Investigating the Antibacterial Characteristics of Japanese Bamboo
Abstract
:1. Introduction
2. Materials and Methods
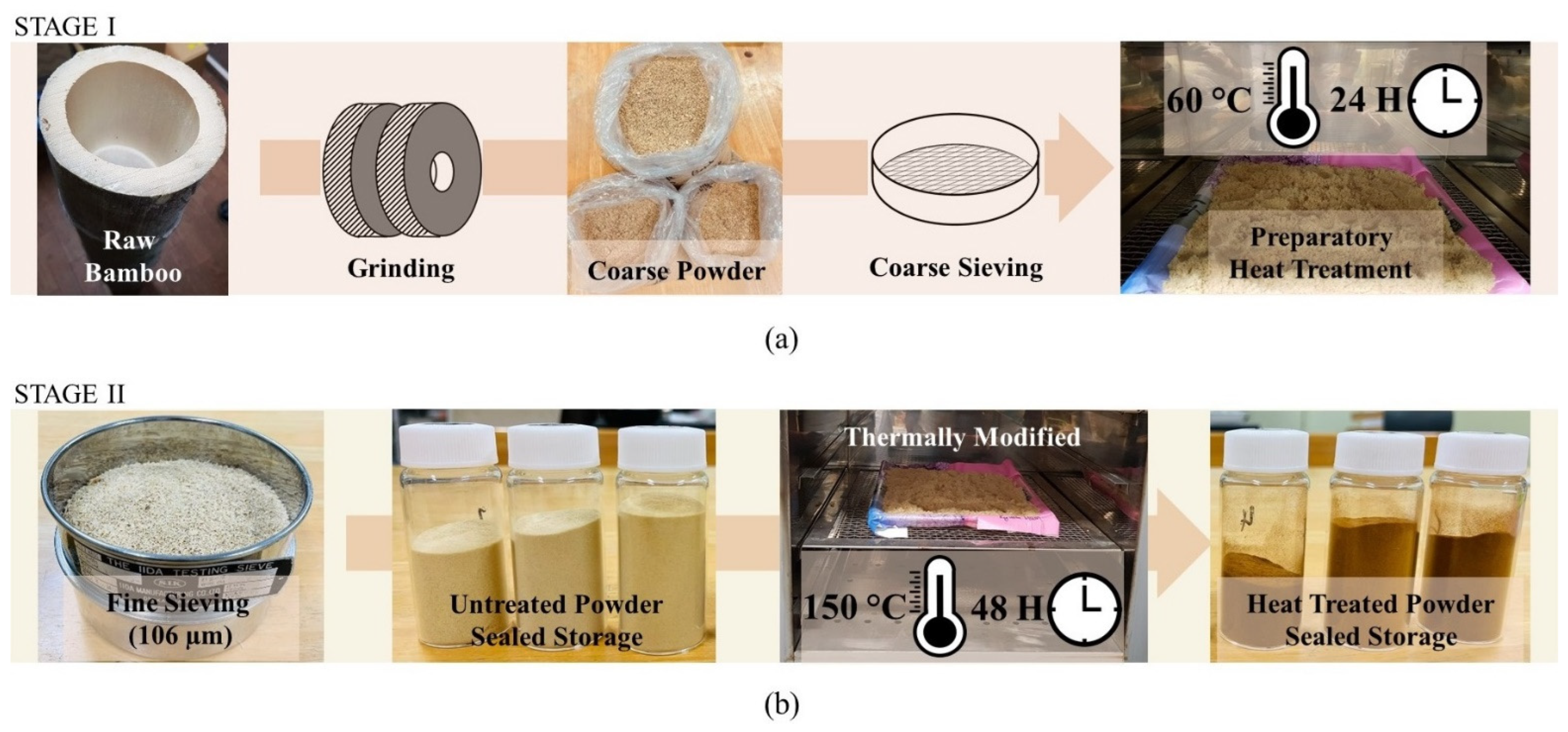
2.1. Sample Preparation
2.2. Antibacterial Experiment
2.2.1. Extraction Method
2.2.2. Non-Extraction Method
2.3. Sample Characterization
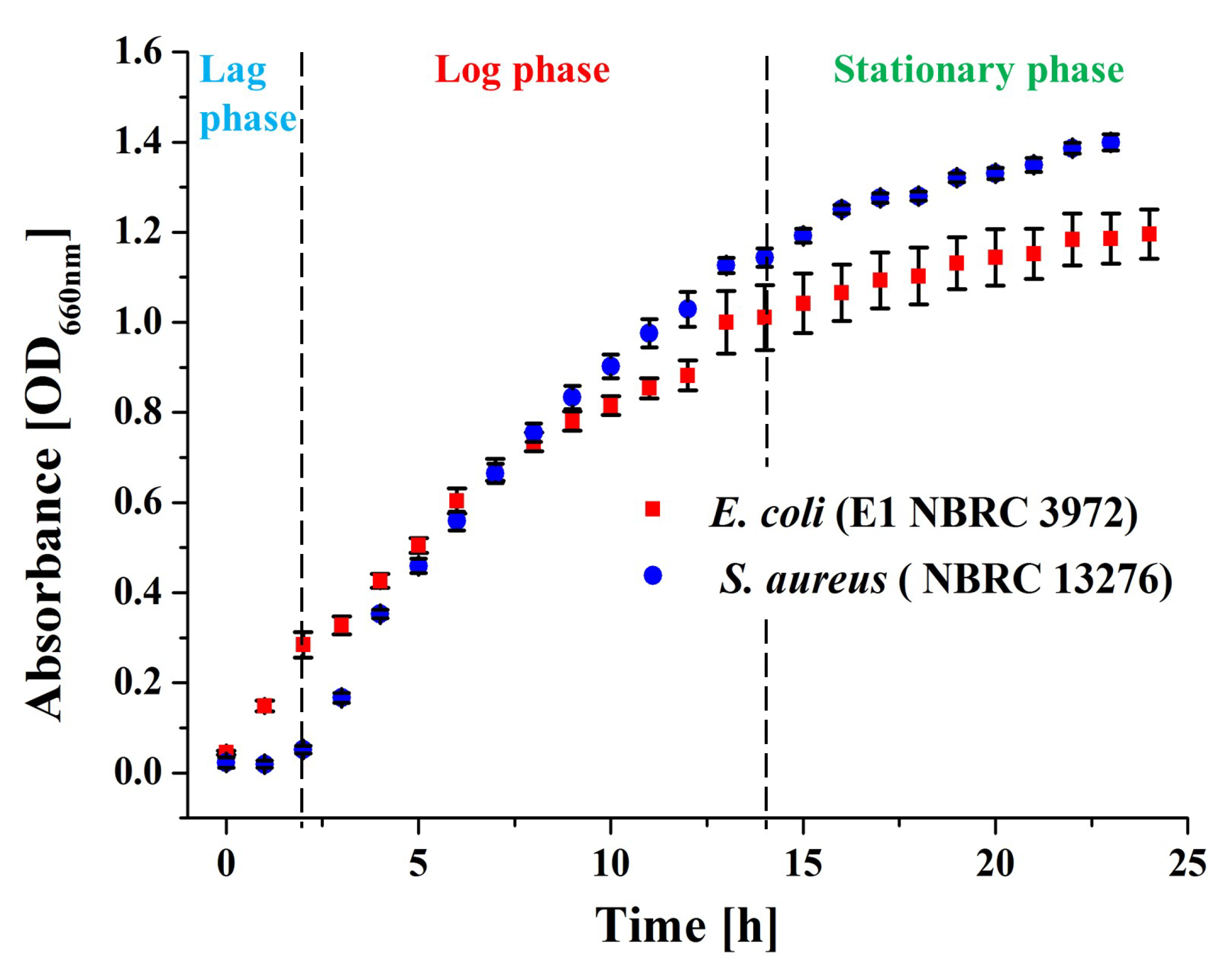
2.3.1. In Vitro Bacteria Culture
2.3.2. Pre-Characterization of Bacterial Culture
2.3.3. Antibacterial Efficacy—Microbial Growth Count Method (Biological Characterization—Colony Forming Units) Preparation
2.4. Fourier Transformed Infrared Spectroscopy (FTIR)
3. Experimental Results
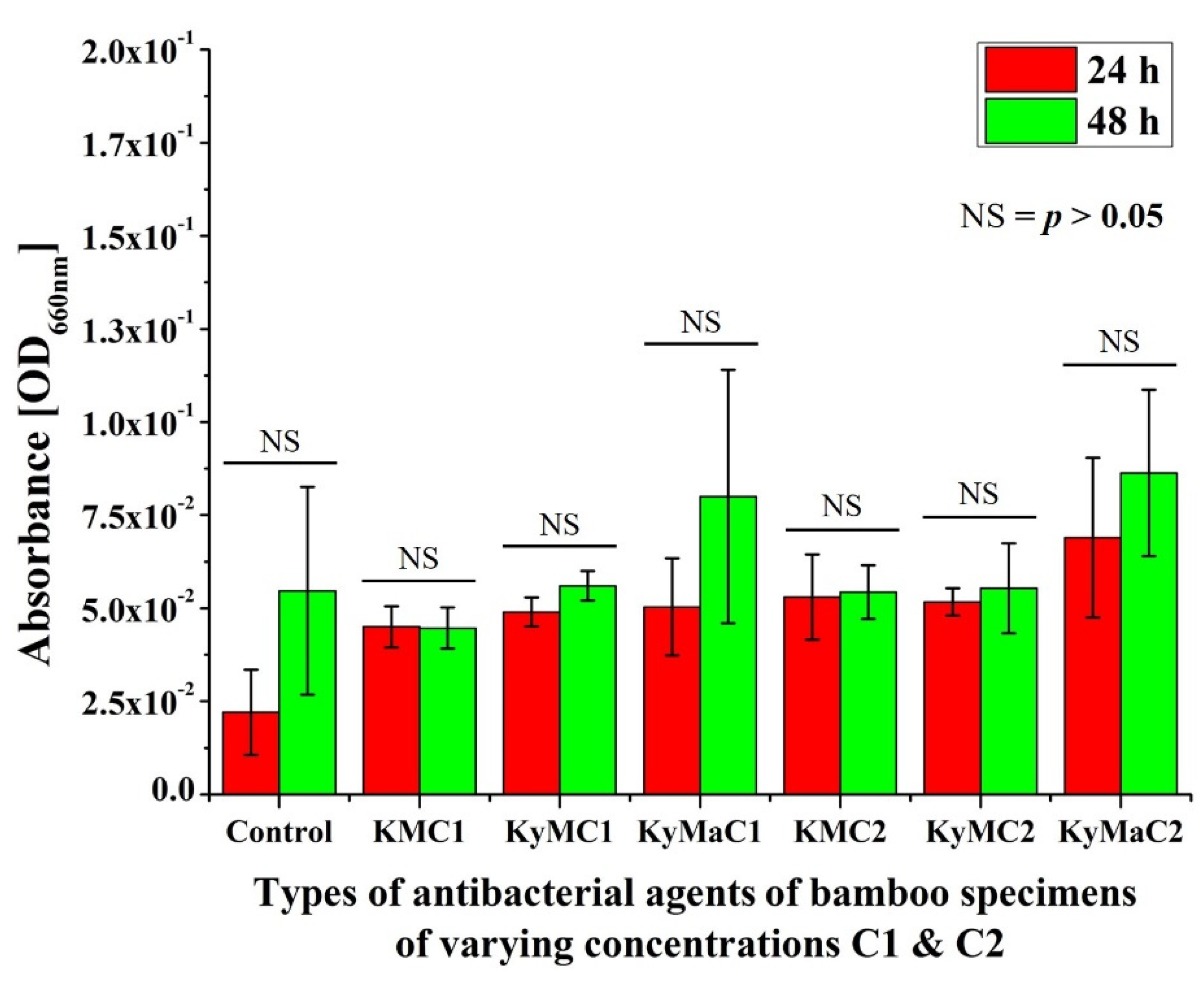
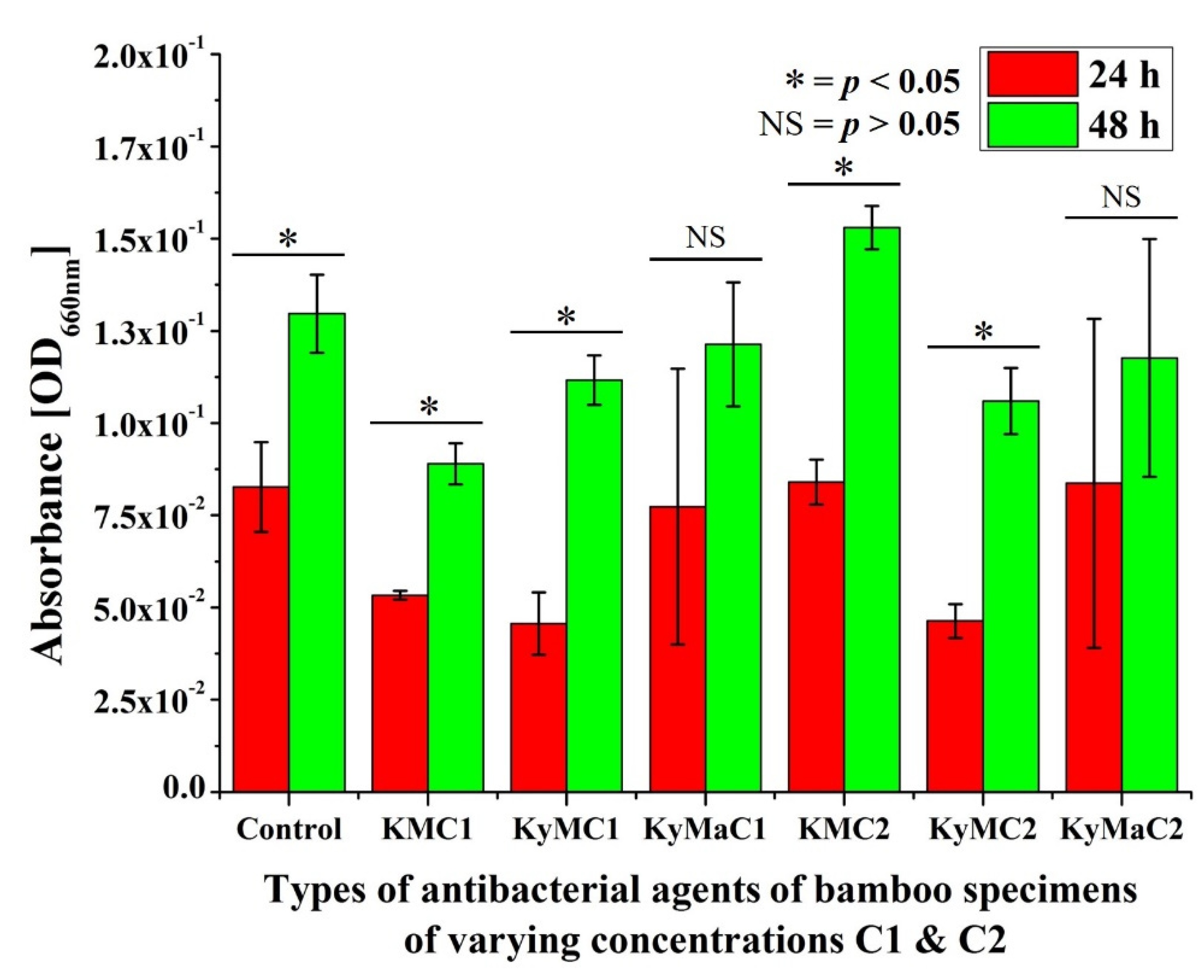
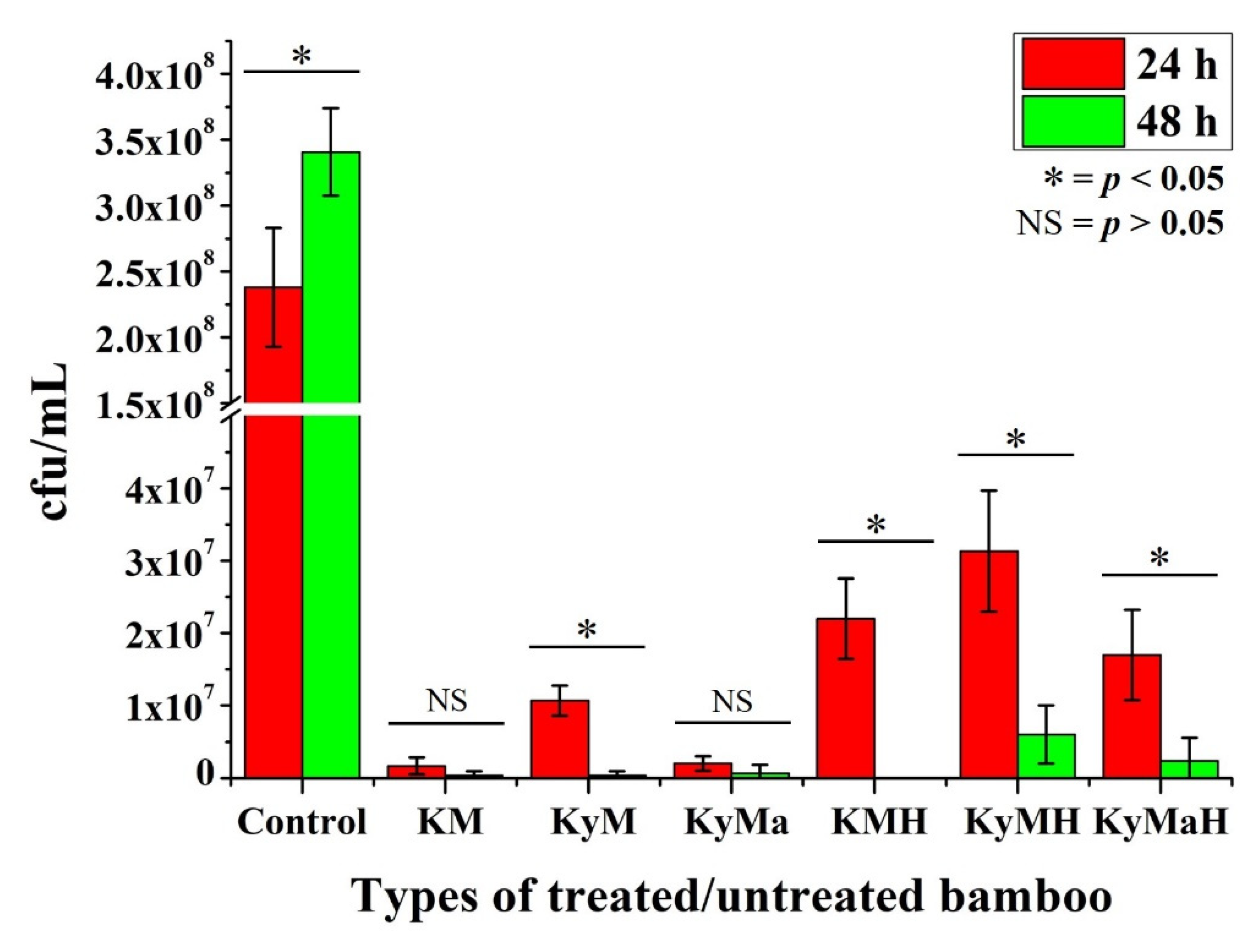
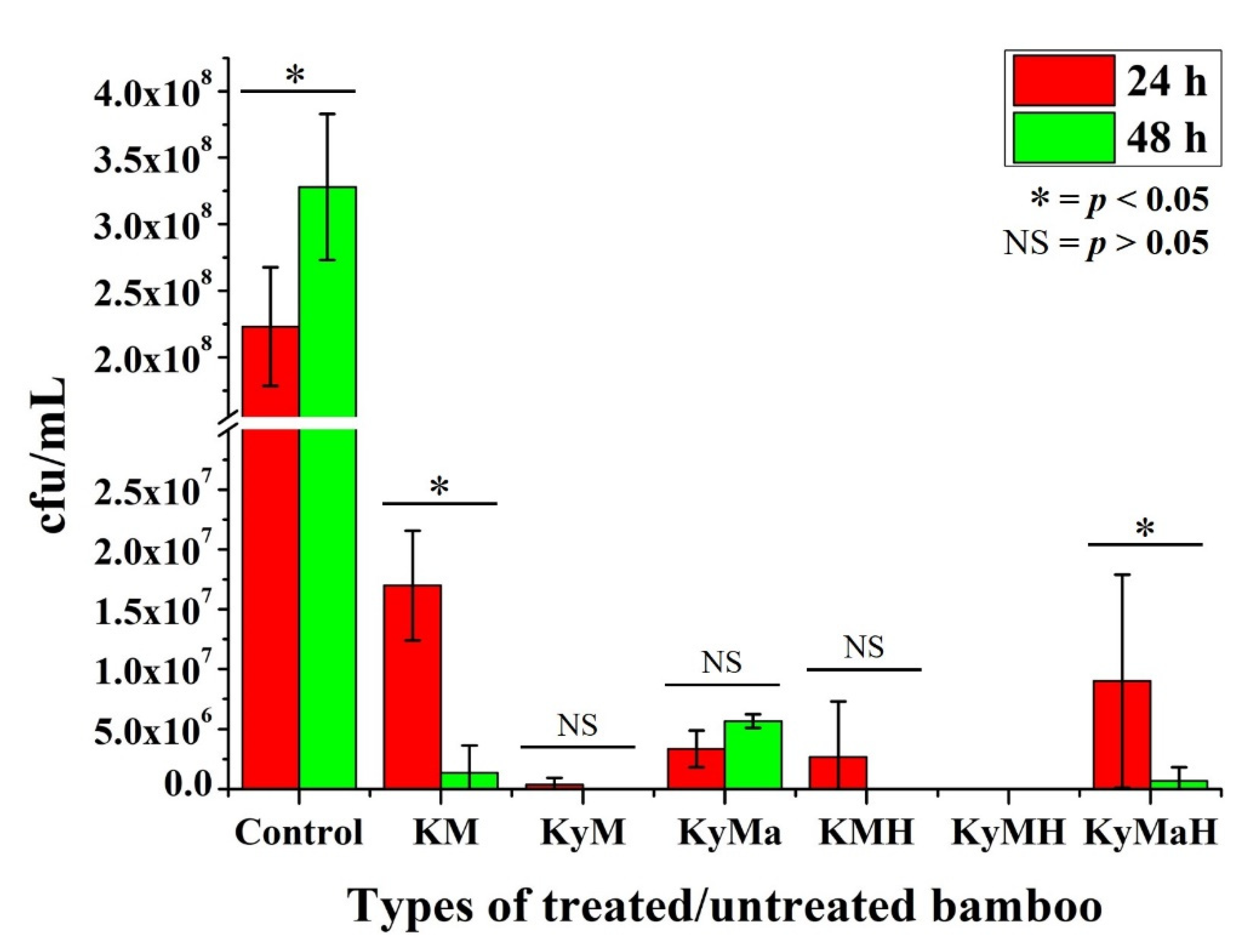
3.1. Extraction Method
3.2. Non-Extraction Method
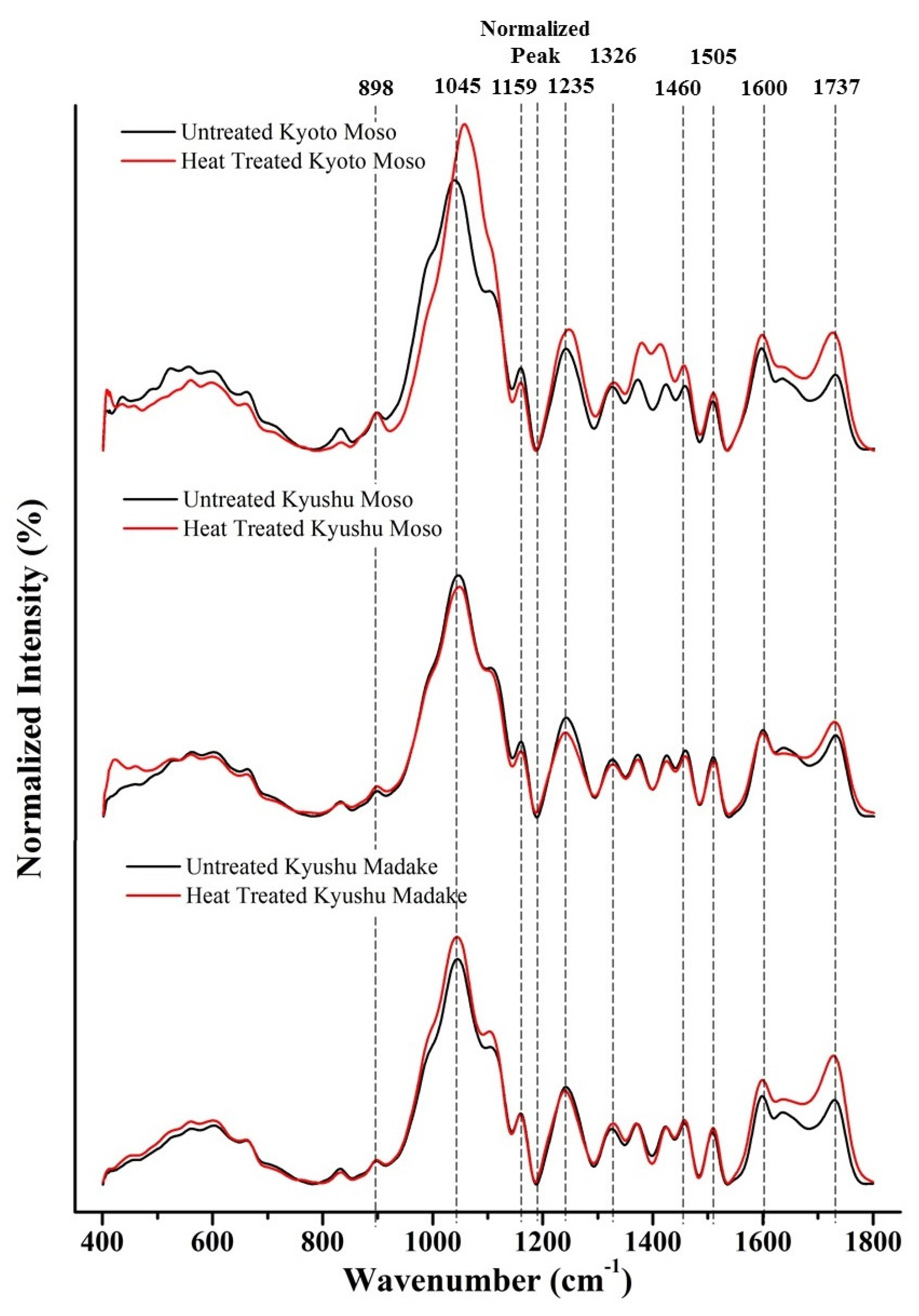
3.3. FTIR Analysis
4. Discussion
4.1. Antibacterial Mechanism in Bamboo
4.2. Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afrin, T.; Tsuzuki, T.; Kanwar, R.K.; Wang, X. The origin of the antibacterial property of bamboo. J. Text. Inst. 2012, 103, 