Rhamnolipid Nano-Micelles versus Alcohol-Based Hand Sanitizer: A Comparative Study for Antibacterial Activity against Hospital-Acquired Infections and Toxicity Concerns
Abstract
1. Introduction
2. Results
2.1. Production of Rha(s)
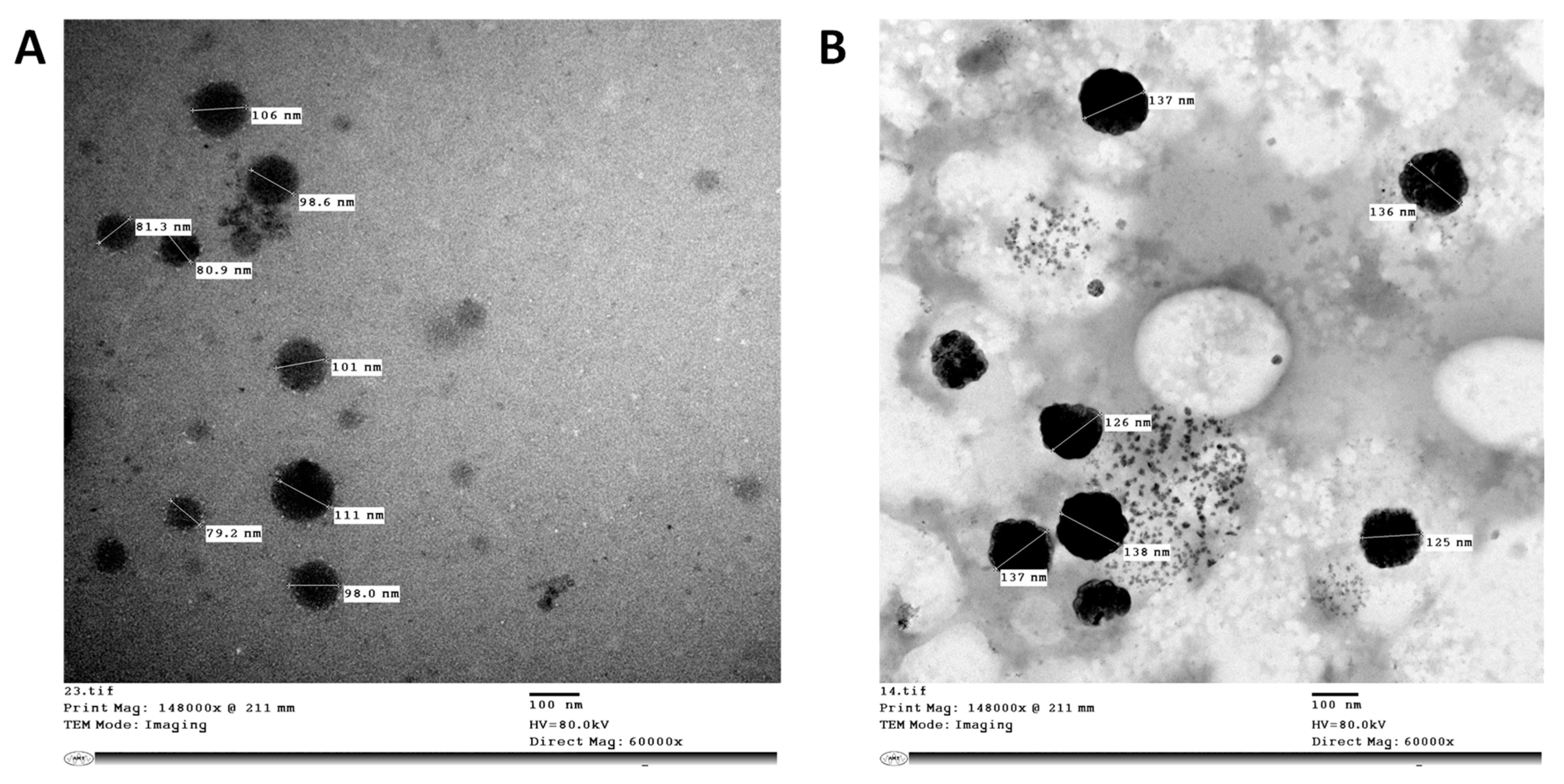
2.2. Preparation and Characterization of Antimicrobial Formulations
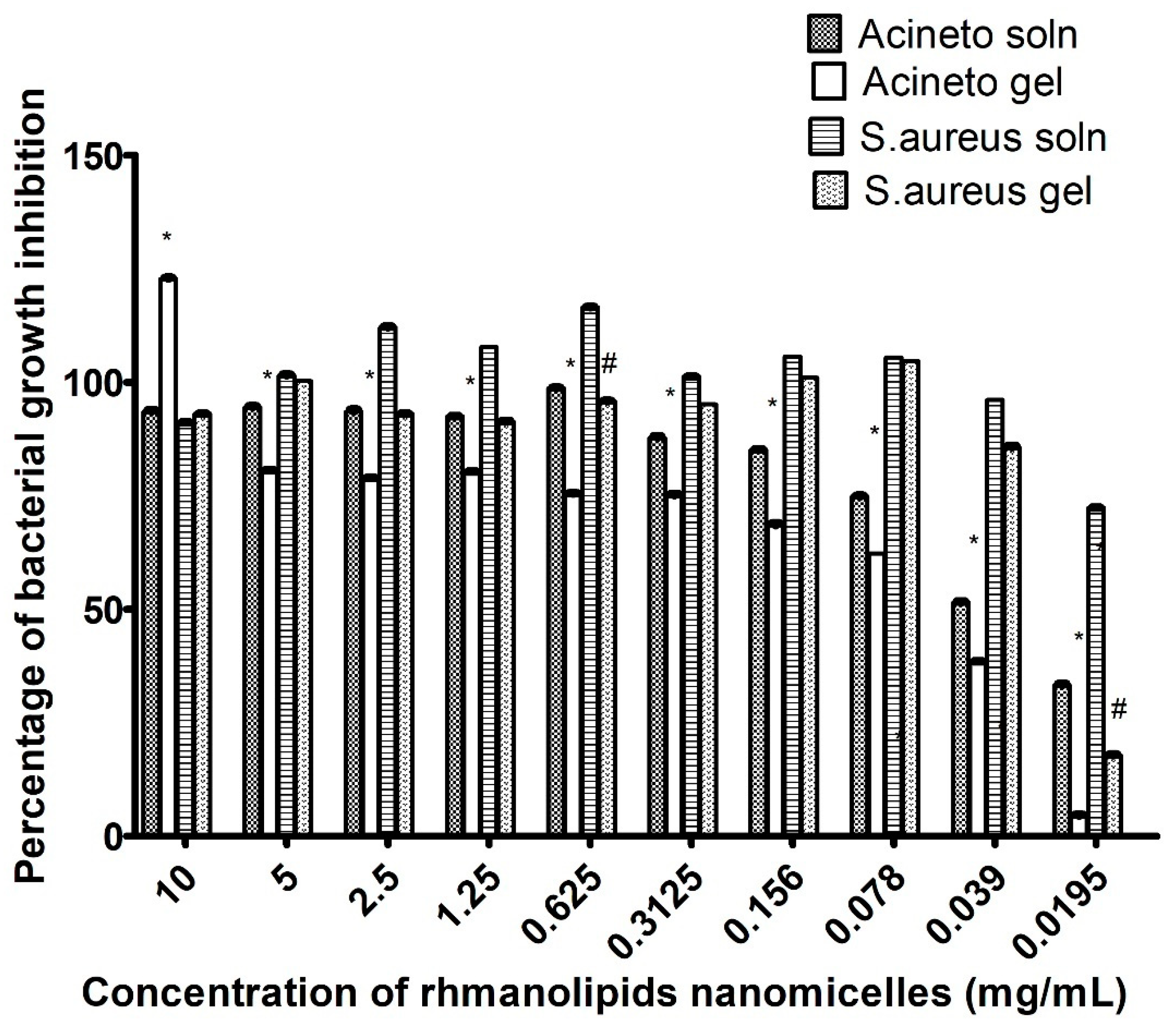
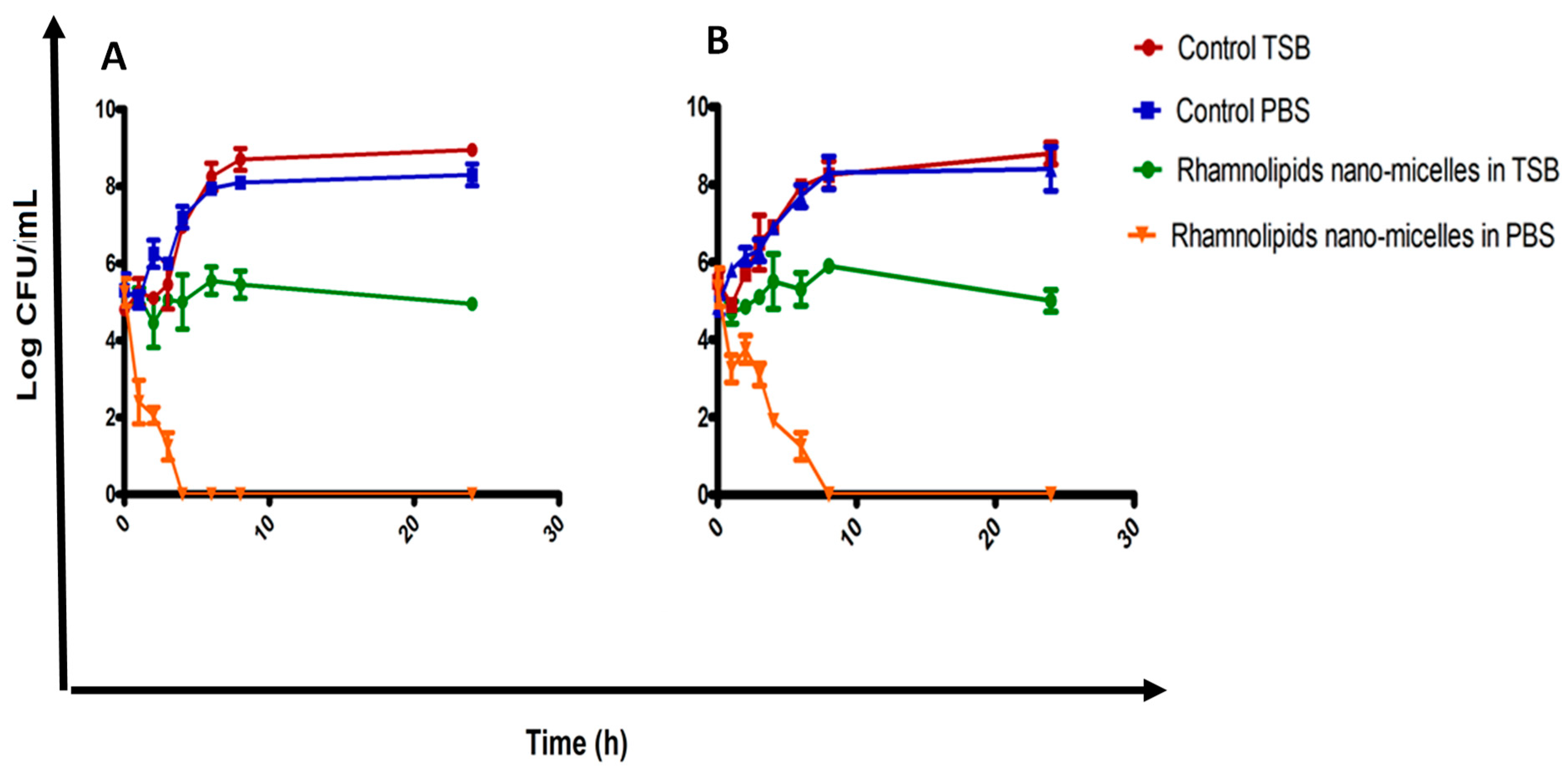
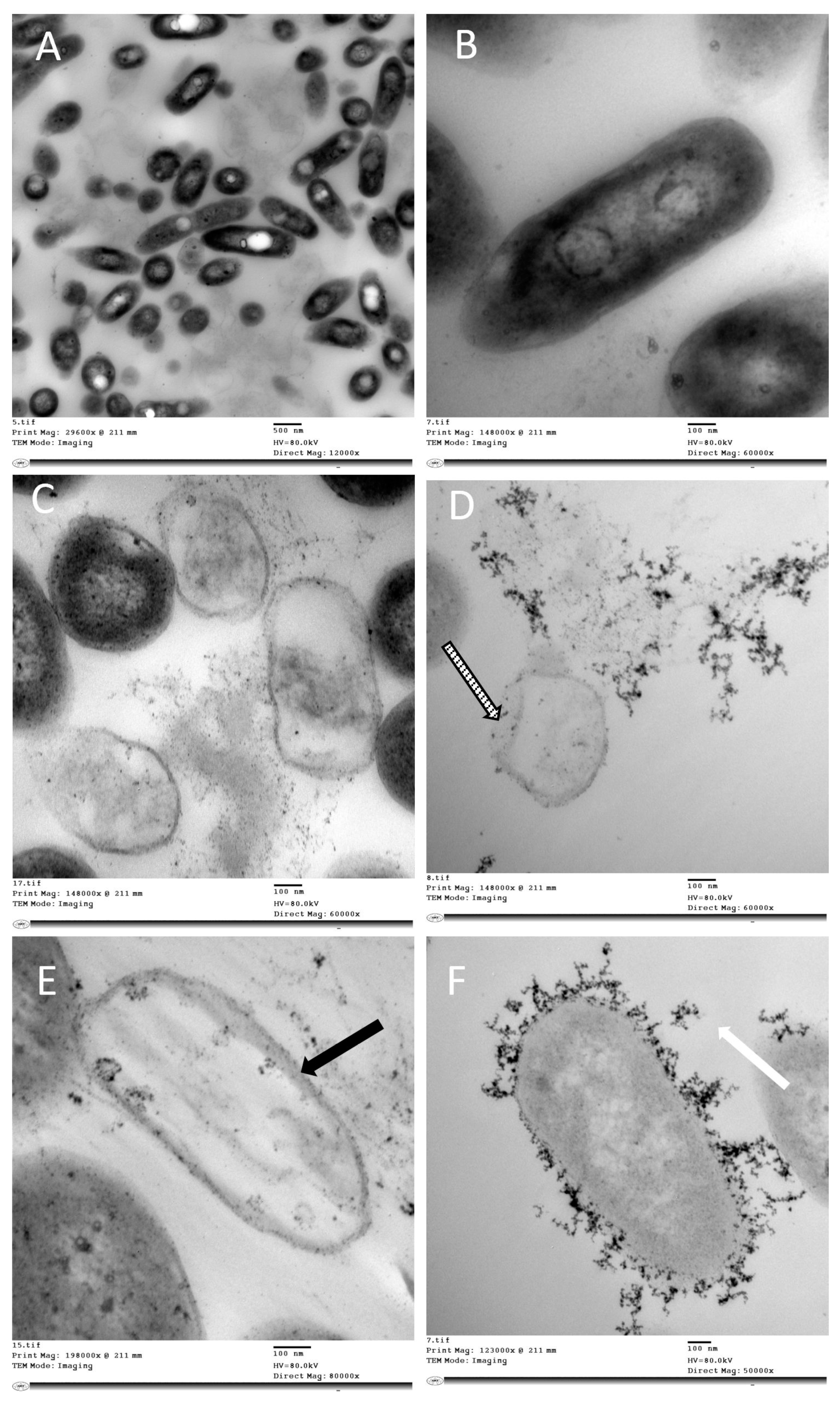
2.3. Determination of Antibacterial Activity
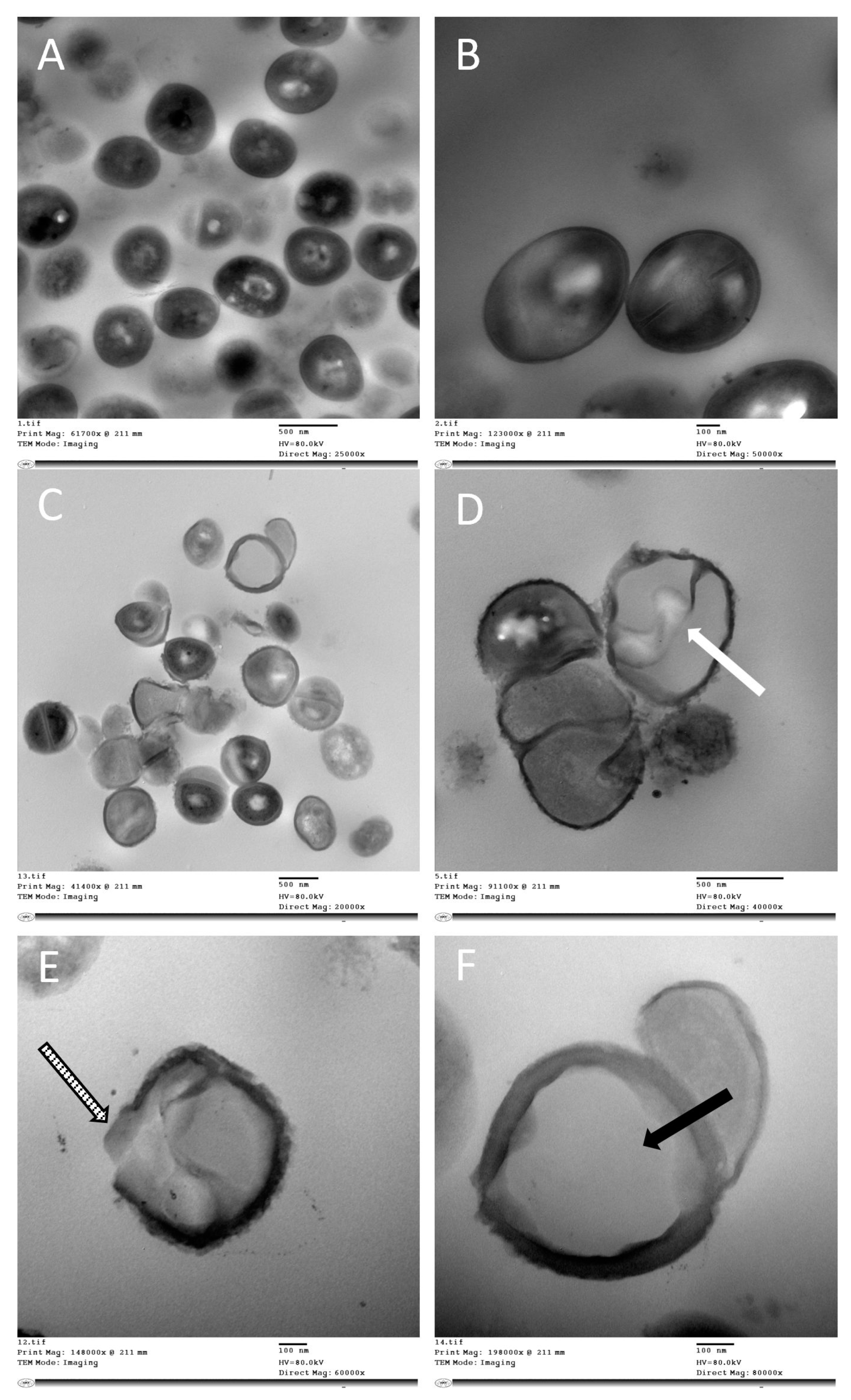
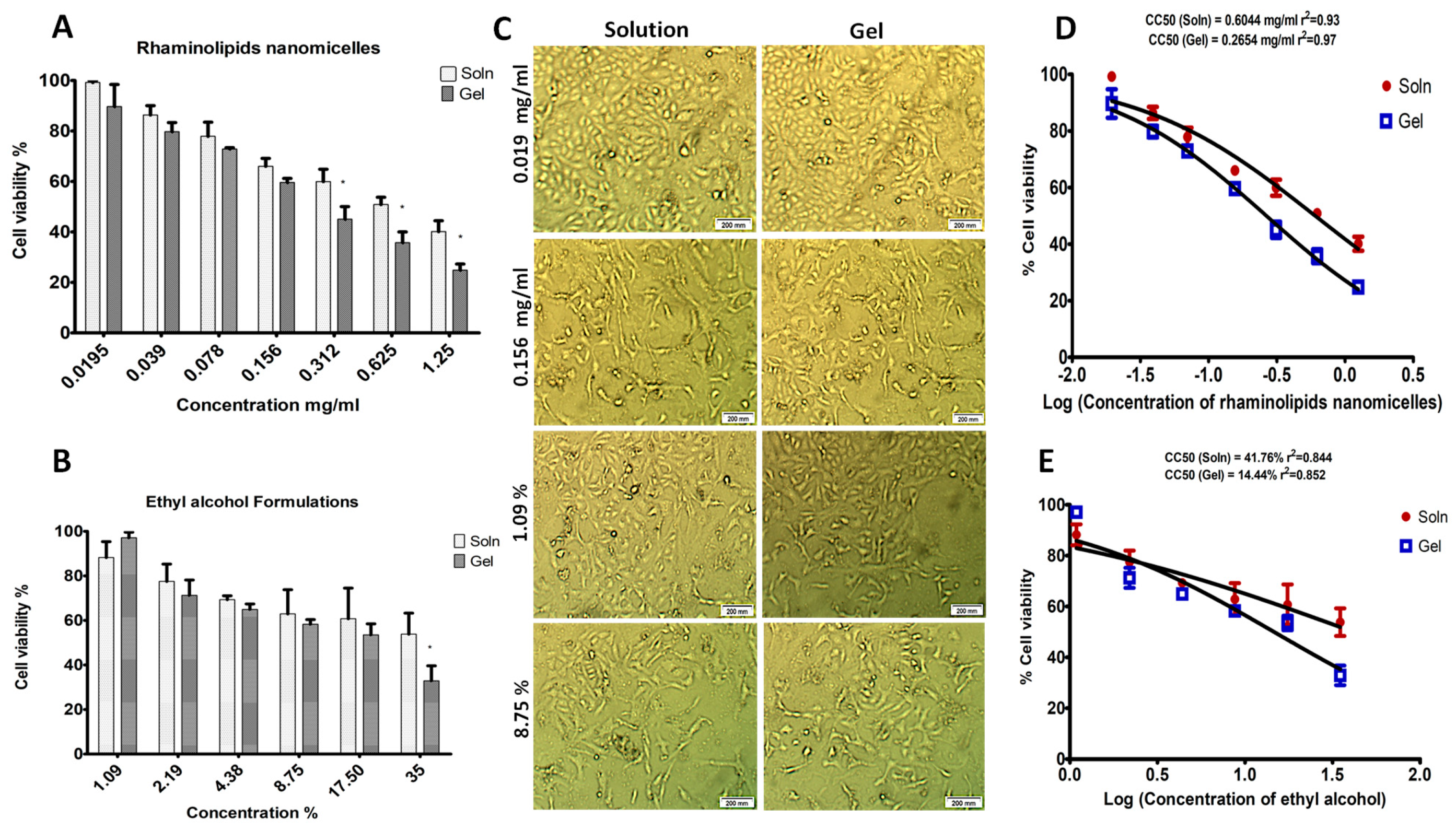
2.4. Cell Viability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methodology
4.2.1. Production of Rhamnolipids
4.2.2. Preparation of Antimicrobial Solutions
4.2.3. Preparation of Antimicrobial Gels
4.2.4. Characterization of Antimicrobial Formulations
Particle Size and Zeta Potential
Transmission Electron Microscopy
Determination of pH and Viscosity
4.2.5. Antimicrobial Activity
Determination of Minimum Inhibitory Concentration (MIC)
Time–Kill Curve Study of the Rhamnolipid Nano-Micelles Solution
Transmission Electron Microscopy Studies
4.2.6. Cytotoxicity Assay
Cell Culture
MTT Cytotoxicity Assay
4.2.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, M.; McKimm, J.; Sartelli, M.; Dhingra, S.; Labricciosa, F.M.; Islam, S.; Jahan, D.; Nusrat, T.; Chowdhury, T.S.; Coccolini, F.; et al. Strategies to prevent healthcare-associated infections: A narrative overview. Risk Manag. Healthc. Policy 2020, 13, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Alothman, A.; Al Thaqafi, A.; Al Ansary, A.; Zikri, A.; Fayed, A.; Khamis, F.; Al Salman, J.; Al Dabal, L.; Khalife, N.; AlMusawi, T.; et al. Prevalence of infections and antimicrobial use in the acute-care hospital setting in the Middle East: Results from the first point-prevalence survey in the region. Int. J. Infect. Dis. 2020, 101, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Talaat, M.; El-Shokry, M.; El-Kholy, J.; Ismail, G.; Kotb, S.; Hafez, S.; Attia, E.; Lessa, F.C. National surveillance of health care–associated infections in Egypt: Developing a sustainable program in a resource-limited country. Am. J. Infect. Control 2016, 44, 1296–1301. [Google Scholar] [CrossRef]
- Ahmad Khan, M. Bacterial Spectrum and Susceptibility patterns of Pathogens in ICU and IMCU of a Secondary Care Hospital in Kingdom of Saudi Arabia. Int. J. Pathol. 2012, 10, 64–70. [Google Scholar]
- Lorina, B.-E.; Abdulrahman, A.A.-S.; Abdullatif, S.A.; Mohammed, S.A.; Afnan, K.A.-B. Antimicrobial susceptibility pattern of Gram negative bacteria isolated from intensive care units in Al-Ahsa, Kingdom of Saudi Arabia. Afr. J. Microbiol. Res. 2018, 12, 747–753. [Google Scholar] [CrossRef][Green Version]
- Al-Hajje, A.; Ezedine, M.; Hammoud, H.; Awada, S.; Rachidi, S.; Zein, S.; Salameh, P. Aspects actuels des infections nosocomiales au centre hospitalier libanais de beyrouth. East. Mediterr. Health J. 2012, 18, 495–500. [Google Scholar] [CrossRef]
- Kanj, S.S.; Kanafani, Z.A.; Sidani, N.; Alamuddin, L.; Zahreddine, N.; Rosenthal, V.D. International nosocomial infection control consortium findings of device-associated infections rate in an intensive care unit of a Lebanese university hospital. J. Glob. Infect. Dis. 2012, 4, 15–21. [Google Scholar] [CrossRef]
