Effectiveness of Different Chemotherapeutic Agents for Decontamination of Infected Dental Implant Surface: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rationale and Focused Question for Review
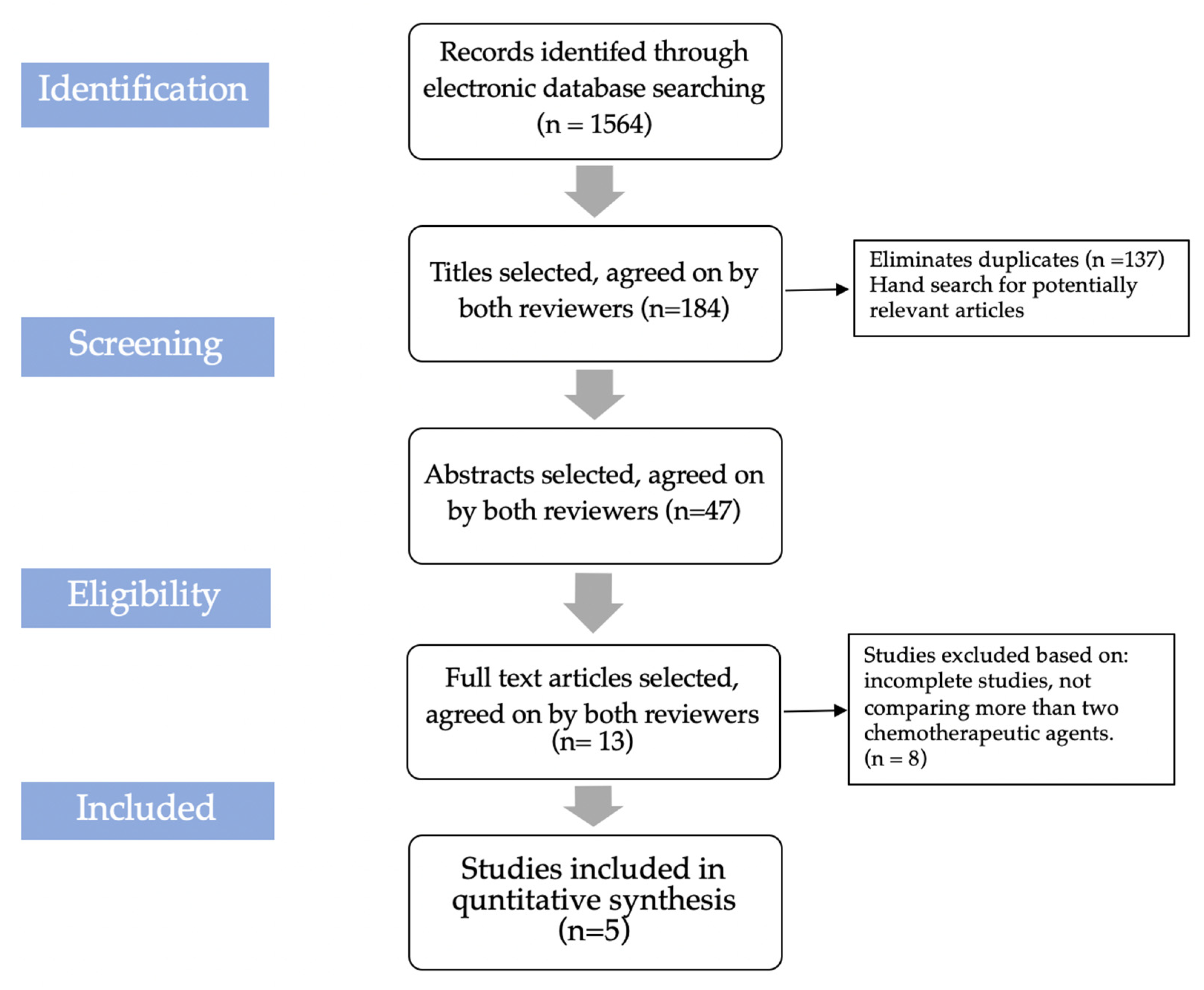
2.2. Sources of Information and Search Strategy
2.3. Study Selection Criteria
2.4. Eligibility Criteria
2.5. Primary and Secondary Outcomes
2.6. Screening and Data Extraction
2.7. Quality Assessment
2.8. Statistical Analysis
3. Results
| Author (Year, with/without Mechanical Cleaning) | Implant Surface Contaminated with | Chemical Used (Application Form, Time)/Type of Intervention | Sample Size | Outcome Measure/Primary Endpoint | Conclusion/Outcome |
|---|---|---|---|---|---|
| Gosau M et al. [19] (2010, without)in vivo | Oral biofilm | 1% sodium hypochlorite (NaOCL) 3% H2O20.2% CHX gluconate Plax (Triclosan 0.3%) Listerine cool mint (alcohol based) Citric acid (Ph 1, 40%) PBS solution(control) (in liquid form, for 1 min) | 8 8 8 8 8 8 8 | The proportion of live/dead bacterial cell | All six antimicrobial agents were effective in reducing oral bacterial biofilm on titanium disc, compared to control. All (except Plax, 40%) showed a significant bactericidal effect on adhering bacteria. |
| Ntrouka et al. [20] (2011, without) In-vitro | 1-streptococcus mutans | 24% EDTA 40% Citric acid 10% H2O2 Ardox-X 0.07% CPC 0.2% CHX digluconate Sterile water(control) (in liquid form, for 5 min) | 6 6 6 6 6 6 6 | 1. Total CFU count 2. Protein measurement (µg) | H2O2, Ardox-X, 40% citric acid Were most effective & Ardox-X, 40% citric acid were most potent in killing streptococcus mutans. 40% citric acid was most effective in bacterial killing, the addition of H2O2,/Ardox-X to Citric acid didn’t have a significant effect |
| 2-saliva to grow polymicrobial biofilm | 40% Citric acid (5 min) Ardox-X (5 min) 10% H2O2 (5 min) Ardox-X then Citric acid (2.5 min each) 10% H2O2 then Citric acid (2.5 min each) | 6 6 6 6 6 | |||
| R Burgers et al. [21] (2012, without) In-vitro | Staphylococcus epidermis | 1% sodium hypochlorite 3% H2O20.2% CHX gluconate Plax (Triclosan 0.3%) Listerine Citric acid (Ph 1, 40%) Saline(control) (in liquid form, for 60 s) (The chemicals used are not categorized well please arrange) | 35 | The proportion of live/dead bacterial cell | Only sodium hypochlorite (1%) was effective against all 3 species. Whereas H2O2 only against Candida albicans. CHX gluconate (0.2%) & Listerine against Candida albicans and Streptococcus sanguis. Plax (0.3%) against Streptococcus sanguis and Staphylococcus epidermis. |
| Candida albicans | 35 | ||||
| Streptococcus sanguis | 35 | ||||
| Georgis A Kotsakis et al. [22] (2016, without) | Multi-species biofilm | 0.12% chlorhexidine 20% citric acid gel 25% EDTA 15% sodium hypochlorite 0.9% NaCl (sterile saline) (Burnished for 20 s with cotton pellet moistened in chemical agent | 6 6 6 6 | 1. CFU count 2. Surface characterization | Antimicrobial effect was greater for citric acid, sodium hypochlorite/EDTA groups followed by Chlorhexidine (0.12%) group as compared to non-contaminated control. sterile saline only had a minimal antimicrobial effect. Chlorhexidine (0.12%) use is not recommended as it produces a cytotoxic effect on the decontaminated surface and compromise the biocompatibility of the titanium surface. |