844–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ge, S.; Liu, Z.; Zhou, Y.; Yang, X.; Yang, W.; Li, D.; Peng, W. Properties of antibacterial bioboard from bamboo macromolecule by hot press. Saudi J. Biol. Sci. 2018, 25, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lou, Z.; Han, X.; Liu, J.; Wang, Z.; Zhang, Y.; Wu, X.; Yuan, C.; Li, Y. Fabrication of a novel magnetic reconstituted bamboo with mildew resistance properties. Mater. Today Commun. 2020, 23, 101086. [Google Scholar] [CrossRef]
- Xi, L.X.; Qin, D.C. The antibacterial performance of natural bamboo fiber and its influencing factors. In Proceedings of the 55th International Convention of Society of Wood Science and Technology, Beijing, China, 27–31 August 2012; pp. 1–8. [Google Scholar]
- Ago, M.; Tardy, B.L.; Wang, L.; Guo, J.; Khakalo, A.; Rojas, O.J. Supramolecular assemblies of lignin into nano-and microparticles. MRS Bull. 2017, 42, 371–378. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and characterization of biodegradable lignin nanoparticles with tunable surface properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef]
- Wang, G.; Pang, T.; Xia, Y.; Liu, X.; Li, S.; Parvez, A.M.; Kong, F.; Si, C. Subdivision of bamboo kraft lignin by one-step ethanol fractionation to enhance its water-solubility and antibacterial performance. Int. J. Biol. Macromol. 2019, 133, 156–164. [Google Scholar] [CrossRef]
- Walker, J.C. Primary Wood Processing: Principles and Practice; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Rzemieniecki, T.; Krawczyk, M.; Malina, D.; Norman, M.; Zdarta, J.; Majchrzak, I.; Dobrowolska, A.; Czaczyk, K.; Jesionowski, T. Kraft lignin/silica–AgNPs as a functional material with antibacterial activity. Colloids Surf. B Biointerfaces 2015, 134, 220–228. [Google Scholar] [CrossRef]
- Ndaba, B.; Roopnarain, A.; Daramola, M.O.; Adeleke, R. Influence of extraction methods on antimicrobial activities of lignin-based materials: A review. Sustain. Chem. Pharm. 2020, 18, 100342. [Google Scholar] [CrossRef]
- Nandi, S.; Maurer, J.J.; Hofacre, C.; Summers, A.O. Gram-positive bacteria are a major reservoir of Class 1 antibiotic resistance integrons in poultry litter. Proc. Natl. Acad. Sci. USA 2004, 101, 7118–7122. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Jin, T.; Nghiem, N.P.; Fan, X.; Qi, P.X.; Jang, C.H.; Shao, L.; Wu, C. Assessment of antioxidant and antimicrobial properties of lignin from corn stover residue pretreated with low-moisture anhydrous ammonia and enzymatic hydrolysis process. Appl. Biochem. Biotechnol. 2018, 184, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Lourençon, T.V.; de Lima, G.G.; Ribeiro, C.S.; Hansel, F.A.; Maciel, G.M.; da Silva, K.; Winnischofer, S.M.; de Muniz, G.I.; Magalhães, W.L. Antioxidant, antibacterial and antitumoural activities of kraft lignin from hardwood fractionated by acid precipitation. Int. J. Biol. Macromol. 2021, 166, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Aradmehr, A.; Javanbakht, V. A novel biofilm based on lignocellulosic compounds and chitosan modified with silver nanoparticles with multifunctional properties: Synthesis and characterization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124952. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Ramful, R.; Sakuma, A. Effect of smoke treatment on flexural strength of bamboo hierarchical structure. BioResources 2021, 16, 387. [Google Scholar] [CrossRef]
- Ramful, R.; Sunthar, T.P.; Zhu, W.; Pezzotti, G. Investigating the Underlying Effect of Thermal Modification on Shrinkage Behavior of Bamboo Culm by Experimental and Numerical Methods. Materials 2021, 14, 974. [Google Scholar] [CrossRef]
- Meng, F.D.; Yu, Y.L.; Zhang, Y.M.; Yu, W.J.; Gao, J.M. Surface chemical composition analysis of heat-treated bamboo. Appl. Surf. Sci. 2016, 371, 383–390. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, D.; Huang, X.; Song, L.; Gu, W.; Liang, X.; Li, Y.; Xu, B. Effect of high-temperature saturated steam treatment on the physical, chemical, and mechanical properties of moso bamboo. J. Wood Sci. 2020, 66, 52. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Kotilainen, R.A.; Toivanen, T.J.; Alén, R.J. FTIR monitoring of chemical changes in softwood during heating. J. Wood Chem. Technol. 2000, 20, 307–320. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F.; Nogués, F.; Garriga, P. Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym. Degrad. Stab. 2003, 80, 543–549. [Google Scholar] [CrossRef]