- Khan, F.Y.; Elshafie, S.S.; Almaslamani, M.; Abu-Khattab, M.; El Hiday, A.H.; Errayes, M.; Almaslamani, E. Epidemiology of bacteraemia in Hamad general hospital, Qatar: A one year hospital-based study. Travel Med. Infect. Dis. 2010, 8, 377–387. [Google Scholar] [CrossRef]
- Garcell, H.G.; Arias, A.V.; Pancorbo Sandoval, C.A.; García, E.G.; Valle Gamboa, M.E.; Sado, A.B.; Alfonso Serrano, R.N. Incidence and etiology of surgical site infections in appendectomies: A 3-year prospective study. Oman Med. J. 2017, 32, 31–35. [Google Scholar] [CrossRef]
- Al-Mousa, H.H.; Omar, A.A.; Rosenthal, V.D.; Salama, M.F.; Aly, N.Y.; El-Dossoky Noweir, M.; Rebello, F.M.; Narciso, D.M.; Sayed, A.F.; Kurian, A.; et al. Device-associated infection rates, bacterial resistance, length of stay, and mortality in Kuwait: International Nosocomial Infection Consortium findings. Am. J. Infect. Control 2016, 44, 444–449. [Google Scholar] [CrossRef]
- Salama, M.F.; Jamal, W.Y.; Al Mousa, H.; Al-AbdulGhani, K.A.; Rotimi, V.O. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J. Infect. Public Health 2013, 6, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hamam, S.; Sakr, A.; Zahran, W.; El Kholy, R.; Kasemy, Z.; Ibrahem, R.; Sakr, M.; Younis, F. Health care-associated infections at an Egyptian tertiary care hospital: A 2-year prospective study. Menoufia Med. J. 2021, 34, 514. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B.; Sax, H.; Dharan, S.; Pessoa-Silva, C.L.; Donaldson, L.; Boyce, J.M. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect. Dis. 2006, 6, 641–652. [Google Scholar] [CrossRef]
- Bakkar, M.R.; Faraag, A.H.I.; Soliman, E.R.S.; Fouda, M.S.; Sarguos, A.M.M.; McLean, G.R.; Hebishy, A.M.S.; Elkhouly, G.E.; Raya, N.R.; Abo-zeid, Y. Rhamnolipids Nano-Micelles as a Potential Hand Sanitizer. Antibiotics 2021, 10, 751. [Google Scholar] [CrossRef]
- Emami, A.; Javanmardi, F.; Keshavarzi, A.; Pirbonyeh, N. Hidden threat lurking behind the alcohol sanitizers in COVID-19 outbreak. Dermatol. Ther. 2020, 33, 17–19. [Google Scholar] [CrossRef]
- Onyedibe, K.I.; Shehu, N.Y.; Pires, D.; Isa, S.E.; Okolo, M.O.; Gomerep, S.S.; Ibrahim, C.; Igbanugo, S.J.; Odesanya, R.U.; Olayinka, A.; et al. Assessment of hand hygiene facilities and staff compliance in a large tertiary health care facility in northern Nigeria: A cross sectional study. Antimicrob. Resist. Infect. Control 2020, 9, 30. [Google Scholar] [CrossRef]
- Chiang, W.L.; Chen, T.W.; Liu, M.Y.; Hsu, C.J. Application and robust H control of PDC fuzzy controller for nonlinear systems with external disturbance. J. Mar. Sci. Technol. 2001, 9, 84–90. [Google Scholar] [CrossRef]
- Slaughter, R.J.; Mason, R.W.; Beasley, D.M.G.; Vale, J.A.; Schep, L.J. Isopropanol poisoning. Clin. Toxicol. 2014, 52, 470–478. [Google Scholar] [CrossRef]
- Mahmood, A.; Eqan, M.; Pervez, S.; Ahmed, H.; Bari, A. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci. Total Environ. 2020, 742, 140561. [Google Scholar] [CrossRef]
- Vogel, L. Hand sanitizers may increase norovirus risk. CMAJ 2011, 183, 799–800. [Google Scholar] [CrossRef][Green Version]
- Blaney, D.D.; Daly, E.R.; Kirkland, K.B.; Tongren, J.E.; Kelso, P.T.; Talbot, E.A. Use of alcohol-based hand sanitizers as a risk factor for norovirus outbreaks in long-term care facilities in northern New England: December 2006 to March 2007. Am. J. Infect. Control 2011, 39, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Pidot, S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R.; Carter, G.P.; Lee, J.Y.H.; Lam, M.M.C.; Grayson, M.L.; Ballard, S.A.; et al. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci. Transl. Med. 2018, 10, eaar6115. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Munnawar, F. Antibacterial Effectiveness of Commercially Available Hand Sanitizers. Int. J. Biol. Biotechnol. 2016, 13, 427–431. [Google Scholar]
