The Role of Point-of-Care C-Reactive Protein Testing in Antibiotic Prescribing for Respiratory Tract Infections: A Survey among Swiss General Practitioners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Survey Instrument and Data Collection
2.3. Clinical Case-Vignettes
2.4. Survey Structure
2.5. Statistical and Content Analyses
3. Results
3.1. Characteristics of Respondents
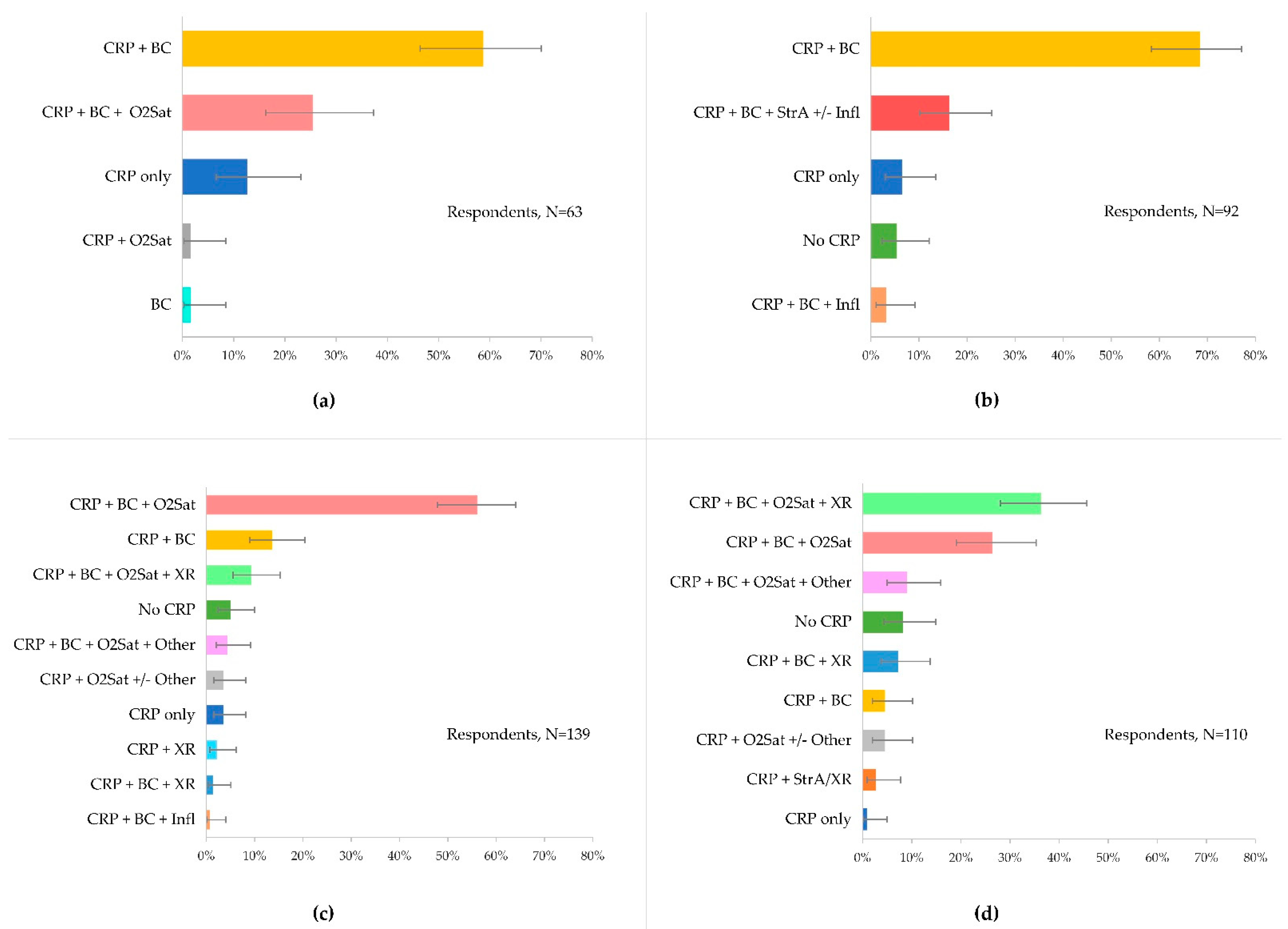
3.2. Use and Choice of Diagnostics to Guide Disease Management
3.3. Use of CRP-POCT for Respiratory Tract Infections
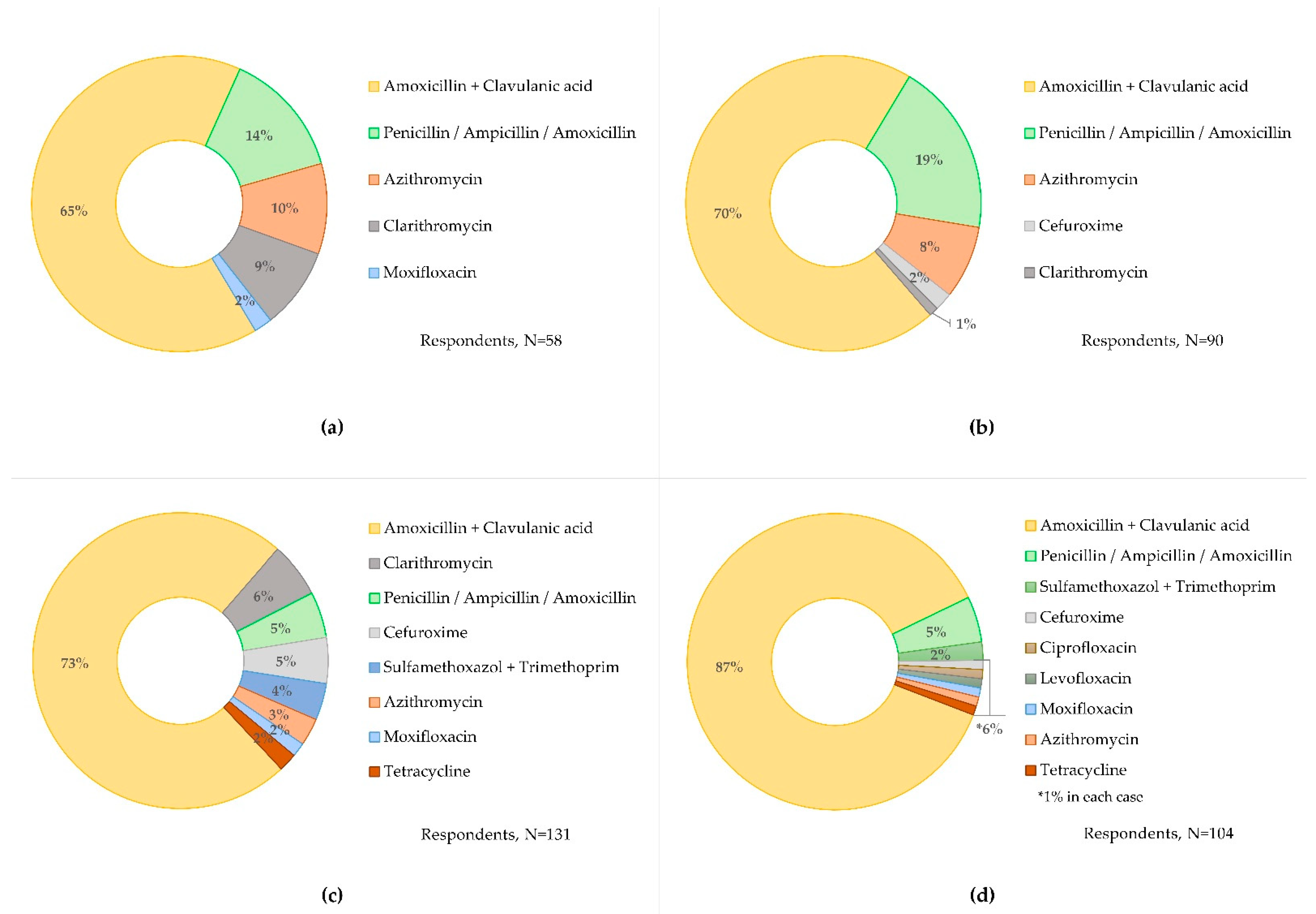
3.3.1. CRP to Guide Antibiotic Prescribing
3.3.2. CRP Cut-Offs and Antibiotics
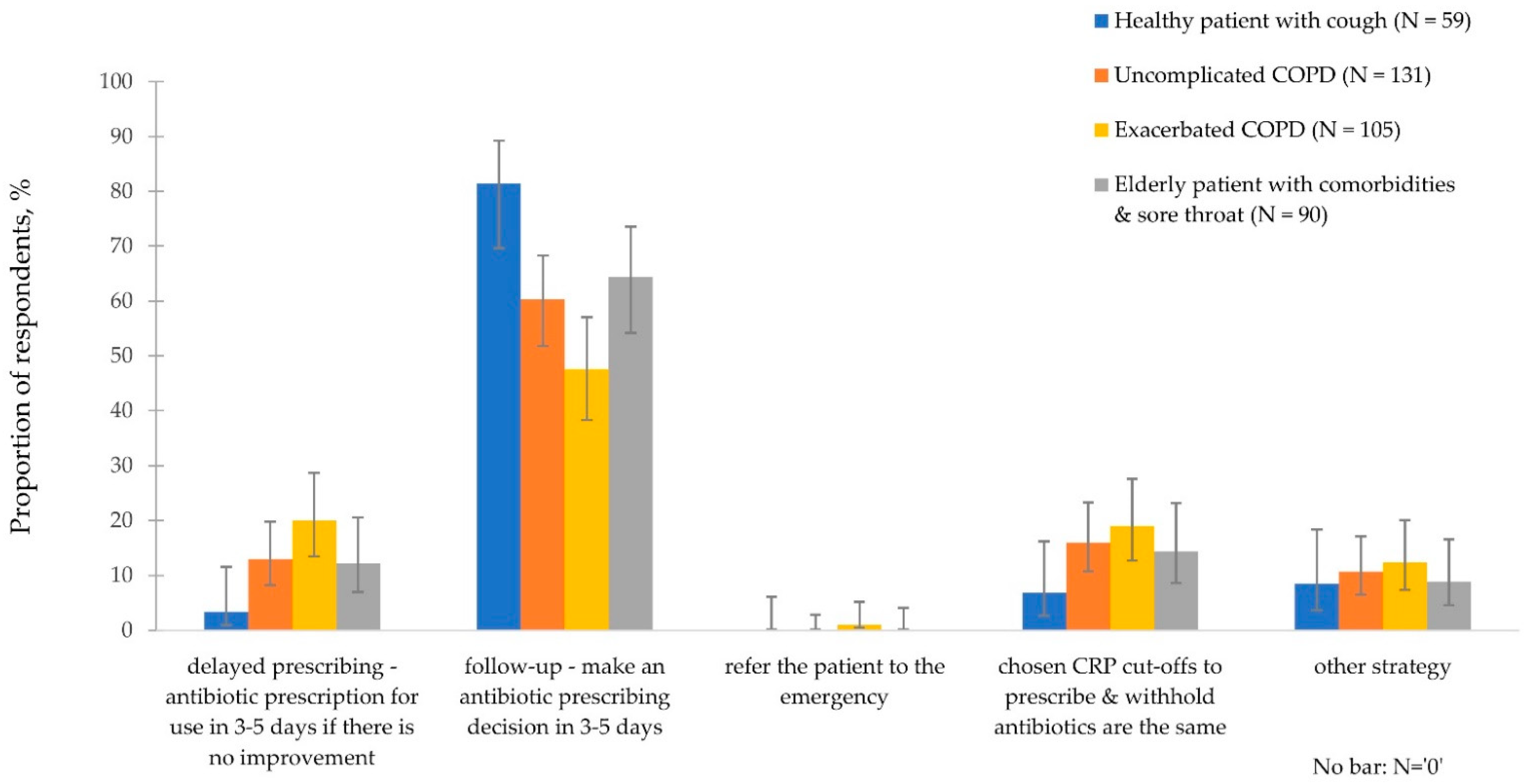
3.3.3. CRP Intermediate Ranges and Disease Management