- Hoseinzadeh, F.; Zabihzadeh, S.M.; Dastoorian, F. Creep behavior of heat treated beech wood and the relation to its chemical structure. Constr. Build. Mater. 2019, 226, 220–226. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Liu, J.; Wu, J. FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl. Surf. Sci. 2013, 280, 799–805. [Google Scholar] [CrossRef]
- Veiga, T.R.L.A.; Lima, J.T.; Dessimoni, A.L.D.A.; Pego, M.F.F.; Soares, J.R.; Trugilho, P.F. Different plant biomass characterizations for biochar production. Cerne 2017, 23, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Sikora, A.; Kačík, F.; Gaff, M.; Vondrová, V.; Bubeníková, T.; Kubovský, I. Impact of thermal modification on color and chemical changes of spruce and oak wood. J. Wood Sci. 2018, 64, 406–416. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial activity of lignin and lignin-derived cellulose and chitosan composites against selected pathogenic and spoilage microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarre, W.W.; Schneewind, O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [CrossRef] [Green Version]
- Díez-Pascual, A.M. Antimicrobial Polymer-Based Materials for Food Packaging Applications. Polymers 2020, 12, 731. [Google Scholar] [CrossRef] [Green Version]
| Types of Antibacterial Agents of Bamboo Specimens of Varying Concentrations C1 & C2 | |||
|---|---|---|---|
| Location | Kyoto | Kyushu | Kyushu |
| Species | Moso (Phyllostachys edulis) | Moso (Phyllostachys edulis) | Madake (Phyllostachys bambusoides) |
| Concentration/μg | |||
| 62.5 | KMC1 | KyMC1 | KyMaC1 |
| 1000 | KMC2 | KyMC2 | KyMaC2 |
| Types of Antibacterial Agents of Bamboo Specimens Subjected to Treatment Modification | |||
|---|---|---|---|
| Location | Kyoto | Kyushu | Kyushu |
| Species | Moso (Phyllostachys edulis) | Moso (Phyllostachys edulis) | Madake (Phyllostachys bambusoides) |
| Treatment Modification | |||
| Stage I—Natural | KM | KyM | KyMa |
| Stage II—Heat Treated | KMH | KyMH | KyMaH |
| Wavenumber (cm−1) | Functional Group | Assignment | References |
|---|---|---|---|
| 898 | C-H | Bending vibration of β-glucosamine bond in cellulose | [20] |
| 1045 | C-O, C-H | Primary alcohol, guaiacyl (lignin) | [21] |
| 1159 | C-O-C | Carbohydrate | [22] |
| 1235 | CO | Guaiacyl ring with CO stretching (lignin and hemicelluloses) | [19] |
| 1326 | O-H | Phenol group (cellulose) | [19] |
| 1460 | C-H | Asymmetric bending in CH3 (lignin) | [23] |