| Dostie S et al. [23] (2017, without) | Multi-species mature oral biofilm | Control group (not rinsed/treated with chemical) | 3 | 1. Bacterial cell count 2. Viability of bacteria after treatment | The double rinse group removed more bacteria compared to the rinse group. But no significant difference between the double rinse and disinfectant group suggests a mechanical effect of rinsing was responsible for the removal of bacteria and not the chemical effect. Proportion of dead cells for CHX group (11.8%), Etch group (6.9%) & tetracycline (3.9%) respectively compared to double saline group. No significant difference was noted between double rinse and the C.C.E. group. |
| Rinse group (0.9% sodium chlorite, 6 increments, total 6 mL) | 3 | ||||
| CHX group (1% Chlorhexidine in methylcellulose gel) | 3 | ||||
| Etch group (35% phosphoric acid gel) | 3 | ||||
| Tetracycline group (250 mg tetracycline with 0.9% NaCl to form thick paste) | 3 | ||||
| C.C.E group (0.3% cetrimide, 0.1% CHX, 0.5% EDTA in 3% methylcellulose gel) | 3 | ||||
| Double-rinse group (12 increments of 1 mL 30.9% NaCl) without any chemical agents (Irrigation/application of gel, 2 min) | 3 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qassadi, W.; AlShehri, T.; Alshehri, A.; ALonazi, K.; Aldhayan, I. Review on Dental Implantology. Egypt. J. Hosp. Med. 2018, 1, 2217–2225. [Google Scholar] [CrossRef]
- Van Velzen, F.J.; Ofec, R.; Schulten, E.A.; Ten Bruggenkate, C.M. 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with an SLA surface: A prospective cohort study in 177 fully and partially edentulous patients. Clin. Oral Implant. Res. 2015, 10, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; Poubel, L.D.; Ferreira, V.F.; dos Sp Barboza, E. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Isidor, F. Consensus report of session IV. In Proceedings of the First European Workshop on Periodontology; Lang, N.P., Karring, T., Eds.; Quintessence Publishing: London, UK, 1994; pp. 365–369. [Google Scholar]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabil. 2014, 6, 443–476. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 4, 501–508. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral microbiome and peri-implant diseases: Where are we now? Ther. Clin Risk Manag. 2017, 13, 1529–1542. [Google Scholar] [CrossRef] [Green Version]
- Quirynen, M.; Vogels, R.; Peeters, W.; van Steenberghe, D.; Naert, I.; Haffajee, A. Dynamics of initial subgingival colonization of ‘pristine’ peri-implant pockets. Clin. Oral Implant. Res. 2006, 17, 25–37. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; Buitrago-Vera, P.; Solá-Ruiz, M.F.; Ferrer-García, J.C. Decontamination of dental implant surface in peri-implantitis treatment: A literature review. Med. Oral Patol. Oral Cir. Bucal 2013, 6, e869–e876. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansåker, A.M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35, 305–315. [Google Scholar] [CrossRef]
- Ntrouka, V.I.; Slot, D.E.; Louropoulou, A.; Van der Weijden, F. The effect of chemotherapeutic agents on contaminated titanium surfaces: A systematic review. Clin. Oral Implant. Res. 2011, 22, 681–690. [Google Scholar] [CrossRef]
- Hentenaar, D.; De Waal, Y.; Strooker, H.; Meijer, H.; Van Winkelhoff, A.J.; Raghoebar, G.M. Implant decontamination with phosphoric acid during surgical peri-implantitis treatment. Int. J. Implant Dent. 2017, 1, 33. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Cordeiro, J.M.; Lima, C.V.; Barão, V.A.R. Citric acid reduces oral biofilm and influences the electrochemical behavior of titanium: An in situ and in vitro study. J. Periodontol. 2018, 90, 149–158. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Claffey, N.; Clarke, E.; Polyzois, I.; Renvert, S. Surgical treatment of peri-implantitis. J. Clin. Periodontol. 2008, 35, 316–332. [Google Scholar] [CrossRef]
- Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; The Cochrane Collaboration: London, UK, 2011; Available online: http://www.cochrane-handbook.org (accessed on 12 January 2019).
- Gosau, M.; Hahnel, S.; Schwarz, F.; Gerlach, T.; Reichert, T.E.; Bürgers, R. Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Clin. Oral Implant. Res. 2010, 21, 866–872. [Google Scholar] [CrossRef]
- Ntrouka, V.; Hoogenkamp, M.; Zaura, E.; van der Weijden, F. The effect of chemotherapeutic agents on titanium-adherent biofilms. Clin. Oral Implant. Res. 2011, 22, 1227–1234. [Google Scholar] [CrossRef]
- Bürgers, R.; Witecy, C.; Hahnel, S.; Gosau, M. The effect of various topical peri-implantitis antiseptics on Staphylococcus epidermidis, Candida albicans, and Streptococcus anguinis. Arch. Oral Biol. 2012, 7, 940–947. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Dostie, S.; Alkadi, L.T.; Owen, G.; Bi, J.; Shen, Y.; Haapasalo, M.; Larjava, H.S. Chemotherapeutic decontamination of dental implants colonized by mature multispecies oral biofilm. J. Clin. Periodontol. 2017, 44, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, L.G.; Ericsson, I.; Berglundh, T.; Lindhe, J. Osseintegration following treatment of periimplantitis and replacement of implant components. An experimental study in the dog. J. Clin. Periodontol. 2001, 28, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Dahlen, G.; Renvert, S. Five-year clinical, microbiological, and radiological outcome following treatment of periimplantitis in man. J. Periodontol. 2003, 74, 1415–1422. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I.; Maguire, R. Reosseointegration on previously contaminated surfaces: A systematic review. Clin. Oral Implant. Res. 2009, 4, 216–227. [Google Scholar] [CrossRef]
- Karring, E.S.; Stavropoulos, A.; Ellegaard, B.; Karring, T. Treatment of peri-implantitis by the Vector system. Clin. Oral Implant. Res. 2005, 16, 288–293. [Google Scholar] [CrossRef]
- Kozlovsky, A.; Artzi, Z.; Moses, O.; Kamin-Belsky, N.; Greenstein, R.B. Interaction of chlorhexidine with smooth and rough types of titanium surfaces. J. Periodontol. 2006, 77, 1194–1200. [Google Scholar] [CrossRef]
- Strooker, H.; Rohn, S.; Van Winkelhoff, A.J. Clinical and microbiologic effects of chemical versus mechanical cleansing in professional supportive implant therapy. Int. J. Oral Maxillofac. Implant. 1998, 13, 845–850. [Google Scholar]
- Ramesh, D.; Sridhar, S.; Siddiqui, D.A.; Valderrama, P.; Rodrigues, D.C. Detoxification of titanium implant surfaces: Evaluation of surface morphology and bone-forming cell compatibility. J. Bio Tribo-Corros. 2017, 3, 50. [Google Scholar] [CrossRef]
- Wheelis, S.E.; Gindri, I.M.; Valderrama, P.; Wilson, T.G., Jr.; Huang, J.; Rodrigues, D.C. Effects of decontamination solutions on the surface of titanium: Investigation of surface morphology, composition, and roughness. Clin. Oral Implant. Res. 2016, 27, 329–340. [Google Scholar] [CrossRef]
- Noronha Oliveira, M.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Guimarães, L.F.; Fidalgo, T.K.; Menezes, G.C.; Primo, L.G.; e Silva-Filho, F.C. Effects of citric acid on cultured human osteoblastic cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 665–669. [Google Scholar] [CrossRef]
