiotics11050666
Chicago/Turabian StyleAyariga, Joseph A., Daniel A. Abugri, Balagopal Amrutha, and Robert Villafane. 2022. "Capsaicin Potently Blocks Salmonella typhimurium Invasion of Vero Cells" Antibiotics 11, no. 5: 666. https://doi.org/10.3390/antibiotics11050666
APA StyleAyariga, J. A., Abugri, D. A., Amrutha, B., & Villafane, R. (2022). Capsaicin Potently Blocks Salmonella typhimurium Invasion of Vero Cells. Antibiotics, 11(5), 666. https://doi.org/10.3390/antibiotics11050666