3.4. Use of Evidence-Based Guidance for Decision-Making
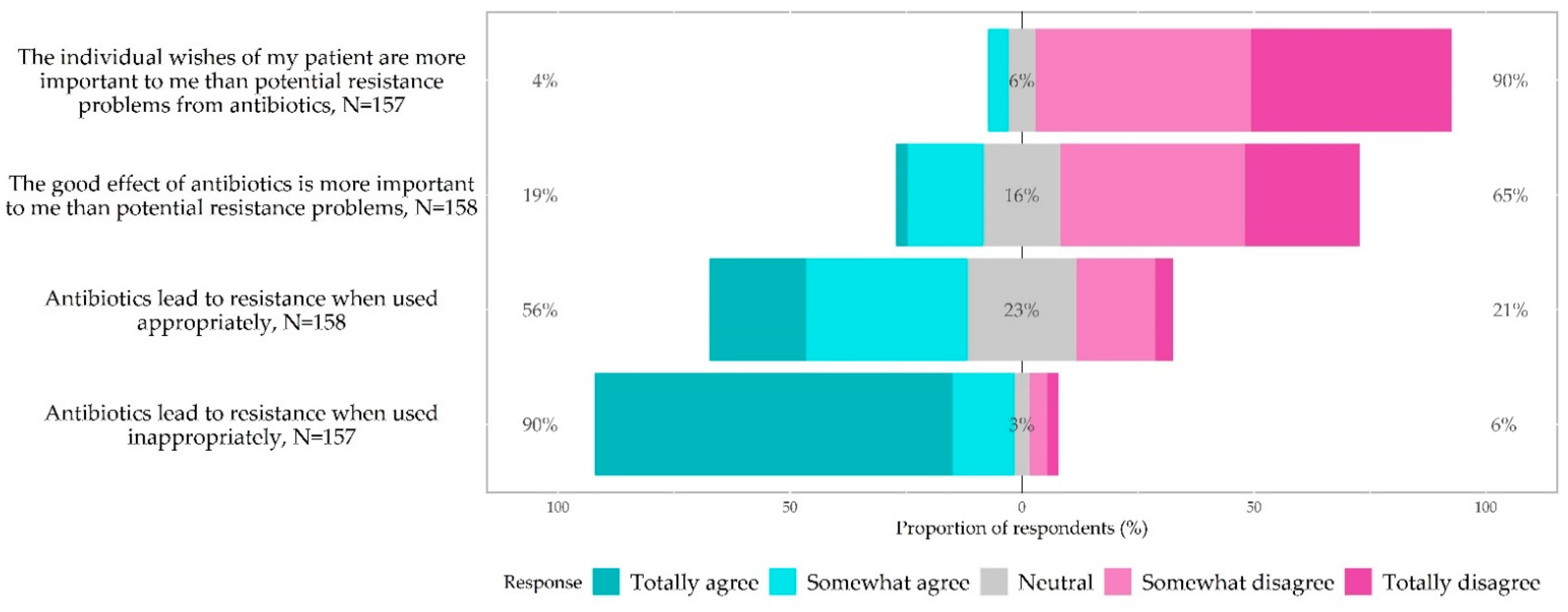
3.5. Knowledge, Awareness and Attitudes of Antibiotic Prescribing and Antibiotic Resistance
3.6. Barriers and Facilitators to Appropriate Antibiotic Prescribing
4. Discussion
4.1. Strengths and Limitations
4.2. Policy Considerations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: http://www.emro.who.int/health-topics/drug-resistance/global-action-plan.html (accessed on 16 May 2021).
- European Center for Disease Prevention and Control. Surveillance and Disease Data for Antimicrobial Consumption. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data (accessed on 16 May 2021).
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Tonkin-Crine, S.K.; Tan, P.S.; van Hecke, O.; Wang, K.; Roberts, N.W.; McCullough, A.; Hansen, M.P.; Butler, C.C.; Del Mar, C.B. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: An overview of systematic reviews. Cochrane Database Syst. Rev. 2017, 9, CD012252. [Google Scholar] [CrossRef] [Green Version]
- Stålsby Lundborg, C.; Tamhankar, A.J. Understanding and changing human behaviour—Antibiotic mainstreaming as an approach to facilitate modification of provider and consumer behaviour. Upsala J. Med. Sci. 2014, 119, 125–133. [Google Scholar] [CrossRef]
- European Commission. A European One Health Action Plan against Antimicrobial Resistance (AMR). Available online: https://ec.europa.eu/health/amr/sites/amr/files/amr_action_plan_2017_en.pdf (accessed on 16 May 2021).