- Lan, W.C.; Lan, W.H.; Chan, C.P.; Hsieh, C.C.; Chang, M.C.; Jeng, J.H. The effects of extracellular citric acid acidosis on the viability, cellular adhesion capacity and protein synthesis of cultured human gingival fibroblasts. Aust. Dent. J. 1999, 44, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Menezes, K.M.; Fernandes-Costa, A.N.; Silva-Neto, R.D.; Calderon, P.S.; Gurgel, B.C. Efficacy of 0.12% chlorhexidine gluconate for non-surgical treatment of peri-implant mucositis. J. Periodontal 2016, 87, 1305–1313. [Google Scholar] [CrossRef]
- Wetzel, A.C.; Vlassis, J.; Caffesse, R.G.; Hämmerle, C.H.; Lang, N.P. Attempts to obtain re-osseointegration following experimental peri-implantitis in dogs. Clin. Oral Implant. Res. 1999, 10, 111–119. [Google Scholar] [CrossRef]
- You, T.M.; Choi, B.H.; Zhu, S.J.; Jung, J.H.; Lee, S.H.; Huh, J.Y.; Lee, H.J.; Li, J. Treatment of experimental peri-implantitis using autogenous bone grafts and platelet enriched fibrin glue in dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, 34–37. [Google Scholar] [CrossRef]
- Schou, S.; Holmstrup, P.; Skovgaard, L.T.; Stoltze, K.; Hjørting-Hansen, E.; Gundersen, H.J. Autogenous bone graft and ePTFEmembrane in the treatment of peri-implantitis. II. Stereologic and histologic observations in cynomolgus monkeys. Clin. Oral Implant. Res. 2003, 14, 404–411. [Google Scholar] [CrossRef]
- De Waal, Y.C.; Raghoebar, G.M.; Huddleston Slater, J.J.; Meijer, H.J.; Winkel, E.G.; van Winkelhoff, A.J. Implant decontamination during surgical peri-implantitis treatment: A randomized, double-blind, placebo-controlled trial. J. Clin. Periodontol. 2013, 40, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.; Bragger, U.; Hammerle, C.H.; Fourmousis, I.; Lang, N.P. Treatment of an early implant failure according to the principles of guided tissue regeneration (GTR). Clin. Oral Implant. Res. 1992, 3, 42–48. [Google Scholar] [CrossRef]
- Giannelli, M.; Chellini, F.; Margheri, M.; Tonelli, P.; Tani, A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol. In Vitro 2008, 22, 308–317. [Google Scholar] [CrossRef]
- Lee, T.H.; Hu, C.C.; Lee, S.S.; Chou, M.Y.; Chang, Y.C. Cytotoxicity of chlorhexidine on human osteoblastic cells is related to intracellular glutathione levels. Int. Endod. J. 2010, 43, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Buergers, R.; Rosentritt, M.; Schneider-Brachert, W.; Behr, M.; Handel, G.; Hahnel, S. Efficacy of denture disinfection methods in controlling Candida albicans colonization in vitro. Acta Odontol. Scand. 2008, 66, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Selection of antimicrobial agents in periodontal therapy. J. Periodontal Res. 2002, 37, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spratt, D.A.; Pratten, J.; Wilson, M.; Gulabivala, K. An in vitro evaluation of the antimicrobial efficacy of irrigants on biofilms of root canal isolates. Int. Endod. J. 2001, 34, 300–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobene, R.R.; Soparkar, P.M.; Hein, J.W.; Quigley, G.A. A study of the effects of antiseptic agents and a pulsating irrigating device on plaque and gingivitis. J. Periodontol. 1972, 43, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Adcock, J.E.; Berry, W.C., Jr.; Kalkwarf, K.L. Effect of sodium hypochlorite solution on the subgingival microflora of juvenile periodontitis lesions. Pediatr. Dent. 1983, 5, 190–194. [Google Scholar]