- Müller, M.M.; Hörmann, B.; Syldatk, C.; Hausmann, R. Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl. Microbiol. Biotechnol. 2010, 87, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Li, Q. Microbial production of rhamnolipids: Opportunities, challenges and strategies. Microb. Cell Fact. 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Kaskatepe, B.; Yildiz, S.; Gumustas, M.; Ozkan, S.A. Biosurfactant production by pseudomonas aeruginosa in kefir and fish meal. Brazilian J. Microbiol. 2015, 46, 855–859. [Google Scholar] [CrossRef]
- Henkel, M.; Geissler, M.; Weggenmann, F.; Hausmann, R. Production of microbial biosurfactants: Status quo of rhamnolipid and surfactin towards large-scale production. Biotechnol. J. 2017, 12, 1600561. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 1–18. [Google Scholar] [CrossRef]
- Eslami, P.; Hajfarajollah, H.; Bazsefidpar, S. Recent advancements in the production of rhamnolipid biosurfactants byPseudomonas aeruginosa. RSC Adv. 2020, 10, 34014–34032. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Invally, K.; Sancheti, A.; Ju, L.K. A new approach for downstream purification of rhamnolipid biosurfactants. Food Bioprod. Process. 2019, 114, 122–131. [Google Scholar] [CrossRef]
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausmann, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Tiso, T.; Thies, S.; Müller, M.; Tsvetanova, L.; Carraresi, L.; Bröring, S.; Jaeger, K. Rhamnolipids: Production, Performance, and Application; Springer: Cham, Germany, 2017; ISBN 9783319504360. [Google Scholar]
- Innovation, C.; Vice, E.; Middle, P. Evonik Builds World’s First Industrial-Scale Production Plant for Rhamnolipids; Evonik Industries AG: Essen, Germany, 2022; pp. 1–3. [Google Scholar]
- Abdelati, A.A.; Sultan, E.A. Incidence and characteristics of health care-associated infection in hospitalized patients with rheumatic diseases in Alexandria Main University Hospital. Egypt. Rheumatol. Rehabil. 2018, 45, 148–152. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, D.B.; Kim, J.I.; Kim, P.Y. In vitro cytotoxicity tests on cultured human skin fibroblasts to predict skin irritation potential of surfactants. Toxicol. Vitr. 2000, 14, 345–349. [Google Scholar] [CrossRef]
- Sotirova, A.; Spasova, D.; Vasileva-Tonkova, E.; Galabova, D. Effects of rhamnolipid-biosurfactant on cell surface of Pseudomonas aeruginosa. Microbiol. Res. 2009, 164, 297–303. [Google Scholar] [CrossRef]
- Raka, L.; Mulliqi-Osmani, G. Infection Control in Developing World. In Infection Control; Sudhakar, C., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 64–78. [Google Scholar]
- Abo-zeid, Y.; Williams, G.R. The potential anti-infective applications of metal oxide nanoparticles: A systematic review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, e1592. [Google Scholar] [CrossRef]
- Quinn, S.C.; Kumar, S. Health inequalities and infectious disease epidemics: A challenge for global health security. Biosecur. Bioterror. 2014, 12, 263–273. [Google Scholar] [CrossRef]
- Yi, Y.; Lagniton, P.N.P.; Ye, S.; Li, E.; Xu, R.H. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020, 16, 1753–1766. [Google Scholar] [CrossRef]