- Martínez-González, N.; Keizer, E.; Plate, A.; Coenen, S.; Valeri, F.; Verbakel, J.; Rosemann, T.; Neuner-Jehle, S.; Senn, O. Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Respiratory Tract Infections in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials. Antibiotics 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Cough (Acute): Antimicrobial Prescribing NICE Clinical Guideline NG120; NICE: London, UK, 2019. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Pneumonia: Diagnosis and Management of Community- and Hospital-Acquired Pneumonia in Adults. NICE Clinical Guideline CG191; NICE: London, UK, 2014. [Google Scholar]
- Verlee, L.; Verheij, T.J.; Hopstaken, R.M.; Prins, J.M.; Salomé, P.L.; Bindels, P.J. Summary of NHG practice guideline “Acute cough”. Ned. Tijdschr. Voor. Geneeskd. 2012, 156, A4188. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Respiratory Tract Infections—Antibiotic Prescribing. NICE Clinical Guideline 69; NICE: London, UK, 2008. [Google Scholar]
- Minnaard, M.C.; de Groot, J.A.H.; Hopstaken, R.M.; Schierenberg, A.; de Wit, N.J.; Reitsma, J.B.; Broekhuizen, B.D.L.; van Vugt, S.F.; Neven, A.K.; Graffelman, A.W.; et al. The added value of C-reactive protein measurement in diagnosing pneumonia in primary care: A meta-analysis of individual patient data. CMAJ 2017, 189, E56–E63. [Google Scholar] [CrossRef] [Green Version]
- Cals, J.; Chappin, F.; Hopstaken, R.; van Leeuwen, M.; Hood, K.; Butler, C.; Dinant, G. C-reactive protein point-of-care testing for lower respiratory tract infections: A qualitative evaluation of experiences by GPs. Fam. Pract. 2010, 27, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Howick, J.; Cals, J.W.L.; Jones, C.; Price, C.P.; Plüddemann, A.; Heneghan, C.; Berger, M.Y.; Buntinx, F.; Hickner, J.; Pace, W.; et al. Current and future use of point-of-care tests in primary care: An international survey in Australia, Belgium, The Netherlands, the UK and the USA. BMJ Open 2014, 4, e005611. [Google Scholar] [CrossRef]
- Briel, M.; Young, J.; Tschudi, P.; Hersberger, K.E.; Hugenschmidt, C.; Langewitz, W.; Bucher, H.C. Prevalence and influence of diagnostic tests for acute respiratory tract infections in primary care. Swiss. Med. Wkly. 2006, 136, 248–253. [Google Scholar]
- Achermann, R.; Suter, K.; Kronenberg, A.; Gyger, P.; Muhlemann, K.; Zimmerli, W.; Bucher, H.C. Antibiotic use in adult outpatients in Switzerland in relation to regions, seasonality and point of care tests. Clin. Microbiol. Infect. 2011, 17, 855–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streit, S.; Frey, P.; Singer, S.; Bollag, U.; Meli, D.N. Clinical and haematological predictors of antibiotic prescribing for acute cough in adults in Swiss practices—An observational study. BMC. Fam. Pract. 2015, 16, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glinz, D.; Leon Reyes, S.; Saccilotto, R.; Widmer, A.F.; Zeller, A.; Bucher, H.C.; Hemkens, L.G. Quality of antibiotic prescribing of Swiss primary care physicians with high prescription rates: A nationwide survey. J. Antimicrob. Chemother. 2017, 72, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.R.; Rathbone, J.; Parekh, S.; Hoffmann, T.C.; Del Mar, C.B. Not in my backyard: A systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2015, 70, 2465–2473. [Google Scholar] [CrossRef] [Green Version]
- Teixeira Rodrigues, A.; Roque, F.; Falcao, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef]
- Tonkin-Crine, S.; Yardley, L.; Little, P. Antibiotic prescribing for acute respiratory tract infections in primary care: A systematic review and meta-ethnography. J. Antimicrob. Chemother. 2011, 66, 2215–2223. [Google Scholar] [CrossRef] [Green Version]
- Wood, F.; Phillips, C.; Brookes-Howell, L.; Hood, K.; Verheij, T.; Coenen, S.; Little, P.; Melbye, H.; Godycki-Cwirko, M.; Jakobsen, K.; et al. Primary care clinicians’ perceptions of antibiotic resistance: A multi-country qualitative interview study. J. Antimicrob. Chemother. 2013, 68, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Eysenbach, G. Correction: Improving the Quality of Web Surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med. Internet Res. 2012, 14, e8. [Google Scholar] [CrossRef]
- Neuner-Jehle, S. Une Fois de Plus: Les Antibiotiques: Aidez-Nous Avec la Recherche en Médecine Générale! Prim. Hosp. Care. Med. Int. Gen. 2020, 20, 307. [Google Scholar] [CrossRef]
- Neuner-Jehle, S. Einmal mehr: Antibiotika: Helfen Sie mit bei hausärztlicher Forschung! Prim. Hosp. Care Med. Int. Gen. 2020, 20, 307. [Google Scholar] [CrossRef]
- Chmiel, C.; Bhend, H.; Senn, O.; Zoller, M.; Rosemann, T. The FIRE project: A milestone for research in primary care in Switzerland. Swiss Med. Wkly. 2011, 140, w13142. [Google Scholar] [PubMed] [Green Version]
- OECD/WHO. OECD Reviews of Health Systems. Available online: https://doi.org/10.1787/9789264120914-en (accessed on 7 February 2020).
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Bryer, J.; Speerschneider, K. Analysis and Visualization Likert Items. R Package Version 1.3.5. Available online: https://CRAN.R-project.org/package=likert (accessed on 15 May 2021).