- Soldatos, N.; Romanos, G.E.; Michaiel, M.; Sajadi, A.; Angelov, N.; Weltman, R. Management of Retrograde Peri-Implantitis Using an Air-Abrasive Device, Er, Cr:YSGG Laser, and Guided Bone Regeneration. Case Rep. Dent. 2018, 2018, 7283240. [Google Scholar] [CrossRef] [Green Version]
- Bain, C.A.; Moy, P.K. The association between the failure of dental implants and cigarette smoking. Int. J. Oral Maxillofac. Implant. 1993, 8, 609–615. [Google Scholar]
- Alsaadi, G.; Quirynen, M.; Komarek, A.; van Steenberghe, D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J. Clin. Periodontol. 2007, 34, 610–617. [Google Scholar] [CrossRef]
- Park, S.H.; Sorensen, W.P.; Wang, H.L. Management and prevention of retrograde peri-implant infection from retained root tips: Two case reports. Int. J. Periodontics Restor. Dent. 2004, 24, 422–433. [Google Scholar] [CrossRef] [Green Version]
- Sarmast, N.D.; Wang, H.H.; Soldatos, N.K.; Angelov, N.; Dorn, S.; Yukna, R.; Iacono, V.J. A Novel Treatment Decision Tree and Literature Review of Retrograde Peri-Implantitis. J. Periodontol. 2016, 87, 1458–1467. [Google Scholar] [CrossRef]
| I. Focus Question | “Which Is the Most Effective Chemotherapeutic Agent for Decontamination of an Infected Dental Implant (with or without Adjunctive Mechanical Cleaning)?” |
|---|---|
| II. Search strategy P—Population I—Intervention C—Comparison O—Outcome | Infected dental implants/different chemotherapeutic agents? Effectiveness of different chemotherapeutic agents used for implant surface decontamination and comparison of them, with or without mechanical cleaning of the implant surface. Use of chemotherapeutic agents along with mechanical cleaning To identify the most effective chemotherapeutic agent (s) for dental implant surface. |
| III. Search keywords | Peri-implantitis treatment, chemotherapeutic agents, implant surface decontamination, chemical disinfectant for implant surface. |
| IV. Database search | PubMed, Google |
| V. Selection criteria Inclusion criteria Exclusion criteria | Studies involving a minimum of two chemotherapeutic agents for implant decontamination Contaminated implant surface Decontamination was done without implantoplasty Only in the English language Experimental human studies Where the full text is not available No access to an English version of the title and abstract. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, C.; Agrawal, A.; Abullais, S.S.; Arora, S.; Khateeb, S.U.; Fadul A. Elagib, M. Effectiveness of Different Chemotherapeutic Agents for Decontamination of Infected Dental Implant Surface: A Systematic Review. Antibiotics 2022, 11, 593. https://doi.org/10.3390/antibiotics11050593
Patil C, Agrawal A, Abullais SS, Arora S, Khateeb SU, Fadul A. Elagib M. Effectiveness of Different Chemotherapeutic Agents for Decontamination of Infected Dental Implant Surface: A Systematic Review. Antibiotics. 2022; 11(5):593. https://doi.org/10.3390/antibiotics11050593
Chicago/Turabian StylePatil, Chayya, Amit Agrawal, Shahabe Saquib Abullais, Suraj Arora, Shafait Ullah Khateeb, and Mohamed Fadul A. Elagib. 2022. "Effectiveness of Different Chemotherapeutic Agents for Decontamination of Infected Dental Implant Surface: A Systematic Review" Antibiotics 11, no. 5: 593. https://doi.org/10.3390/antibiotics11050593
APA StylePatil, C., Agrawal, A., Abullais, S. S., Arora, S., Khateeb, S. U., & Fadul A. Elagib, M. (2022). Effectiveness of Different Chemotherapeutic Agents for Decontamination of Infected Dental Implant Surface: A Systematic Review. Antibiotics, 11(5), 593. https://doi.org/10.3390/antibiotics11050593