| 1505 | C=C | Aromatic ring (lignin), guaiacyl elements stronger than syringyl | [19] |
| 1600 | C=C | Aromatic ring (lignin) | [19] |
| 1737 | C=O | Carbonyl groups in lignin, Stretching of acetyl or carboxylic acid (hemicelluloses) | [24,25,26,27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramful, R.; Sunthar, T.P.M.; Kamei, K.; Pezzotti, G. Investigating the Antibacterial Characteristics of Japanese Bamboo. Antibiotics 2022, 11, 569. https://doi.org/10.3390/antibiotics11050569
Ramful R, Sunthar TPM, Kamei K, Pezzotti G. Investigating the Antibacterial Characteristics of Japanese Bamboo. Antibiotics. 2022; 11(5):569. https://doi.org/10.3390/antibiotics11050569
Chicago/Turabian StyleRamful, Raviduth, Thefye P. M. Sunthar, Kaeko Kamei, and Giuseppe Pezzotti. 2022. "Investigating the Antibacterial Characteristics of Japanese Bamboo" Antibiotics 11, no. 5: 569. https://doi.org/10.3390/antibiotics11050569
APA StyleRamful, R., Sunthar, T. P. M., Kamei, K., & Pezzotti, G. (2022). Investigating the Antibacterial Characteristics of Japanese Bamboo. Antibiotics, 11(5), 569. https://doi.org/10.3390/antibiotics11050569