- Wilson, E.B. Probable Inference, the Law of Succession, and Statistical Inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Björkman, I.; Berg, J.; Viberg, N.; Stålsby Lundborg, C. Awareness of antibiotic resistance and antibiotic prescribing in UTI treatment: A qualitative study among primary care physicians in Sweden. Scand. J. Prim. Health Care 2013, 31, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, L.D.; Jäger, L.; Meier, R.; Rachamin, Y.; Senn, O.; Rosemann, T.; Markun, S. Trends and between-Physician Variation in Laboratory Testing: A Retrospective Longitudinal Study in General Practice. J. Clin. Med. 2020, 9, 1787. [Google Scholar] [CrossRef]
- van der Velden, A.; van de Pol, A.C.; Bongard, E.; Cianci, D.; Aabenhus, R.; Balan, A.; Böhmer, F.; Bralic Lang, V.; Bruno, P.; Chlabicz, S.; et al. Point of care testing, antibiotic prescribing and prescribing confidence for respiratory tract infections in primary care: Prospective audit in 18 European countries. BJGP Open 2022. [Google Scholar] [CrossRef]
- Moberg, A.B.; Cronberg, O.; Falk, M.; Hedin, K. Change in the use of diagnostic tests in the management of lower respiratory tract infections: A register-based study in primary care. BJGP Open 2020, 4, bjgpopen20X101015. [Google Scholar] [CrossRef] [Green Version]
- Haldrup, S.; Thomsen, R.W.; Bro, F.; Skov, R.; Bjerrum, L.; Søgaard, M. Microbiological point of care testing before antibiotic prescribing in primary care: Considerable variations between practices. BMC Fam. Pract. 2017, 18, 9. [Google Scholar] [CrossRef] [Green Version]
- Neumark, T.; Brudin, L.; Mölstad, S. Use of rapid diagnostic tests and choice of antibiotics in respiratory tract infections in primary healthcare—A 6-y follow-up study. Scand. J. Infect. Dis. 2010, 42, 90–96. [Google Scholar] [CrossRef]
- Engström, S.; Mölstad, S.; Lindström, K.; Nilsson, G.; Borgquist, L. Excessive use of rapid tests in respiratory tract infections in Swedish primary health care. Scand. J. Infect. Dis. 2004, 36, 213–218. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA–Annual Epidemiological Report 2019; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Steurer-Stey, C. mediX. Guidelines. Chronic. Obstructive. Pulmonary. Disease. (COPD.)—2021 Update. Available online: https://www.medix.ch/wissen/guidelines/lungenkrankheiten/copd/ (accessed on 16 May 2021).
- National Institute for Health and Care Excellence (NICE). Exceptional Surveillance of Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management: NICE Clinical Guideline NG115; NICE: London, UK, 2019. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Chronic Obstructive Pulmonary Disease (Acute Exacerbation): Antimicrobial Prescribing NICE Clinical Guideline NG114; NICE: London, UK, 2018. [Google Scholar]
- World Health Organisation. AWaRe—A New WHO Tool to Help Countries Improve Antibiotic Treatment, Increase Access and Reduce Resistance. 2019. Available online: https://adoptaware.org/ (accessed on 8 August 2020).
- ANRESIS. Swiss Antibiotic Resistance Report 2020: Usage of Antibiotics and Occurrence of Antibiotic Resistance in Bacteria from Humans and Animals in Switzerland. Available online: https://www.star.admin.ch/star/en/home/star/Newsletter-Beitraege/swiss-antibiotic-resistance-report-2020.html (accessed on 16 May 2021).
- Martínez-González, N.A.; Di Gangi, S.; Pichierri, G.; Neuner-Jehle, S.; Senn, O.; Plate, A. Time Trends and Factors Associated with Antibiotic Prescribing in Swiss Primary Care (2008 to 2020). Antibiotics 2020, 9, 837. [Google Scholar] [CrossRef]
- Cals, J.W.; Schot, M.J.; de Jong, S.A.; Dinant, G.J.; Hopstaken, R.M. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: A randomized controlled trial. Ann. Fam. Med. 2010, 8, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Cots, J.M.; López-Valcárcel, B.G.; Arranz, J.; García, G.; Ortega, J.; Gómez, M.; Guerra, G.; Monedero, M.J.; Alcántara, J.D.; et al. Interventions to reduce antibiotic prescription for lower respiratory tract infections: Happy Audit study. Eur. Respir. J. 2012, 40, 436–441. [Google Scholar] [CrossRef]
- André, M.; Schwan, A.; Odenholt, I. The use of CRP tests in patients with respiratory tract infections in primary care in Sweden can be questioned. Scand. J. Infect. Dis. 2004, 36, 192–197. [Google Scholar] [CrossRef]
- Cals, J.W.; Hood, K.; Aaftink, N.; Hopstaken, R.M.; Francis, N.A.; Dinant, G.J.; Butler, C.C. Predictors of patient-initiated reconsultation for lower respiratory tract infections in general practice. Br. J. Gen. Pract. 2009, 59, 761–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.L.; Melbye, H.; Santer, M.; Moore, M.; et al. Antibiotic Prescribing for Acute Respiratory Tract Infections 12 Months After Communication and CRP Training: A Randomized Trial. Ann. Fam. Med. 2019, 17, 125–132. [Google Scholar] [CrossRef]
- Peters, S.; Rowbotham, S.; Chisholm, A.; Wearden, A.; Moschogianis, S.; Cordingley, L.; Baker, D.; Hyde, C.; Chew-Graham, C. Managing self-limiting respiratory tract infections: A qualitative study of the usefulness of the delayed prescribing strategy. Br. J. Gen. Pract. 2011, 61, e579–e589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, B.; Hounkpatin, H.; Becque, T.; Yao, G.; Zhu, S.; Alonso-Coello, P.; Altiner, A.; Arroll, B.; Böhning, D.; Bostock, J.; et al. Delayed antibiotic prescribing for respiratory tract infections: Individual patient data meta-analysis. BMJ 2021, 373, n808. [Google Scholar] [CrossRef]
- Little, P.; Moore, M.; Kelly, J.; Williamson, I.; Leydon, G.; McDermott, L.; Mullee, M.; Stuart, B. Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: Pragmatic, factorial, randomised controlled trial. BMJ 2014, 348, g1606. [Google Scholar] [CrossRef] [Green Version]
- Hungenberg, M.; Hansen-Guzman, A.; Jankousky, K.C.; DeSanto, K. Does a delayed antibiotic prescribing strategy for respiratory infections result in fewer prescriptions compared with an immediate prescribing or no-prescribing strategy? Evid. Based Pract. 2019, 22, 1–2. [Google Scholar] [CrossRef]
- Coxeter, P.; Del Mar, C.B.; McGregor, L.; Beller, E.M.; Hoffmann, T.C. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst. Rev. 2015, 2015, Cd010907. [Google Scholar] [CrossRef] [Green Version]