- Ahmad, T.; Haroon; Dhama, K.; Sharun, K.; Khan, F.M.; Ahmed, I.; Tiwari, R.; Musa, T.H.; Khan, M.; Bonilla-Aldana, D.K.; et al. Biosafety and biosecurity approaches to restrain/contain and counter SARS-CoV-2/COVID-19 pandemic: A rapid-review. Turk. J. Biol. 2020, 44, 132–145. [Google Scholar] [CrossRef]
- Haque, M. The COVID-19 pandemic-A global public health crisis: A brief overview regarding pharmacological interventions. Pesqui. Bras. Odontopediatria Clin. Integr. 2020, 20, e109059. [Google Scholar] [CrossRef]
- Haque, M. Handwashing in averting infectious diseases: Relevance to COVID-19. J. Popul. Ther. Clin. Pharmacol. 2020, 27, e37–e52. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Islam, S.; Iqbal, S.; Urmi, U.L.; Kamal, Z.M.; Rahman, A.; Kamal, M.; Haque, M.; Jahan, I.; Islam, Z.; et al. Availability and price changes of potential medicines and equipment for the prevention and treatment of COVID-19 among pharmacy and drug stores in bangladesh; findings and implications. Bangladesh J. Med. Sci. 2020, 19, S36–S50. [Google Scholar] [CrossRef]
- Haque, M. Combating COVID-19: A coordinated efforts of healthcare providers and policy makers with global participation are needed to achieve the desired goals. Bangladesh J. Med. Sci. 2020, 19, S1–S5. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Mantovani, G.; Irving, W.L.; Garnett, M.C. Synthesis of nucleoside-boronic esters hydrophobic pro-drugs: A possible route to improve hydrophilic nucleoside drug loading into polymer nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 46, 354–364. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Williams, G.R.; Touabi, L.; Mclean, G.R. An investigation of rhinovirus infection on cellular uptake of poly (glycerol-adipate) nanoparticles. Int. J. Pharm. 2020, 589, 119826. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.; McLean, G.R.; Hamdy, N.M. A Molecular Docking Study Repurposes FDA Approved Iron Oxide Nanoparticles to Treat and Control COVID-19 Infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Urbanowicz, R.A.; Thomson, B.J.; William, L.; Tarr, A.W.; Garnett, M.C. Enhanced Nanoparticle Uptake into Virus Infected Cells: Could Nanoparticles be useful in antiviral therapy? Int. J. Pharm. 2018, 547, 572–581. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Garnett, M.C. Polymer nanoparticle as a delivery system for ribavirin: Do nanoparticle avoid uptake by Red Blood Cells? J. Drug Deliv. Sci. Technol. 2020, 56, 101552. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Amer, A.; El-Houssieny, B.; Mahmoud, M.; Sakran, W. Overview on bacterial resistance and nanoparticles to overcome bacterial resistance. J. Adv. Pharm. Res. 2021, 5, 312–326. [Google Scholar] [CrossRef]
- Israel Nii-Trebi, N. Emerging and Neglected Infectious Diseases: Insights, Advances, and Challenges. Biomed Res. Int. 2017, 2017, 5245021. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.A.; Eman, M.; Ishijima, H.; Noriyuki, M. Health Sector Cooperation Planning Survey in Arab Republic of Egypt Final Report; Japan International Cooperation Agency: Cairo, Egypt, 2017.
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Pittet, D.; Hugonnet, S.; Harbarth, S.; Mourouga, P.; Sauvan, V.; Touveneau, S.; Perneger, T.V. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Lancet 2000, 356, 1307–1312. [Google Scholar] [CrossRef]