- Høye, S.; Frich, J.C.; Lindbæk, M. Use and feasibility of delayed prescribing for respiratory tract infections: A questionnaire survey. BMC. Fam. Pract. 2011, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altiner, A.; Brockmann, S.; Sielk, M.; Wilm, S.; Wegscheider, K.; Abholz, H.H. Reducing antibiotic prescriptions for acute cough by motivating GPs to change their attitudes to communication and empowering patients: A cluster-randomized intervention study. J. Antimicrob. Chemother. 2007, 60, 638–644. [Google Scholar] [CrossRef] [Green Version]
- Hardy-Holbrook, R.; Aristidi, S.; Chandnani, V.; DeWindt, D.; Dinh, K. Antibiotic resistance and prescribing in Australia: Current attitudes and practice of GPs. Healthc. Infect. 2013, 18, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Abera, B.; Kibret, M.; Mulu, W. Knowledge and beliefs on antimicrobial resistance among physicians and nurses in hospitals in Amhara Region, Ethiopia. BMC Pharmacol. Toxicol. 2014, 15, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.C.; Reveles, K.R.; Attridge, R.T.; Lawson, K.A.; Mansi, I.A.; Lewis, J.S.; Frei, C.R. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med. 2014, 12, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, P.; Dorward, M.; Warner, G.; Stephens, K.; Senior, J.; Moore, M. Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: Nested observational study. BMJ 2004, 328, 444. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Liu, C.; Zhang, X.; Liu, C. Does diagnostic uncertainty increase antibiotic prescribing in primary care? NPJ Prim. Care Respir. Med. 2021, 31, 17. [Google Scholar] [CrossRef]
- Shallcross, L.J.; Rockenschaub, P.; McNulty, D.; Freemantle, N.; Hayward, A.; Gill, M.J. Diagnostic uncertainty and urinary tract infection in the emergency department: A cohort study from a UK hospital. BMC Emerg. Med. 2020, 20, 40. [Google Scholar] [CrossRef]
- Jeffs, L.; McIsaac, W.; Zahradnik, M.; Senthinathan, A.; Dresser, L.; McIntyre, M.; Tannenbaum, D.; Bell, C.; Morris, A. Barriers and facilitators to the uptake of an antimicrobial stewardship program in primary care: A qualitative study. PLoS ONE 2020, 15, e0223822. [Google Scholar] [CrossRef] [Green Version]
- Lum, E.P.M.; Page, K.; Whitty, J.A.; Doust, J.; Graves, N. Antibiotic prescribing in primary healthcare: Dominant factors and trade-offs in decision-making. Infect. Dis. Health 2018, 23, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S.; Bottle, A.; Gilbert, R.; Sharland, M. Increasing short-stay unplanned hospital admissions among children in England; time trends analysis ’97-’06. PLoS ONE 2009, 4, e7484. [Google Scholar] [CrossRef]
- Borek, A.J.; Anthierens, S.; Allison, R.; McNulty, C.A.M.; Anyanwu, P.E.; Costelloe, C.; Walker, A.S.; Butler, C.C.; Tonkin-Crine, S.; On Behalf of The Step-Up Study, T. Social and Contextual Influences on Antibiotic Prescribing and Antimicrobial Stewardship: A Qualitative Study with Clinical Commissioning Group and General Practice Professionals. Antibiotics 2020, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.J.; Halls, A.V.; Tonkin-Crine, S.; Moore, M.V.; Latter, S.E.; Little, P.; Eyles, C.; Postle, K.; Leydon, G.M. General practitioner and nurse prescriber experiences of prescribing antibiotics for respiratory tract infections in UK primary care out-of-hours services (the UNITE study). J. Antimicrob. Chemother. 2018, 73, 795–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher-Lartey, S.; Yee, M.; Gaarslev, C.; Khan, R. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: A mixed methods study. BMJ Open 2016, 6, e012244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwolsman, S.; te Pas, E.; Hooft, L.; Wieringa-de Waard, M.; van Dijk, N. Barriers to GPs’ use of evidence-based medicine: A systematic review. Br. J. Gen. Pract. 2012, 62, e511–e521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabana, M.D.; Rand, C.S.; Powe, N.R.; Wu, A.W.; Wilson, M.H.; Abboud, P.A.; Rubin, H.R. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999, 282, 1458–1465. [Google Scholar] [CrossRef]
- Wood, F.; Brookes-Howell, L.; Hood, K.; Cooper, L.; Verheij, T.; Goossens, H.; Little, P.; Godycki-Cwirko, M.; Adriaenssens, N.; Jakobsen, K.; et al. A multi-country qualitative study of clinicians’ and patients” views on point of care tests for lower respiratory tract infection. Fam. Pract. 2011, 28, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Cals, J.W.; Butler, C.C.; Hopstaken, R.M.; Hood, K.; Dinant, G.J. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: Cluster randomised trial. BMJ 2009, 338, b1374. [Google Scholar] [CrossRef] [Green Version]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.; Melbye, H.; Santer, M.; Moore, M.; et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013, 382, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Cals, J.; De Bock, L.; Beckers, P.-J.; Francis, N.; Hopstaken, R.; Hood, K.; De Bont, E.; Butler, C.; Dinant, G.-J. Enhanced communication skills and C-reactive protein point-of-care testing for respiratory tract Infection: 3.5-year follow-up of a cluster randomized trial. Ann. Fam. Med. 2013, 11, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Butler, C.C.; Simpson, S.A.; Dunstan, F.; Rollnick, S.; Cohen, D.; Gillespie, D.; Evans, M.R.; Alam, M.F.; Bekkers, M.J.; Evans, J.; et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: Practice based randomised controlled trial. BMJ 2012, 344, d8173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, N.A.; Butler, C.C.; Hood, K.; Simpson, S.; Wood, F.; Nuttall, J. Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on reconsulting and antibiotic prescribing: A cluster randomised controlled trial. BMJ 2009, 339, b2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boere, T.M.; van Buul, L.W.; Hopstaken, R.M.; van Tulder, M.W.; Twisk, J.; Verheij, T.J.M.; Hertogh, C. Effect of C reactive protein point-of-care testing on antibiotic prescribing for lower respiratory tract infections in nursing home residents: Cluster randomised controlled trial. BMJ 2021, 374, n2198. [Google Scholar] [CrossRef] [PubMed]
- le News. Switzerland Launches New Campaign against Antibiotic Abuse. Available online: https://lenews.ch/2018/11/16/switzerland-launches-new-campaign-against-antibiotic-abuse/ (accessed on 10 June 2019).
- Federal Office of Public Health. National Strategy on Antibiotic Resistance (StAR). Raising Awareness on the Use of Antibiotics Campaign: “Antibiotics: Use Wisely, Take Precisely”. Available online: https://www.bag.admin.ch/bag/en/home/das-bag/aktuell/news/news-09-11-2018.htmlandhttps://www.use-wisely-take-precisely.ch/campaign/ (accessed on 10 June 2019).
- Llor, C.; Bjerrum, L.; Munck, A.; Hansen, M.P.; Córdoba, G.C.; Strandberg, E.L.; Ovhed, I.; Radzeviciene, R.; Cots, J.M.; Reutskiy, A.; et al. Predictors for antibiotic prescribing in patients with exacerbations of COPD in general practice. Ther. Adv. Respir. Dis. 2013, 7, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callegaro, M.; Manfreda, K.L.; Vehovar, V. Web Survey Methodology; Sage: Beverly Hills, CA, USA, 2015. [Google Scholar]
- Hofmann, Y.; Berger, H.; Wingeier, B.; Huber, B.; Boggian, K.; Hug-Batschelet, H.; Rosamilia, C.; Mosimann, P.; Bielicki, J.; Horvath, L.; et al. Behandlung der Streptokokken Angina. Swiss Med. Forum 2019, 19, 481–488. [Google Scholar] [CrossRef]
- Swiss Society for Infectious Diseases. SSI Guidelines and StAR Guidelines. Available online: https://www.sginf.ch/guidelines/guidelines-overview.html (accessed on 7 July 2021).
- Puhan, D.; Vilan, S.; Huber, F. mediX Guideline Infektiologie 2019: Therapieempfehlungen. Available online: https://www.medix.ch/media/medix_gl_infektiologie_8-2019.pdf (accessed on 7 July 2021).
- Neuner-Jehle, S.; Grischott, T.; Markun, S.; Rosemann, T.; Senn, O.; Maeder, M. What interventions do general practitioners recommend avoiding? A nationwide survey from Switzerland. Swiss Med. Wkly. 2020, 150, w20283. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Summary of the Latest Data on Antibiotic Consumption in the European Union. ESAC-Net Surveillance Data. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial-consumption-in-the-EU-Annual-Epidemiological-Report-2019.pdf (accessed on 3 August 2020).
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections—Full version. Clin. Microbiol. Infect. 2011, 17 (Suppl. 6), E1–E59. [Google Scholar] [CrossRef] [Green Version]
- Miravitlles, M.; Moragas, A.; Hernández, S.; Bayona, C.; Llor, C. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest 2013, 144, 1571–1577. [Google Scholar] [CrossRef]
- Butler, C.C.; Gillespie, D.; White, P.; Bates, J.; Lowe, R.; Thomas-Jones, E.; Wootton, M.; Hood, K.; Phillips, R.; Melbye, H.; et al. C-Reactive Protein Testing to Guide Antibiotic Prescribing for COPD Exacerbations. N. Engl. J. Med. 2019, 381, 111–120. [Google Scholar] [CrossRef]
- Mata, Á.N.S.; de Azevedo, K.P.M.; Braga, L.P.; de Medeiros, G.; de Oliveira Segundo, V.H.; Bezerra, I.N.M.; Pimenta, I.; Nicolás, I.M.; Piuvezam, G. Training in communication skills for self-efficacy of health professionals: A systematic review. Hum. Resour. Health 2021, 19, 30. [Google Scholar] [CrossRef]
- Boissy, A.; Windover, A.K.; Bokar, D.; Karafa, M.; Neuendorf, K.; Frankel, R.M.; Merlino, J.; Rothberg, M.B. Communication Skills Training for Physicians Improves Patient Satisfaction. J. Gen. Intern. Med. 2016, 31, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Lambe, K.A.; O’Reilly, G.; Kelly, B.D.; Curristan, S. Dual-process cognitive interventions to enhance diagnostic reasoning: A systematic review. BMJ Qual. Saf. 2016, 25, 808–820. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, M.S.; Peterson, K.; Winthrop, K.; Cantor, A.; Lazur, B.H.; Buckley, D.I. Interventions to reduce inappropriate prescribing of antibiotics for acute respiratory tract infections: Summary and update of a systematic review. J. Int. Med. Res. 2018, 46, 3337–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, S.R.; Straus, S.E. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst. Rev. 2005, CD003539. [Google Scholar] [CrossRef]


| Participants Characteristics | p-Value | |
|---|---|---|
| Responders with demographic data, n | 151 | |
| Gender, n (%) | ||
| Female | 74 (49.0) | |
| Male | 77 (51.0) | |
| Age in years, mean (SD) | 0.001 | |
| Overall | 52 (10.1) | |
| Female | 49 (9.0) | |
| Male | 54 (10.5) | |
| Years of professional experience, mean (SD) | 0.001 | |
| Overall | 22 (10.8) | |
| Female | 19 (9.7) | |
| Male | 25 (11) | |
| Percentage of employment, % mean (SD) | <0.001 | |
| Overall | 74 (23.5) | |
| Female | 66 (19.4) | |
| Male | 81 (25.0) | |
| Number of patients per day, mean (SD) | 0.003 | |
| Overall | 22 (7.0) | |
| Female | 19 (9.7) | |
| Male | 25 (11.0) | |
| Type of practice, n (%) | ||
| Individual practice | 34 (22.5) | |
| Dual practice | 30 (19.9) | |
| Group practice | 80 (53.0) | |
| Hospital outpatient consultation | 7 (4.6) | |
| Network affiliation practice, n (%) | ||
| Network affiliated practice | 109 (72.2) | |