- Garner, J.S.; Favero, M.S. Guideline for handwashing and hospital environmental control, 1985 supersedes guideline for hospital environmental control published in 1981. Am. J. Infect. Control 1986, 14, 110–126. [Google Scholar] [CrossRef]
- Pugliese, G. Recommendations for Preventing the Spread of Vancomycin Resistance. Infect. Control Hosp. Epidemiol. 1995, 16, 498. [Google Scholar] [CrossRef]
- Burgess, K.; Li, H.; Abo-Zeid, Y.; Fatimah; Williams, G.R. The effect of molecular properties on active ingredient release from electrospun eudragit fibers. Pharmaceutics 2018, 10, 103. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Abo-zeid, Y.; Bear, J.C.; Davies, G.; Lei, X.; Williams, G.R. SiO2-coated layered gadolinium hydroxides for simultaneous drug delivery and magnetic resonance imaging. J. Solid State Chem. 2020, 286, 121291. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Sai, K.M.; Nalluru, S.; Sampath, S.K. Nanotechnology: An emerging approach to combat COVID-19. Emergent Mater. 2021, 4, 119–130. [Google Scholar] [CrossRef]
- Rangayasami, A.; Kannan, K.; Murugesan, S.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Influence of nanotechnology to combat against COVID-19 for global health emergency: A review. Sensors Int. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Pereira, A.E.S.; De Oliveira, J.L.; Carvalho, L.B.; Guilger-Casagrande, M.; De Lima, R.; Fraceto, L.F. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnol. 2020, 18, 125. [Google Scholar] [CrossRef]
- Hashim, F.; El-Ridy, M.; Nasr, M.; Abdallah, Y. Preparation and characterization of niosomes containing ribavirin for liver targeting. Drug Deliv. 2010, 17, 282–287. [Google Scholar] [CrossRef] [PubMed]
- AlMatar, M.; Makky, E.A.; Var, I.; Koksal, F. The Role of Nanoparticles in the Inhibition of Multidrug-resistant Bacteria and Biofilms. Curr. Drug Deliv. 2017, 15, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria-“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Ejobi, F.; Tweyongyere, R.; Lubwama, M.; Kirabira, J.B.; Wampande, E.M. Nanotechnological solutions for controlling transmission and emergence of antimicrobial-resistant bacteria, future prospects, and challenges: A systematic review. J. Nanopart. Res. 2020, 22, 117. [Google Scholar] [CrossRef]
- Rosenberg, E.; DeLong, E.F.; Thompson, F.; Lory, S.; Stackebrandt, E. The prokaryotes: Applied bacteriology and biotechnology. Prokaryotes Appl. Bacteriol. Biotechnol. 2013, 9783642313, 1–393. [Google Scholar] [CrossRef]
- Kumar, R.; Das, A.J. Rhamnolipid Biosurfactant: Recent Trends in Production and Application; Springer: Singapore, 2018; ISBN 9789811312892. [Google Scholar]
- Johann, S.; Seiler, T.B.; Tiso, T.; Bluhm, K.; Blank, L.M.; Hollert, H. Mechanism-specific and whole-organism ecotoxicity of mono-rhamnolipids. Sci. Total Environ. 2016, 548–549, 155–163. [Google Scholar] [CrossRef]
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef]
- Lotfabad, T.B.; Shahcheraghi, F.; Shooraj, F. Assessment of antibacterial capability of rhamnolipids produced by two indigenous Pseudomonas aeruginosa strains. Jundishapur J. Microbiol. 2013, 6, 29–35. [Google Scholar] [CrossRef]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 2017, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ouyang, W.-Q.; Wei, Y.-P.; Faraz Syed, S.; Hao, C.; Wang, B.-Z.; Shang, Y. effects of carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int. J. Nanomed. 2016, 11, 5971–5987. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv. 2016, 23, 652–667. [Google Scholar] [CrossRef]
- Tan, Y.T.F.; Peh, K.K.; Al-Hanbali, O. Investigation of interpolymer complexation Carbopol and various grades of polyvinylpyrrolidone and effects on adhesion strength and swelling properties. J. Pharm. Pharm. Sci. 2001, 4, 7–14. [Google Scholar]
- Putri, D.C.A.; Dwiastuti, R.; Marchaban, M.; Nugroho, A.K. Optimization of mixing temperature and sonication duration in liposomes preparation. J. Pharm. Sci. Community 2017, 14, 79–85. [Google Scholar] [CrossRef]
- Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Landucci, E.; Bilia, A.R.; Bergonzi, M.C. Formulation of nanomicelles to improve the solubility and the oral absorption of silymarin. Molecules 2019, 24, 1688. [Google Scholar] [CrossRef]
- Das, P.; Yang, X.P.; Ma, L.Z. Analysis of biosurfactants from industrially viable Pseudomonas strain isolated from crude oil suggests how rhamnolipids congeners affect emulsification property and antimicrobial activity. Front. Microbiol. 2014, 5, 696. [Google Scholar] [CrossRef]
- Diviyagahage, C.M.; Thuvaragan, S.; Gnanakarunyan, T.J.; Srikaran, R. Formulation and Characterization of Essential Oils Based Antibacterial Hand Sanitizer Gels. Pharm. J. Sri Lanka 2021, 11, 14. [Google Scholar] [CrossRef]
- Surini, S.; Amirtha, N.I.; Lestari, D.C. Formulation and effectiveness of a hand sanitizer gel produced using Salam bark extract. Int. J. Appl. Pharm. 2018, 10, 216–220. [Google Scholar] [CrossRef][Green Version]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Aodah, A.H.; Bakr, A.A.; Booq, R.Y.; Rahman, M.J.; Alzahrani, D.A.; Alsulami, K.A.; Alshaya, H.A.; Alsuabeyl, M.S.; Alyamani, E.J.; Tawfik, E.A. Preparation and evaluation of benzalkonium chloride hand sanitizer as a potential alternative for alcohol-based hand gels. Saudi Pharm. J. 2021, 29, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Booq, R.Y.; Alshehri, A.A.; Almughem, F.A.; Zaidan, N.M.; Aburayan, W.S.; Bakr, A.A.; Kabli, S.H.; Alshaya, H.A.; Alsuabeyl, M.S.; Alyamani, E.J.; et al. Formulation and evaluation of alcohol-free hand sanitizer gels to prevent the spread of infections during pandemics. Int. J. Environ. Res. Public Health 2021, 18, 6252. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Danby, S.G.; Cork, M.J. PH in Atopic Dermatitis. Curr. Probl. Dermatol. 2018, 54, 95–107. [Google Scholar] [CrossRef]
- Panther, D.J.; Jacob, S.E. The importance of acidification in atopic eczema: An underexplored avenue for treatment. J. Clin. Med. 2015, 4, 970–978. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.; Kamalanathan, I.D.; Martin, P.J. Comparative study of the production of rhamnolipid biosurfactants by B. thailandensis E264 and P. aeruginosa ATCC 9027 using foam fractionation. Process Biochem. 2016, 51, 820–827. [Google Scholar] [CrossRef]
- Bharali, P.; Saikia, J.P.; Ray, A.; Konwar, B.K. Rhamnolipid (RL) from Pseudomonas aeruginosa OBP1: A novel chemotaxis and antibacterial agent. Colloids Surfaces B Biointerfaces 2013, 103, 502–509. [Google Scholar] [CrossRef]
- Chojnacki, M.; Dobrotka, C.; Osborn, R.; Johnson, W.; Young, M.; Meyer, B.; Laskey, E.; Wozniak, R.A.F.; Dewhurst, S.; Dunma, P.M. Evaluating the Antimicrobial Properties of Commercial Hand Sanitizers. Am. Soc. Microbiol. 2021, 6, e00062-21. [Google Scholar] [CrossRef]