| Non-network affiliated practice | 38 (25.2) | |
| Hospital outpatient consultation | 4 (2.6) | |
| Dispensing type practice, n (%) | ||
| Self-dispensing practice | 92 (60.9) | |
| Non-self-dispensing practice | 56 (37.1) | |
| Hospital outpatient consultation | 3 (2.0) | |
| Theme/Subtheme | Factors, n (%) | |
|---|---|---|
| BARRIERS * | Total | 354 (44) |
| Patient-related | 111 | |
| patient wish, request, demand, pressure or expectation to receive antibiotics | 79 (71.2) | |
| negative or defensive patient attitude, rejection of antibiotics by patient | 8 (7.2) | |
| GP-related | 116 | |
| fear of complications or side effects of treatment | 40 (34.5) | |
| lack of knowledge, awareness, consciousness or understanding of antibiotics and judicious antibiotic use, prescribing and ABR | 38 (32.8) | |
| Clinical-related | 116 | |
| diagnostic uncertainty, uncertain or unclear clinical picture | 47 (40.5) | |
| clinical or practice resources: consultation time too short or time pressure | 16 (13.8) | |
| clinical or practice resources: follow-up consultations under time pressure or not possible | 11 (9.5) | |
| Regulation measures/sources | 8 | |
| lack of guidance or clear recommendations for treatment and management of disease, or guidance not available in a timely fashion (immediately, when it is needed) | 3 (37.5) | |
| lack of effective or stricter measures or procedures to appropriately moderate antibiotics use | 2 (25.0) | |
| Society-related | 2 | |
| environmental impact | 1 (50.0) | |
| pressure from others or the society to be fit | 1 (50.0) | |
| Evidence-based | 1 | |
| need for better, new or updated evidence-based medical resources and information | 1 (100) | |
| FACILITATORS ‡ | Total | 445 (56) |
| Patient-related | 33 | |
| (well) informed patient or (good) patient information e.g., leaflets, websites, etc | 10 (30.3) | |
| patient wish, request or expectation to be treated without antibiotics or according to the evidence | 5 (15.2) | |
| patient consent or patient collaboration or patient cooperation | 5 (15.2) | |
| GP-related | 87 | |
| physicians’ experience | 27 (31.0) | |
| (more) education (prevention) and (good) training for GPs about judicious antibiotic consumption, prescribing and ABR, e.g., leaflets and programs | 13 (14.9) | |
| knowledge, awareness or perception of disease, e.g., risks, effects, treatment choice, complications (e.g., hospitalisation), effects of broad-spectrum antibiotics | 8 (9.2) | |
| Clinical-related | 233 | |
| good access to or availability of (additional, specific or appropriate choice of) diagnostic tests or laboratory in the practice setting | 64 (27.5) | |
| clear or accurate clinical picture, diagnosis or course of disease | 43 (18.5) | |
| clear symptomatology, underlying condition, severity of disease or comorbidities | 32 (13.7) | |
| Regulation measures/sources | 76 | |
| clear, effective, properly updated or rapidly accessible local and international guidelines for disease management and routine prescribing procedures | 56 (73.7) | |
| clear, effective or properly updated guidance specific for antibiotic prescribing and use | 8 (10.5) | |
| Society-related | 5 | |
| Media or media reports “available” to the whole population and society | 2 (40.0) | |
| (increasing or growing) population knowledge or understanding of antibiotics and ABR | 1 (20.0) | |
| Evidence-based | 11 | |
| use of new or updated evidence-based resources, information, science and research | 10 (90.9) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-González, N.A.; Plate, A.; Jäger, L.; Senn, O.; Neuner-Jehle, S. The Role of Point-of-Care C-Reactive Protein Testing in Antibiotic Prescribing for Respiratory Tract Infections: A Survey among Swiss General Practitioners. Antibiotics 2022, 11, 543. https://doi.org/10.3390/antibiotics11050543
Martínez-González NA, Plate A, Jäger L, Senn O, Neuner-Jehle S. The Role of Point-of-Care C-Reactive Protein Testing in Antibiotic Prescribing for Respiratory Tract Infections: A Survey among Swiss General Practitioners. Antibiotics. 2022; 11(5):543. https://doi.org/10.3390/antibiotics11050543
Chicago/Turabian StyleMartínez-González, Nahara Anani, Andreas Plate, Levy Jäger, Oliver Senn, and Stefan Neuner-Jehle. 2022. "The Role of Point-of-Care C-Reactive Protein Testing in Antibiotic Prescribing for Respiratory Tract Infections: A Survey among Swiss General Practitioners" Antibiotics 11, no. 5: 543. https://doi.org/10.3390/antibiotics11050543
APA StyleMartínez-González, N. A., Plate, A., Jäger, L., Senn, O., & Neuner-Jehle, S. (2022). The Role of Point-of-Care C-Reactive Protein Testing in Antibiotic Prescribing for Respiratory Tract Infections: A Survey among Swiss General Practitioners. Antibiotics, 11(5), 543. https://doi.org/10.3390/antibiotics11050543