- Abbasi, H.; Noghabi, K.A.; Hamedi, M.M.; Zahiri, H.S.; Moosavi-Movahedi, A.A.; Amanlou, M.; Teruel, J.A.; Ortiz, A. Physicochemical characterization of a monorhamnolipid secreted by Pseudomonas aeruginosa MA01 in aqueous media. An experimental and molecular dynamics study. Colloids Surfaces B Biointerfaces 2013, 101, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Gilligan, P.; Jaynes, J.; Henke, D. In vitro activities of designed antimicrobial peptides against multidrug-resistant cystic fibrosis pathogens. Antimicrob. Agents Chemother. 1999, 43, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Rikalović, M.G.; Gojgić-Cvijović, G.; Vrvić, M.M.; Karadžić, I. Production and characterization of rhamnolipids from Pseudomonas aeruginosa san-ai. J. Serbian Chem. Soc. 2012, 77, 27–42. [Google Scholar] [CrossRef]
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb. Cell Fact. 2021, 20, 1. [Google Scholar] [CrossRef]
- Jing, J.L.J.; Yi, T.P.; Bose, R.J.C.; McCarthy, J.R.; Tharmalingam, N.; Madheswaran, T. Hand sanitizers: A review on formulation aspects, adverse effects, and regulations. Int. J. Environ. Res. Public Health 2020, 17, 3326. [Google Scholar] [CrossRef]
- Suchomel, M.; Rotter, M. Ethanol in pre-surgical hand rubs: Concentration and duration of application for achieving European Norm EN 12791. J. Hosp. Infect. 2011, 77, 263–266. [Google Scholar] [CrossRef]
- Hall, T.J.; Wren, M.W.D.; Jeanes, A.; Gant, V.A. A comparison of the antibacterial efficacy and cytotoxicity to cultured human skin cells of 7 commercial hand rubs and Xgel, a new copper-based biocidal hand rub. Am. J. Infect. Control 2009, 37, 322–326. [Google Scholar] [CrossRef]
- Lydon, H.L.; Baccile, N.; Callaghan, B.; Marchant, R.; Mitchell, C.A.; Banat, I.M. Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing. Antimicrob. Agents Chemother. 2017, 61, e02547-16. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Preservative and irritant capacity of biosurfactants from different sources: A comparative study. J. Pharm. Sci. 2019, 108, 2296–2304. [Google Scholar] [CrossRef]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial biosurfactants in cosmetic and personal skincare pharmaceutical formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ohadi, M.; Forootanfar, H.; Rahimi, H.R.; Jafari, E.; Shakibaie, M.; Eslaminejad, T.; Dehghannoudeh, G. Antioxidant potential and wound healing activity of biosurfactant produced by Acinetobacter junii B6. Curr. Pharm. Biotechnol. 2017, 18, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.; Pasirayi, G.; Auger, V.; Ali, Z. Production of rhamnolipid biosurfactants by Pseudomonas aeruginosa DS10-129 in a microfluidic bioreactor. Biotechnol. Appl. Biochem. 2010, 55, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A.H. Characterization of rhamnolipid produced by pseudomonas aeruginosa isolate Bs20. Appl. Biochem. Biotechnol. 2009, 157, 329–345. [Google Scholar] [CrossRef]
- Ubaid, M.; Ilyas, S.; Mir, S.; Khan, A.K.; Rashid, R.; Khan, M.Z.U.; Kanwal, Z.G.; Nawaz, A.; Shah, A.; Murtaza, G. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An. Acad. Bras. Cienc. 2016, 88, 2303–2317. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, H.; Rohilla, A.; Rathee, P.; Kumar, V. Optimization and formulation design of carbopol loaded Piroxicam gel using novel penetration enhancers. Int. J. Biol. Macromol. 2013, 55, 246–253. [Google Scholar] [CrossRef]
- Osei-Asare, C.; Eshun Oppong, E.; Apenteng, J.A.; Adi-Dako, O.; Kumadoh, D.; Akosua, A.A.; Ohemeng, K.A. Managing Vibrio cholerae with a local beverage: Preparation of an affordable ethanol based hand sanitizer. Heliyon 2020, 6, e03105. [Google Scholar] [CrossRef]
- Vilka, I.; Skudra, L. Development and analysis of the hand alcohol disinfectant. In Proceedings of the 3rd Baltic Conference on Food Science and Technology FOODBALT-2008, Jelgava, Latvia, 17–18 April 2008; pp. 36–40. [Google Scholar]
- Padsalgi, A.; Jain, D.; Bidkar, S.; Harinarayana, D.; Jadhav, V. Preparation and evaluation of hand rub disinfectant. Asian J. Pharm. 2008, 2, 18. [Google Scholar] [CrossRef]
- CLSI. M100-ED29; Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019.
- ASTM-E2315; Standard Guide for Assessment of Antimicrobial Activity Using a Time-Kill Procedure. ASTM International: West Conshohocken, PA, USA, 2012.
| Concentration (mg/mL) | Rhamnolipid Nano-Micelles Solution | Rhamnolipid Nano-Micelles Gel | ||
|---|---|---|---|---|
| * Particle Size (nm) ± SD (PDI) | Zeta Potential (mv) ± SD | Particle Size (nm) ± SD (PDI) | Zeta Potential (mv) ± SD | |
| 10 | 129 ± 4.41 (0.25) | −67.97 ± 2.56 | 263 ± 19.13 (0.29) | −38.03 ± 9.24 |
| 5 | 165 ± 0.97 (0.30) | −61.57 ± 3.23 | 265 ± 1.37 (0.36) | −35.13 ± 3.23 |
| 2.5 | 169 ± 1.81 (0.267 | −78.67 ± 8.61 | 310 ± 9.72 (0.38) | −42.53 ± 4.39 |
| 1.25 | 183 ± 23.08 (0.36) | −75.43 ± 4.74 | 308 ± 13.37 (0.47) | −39.83 ± 0.70 |
| 0.625 | 206 ± 5.23 (0.35) | −66.12 ± 5.60 | 206 ± 8.00 (0.45) | −43.43 ± 2.55 |
| 0.313 | 188 ± 46.98 (0.36) | −41.57 ± 13.70 | 193 ± 8.74 (0.56) | −36.00 ± 1.15 |
| 0.156 | 166 ± 21.70 (0.46) | −61.17 ± 3.59 | 215 ± 13.60 (0.39) | −33.23 ± 1.04 |
| 0.078 | 265 ± 33.56 (0.30) | −35.23 ± 4.32 | 192 ± 2.94 (0.47) | −33.93 ± 3.20 |
| 0.039 | 204 ± 12.54 (0.25) | −45.20 ± 3.08 | 170 ± 1.00 (0.46) | −36.43 ± 0.70 |
| 0.0195 | 191 ± 22.16 (0.33) | −47.93 ± 1.81 | 195 ± 17.44 (0.45) | −37.77 ± 1.96 |
| Bacteria | Rhamnolipid Nano-Micelles Solution | Rhamnolipid Nano-Micelles Gel | ||
|---|---|---|---|---|
| MIC (mg/mL) | Cell Viability% ± SD | MIC (mg/mL) | Cell Viability% ± SD | |
| S. aureus | 0.039 | 86.5 ± 3.69 | 0.078 | 72.8 ±0.5 |
| A. baumannii | 0.312 | 60 ± 4.9 | 0.625 | 35.7 ± 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo-zeid, Y.; Bakkar, M.R.; Elkhouly, G.E.; Raya, N.R.; Zaafar, D. Rhamnolipid Nano-Micelles versus Alcohol-Based Hand Sanitizer: A Comparative Study for Antibacterial Activity against Hospital-Acquired Infections and Toxicity Concerns. Antibiotics 2022, 11, 605. https://doi.org/10.3390/antibiotics11050605
Abo-zeid Y, Bakkar MR, Elkhouly GE, Raya NR, Zaafar D. Rhamnolipid Nano-Micelles versus Alcohol-Based Hand Sanitizer: A Comparative Study for Antibacterial Activity against Hospital-Acquired Infections and Toxicity Concerns. Antibiotics. 2022; 11(5):605. https://doi.org/10.3390/antibiotics11050605
Chicago/Turabian StyleAbo-zeid, Yasmin, Marwa Reda Bakkar, Gehad E. Elkhouly, Nermeen R. Raya, and Dalia Zaafar. 2022. "Rhamnolipid Nano-Micelles versus Alcohol-Based Hand Sanitizer: A Comparative Study for Antibacterial Activity against Hospital-Acquired Infections and Toxicity Concerns" Antibiotics 11, no. 5: 605. https://doi.org/10.3390/antibiotics11050605
APA StyleAbo-zeid, Y., Bakkar, M. R., Elkhouly, G. E., Raya, N. R., & Zaafar, D. (2022). Rhamnolipid Nano-Micelles versus Alcohol-Based Hand Sanitizer: A Comparative Study for Antibacterial Activity against Hospital-Acquired Infections and Toxicity Concerns. Antibiotics, 11(5), 605. https://doi.org/10.3390/antibiotics11050605







