The Role of Antimicrobial Resistance in Refractory and Recurrent Bacterial Vaginosis and Current Recommendations for Treatment
Abstract
:1. Introduction
2. In Vitro Data on Antibiotic Drug Resistance in BV-Associated Bacteria
3. In Vivo Data on Antibiotic Drug Resistance in Women with Recurrent and Refractory BV
4. Treatment of Women with Refractory and Recurrent BV
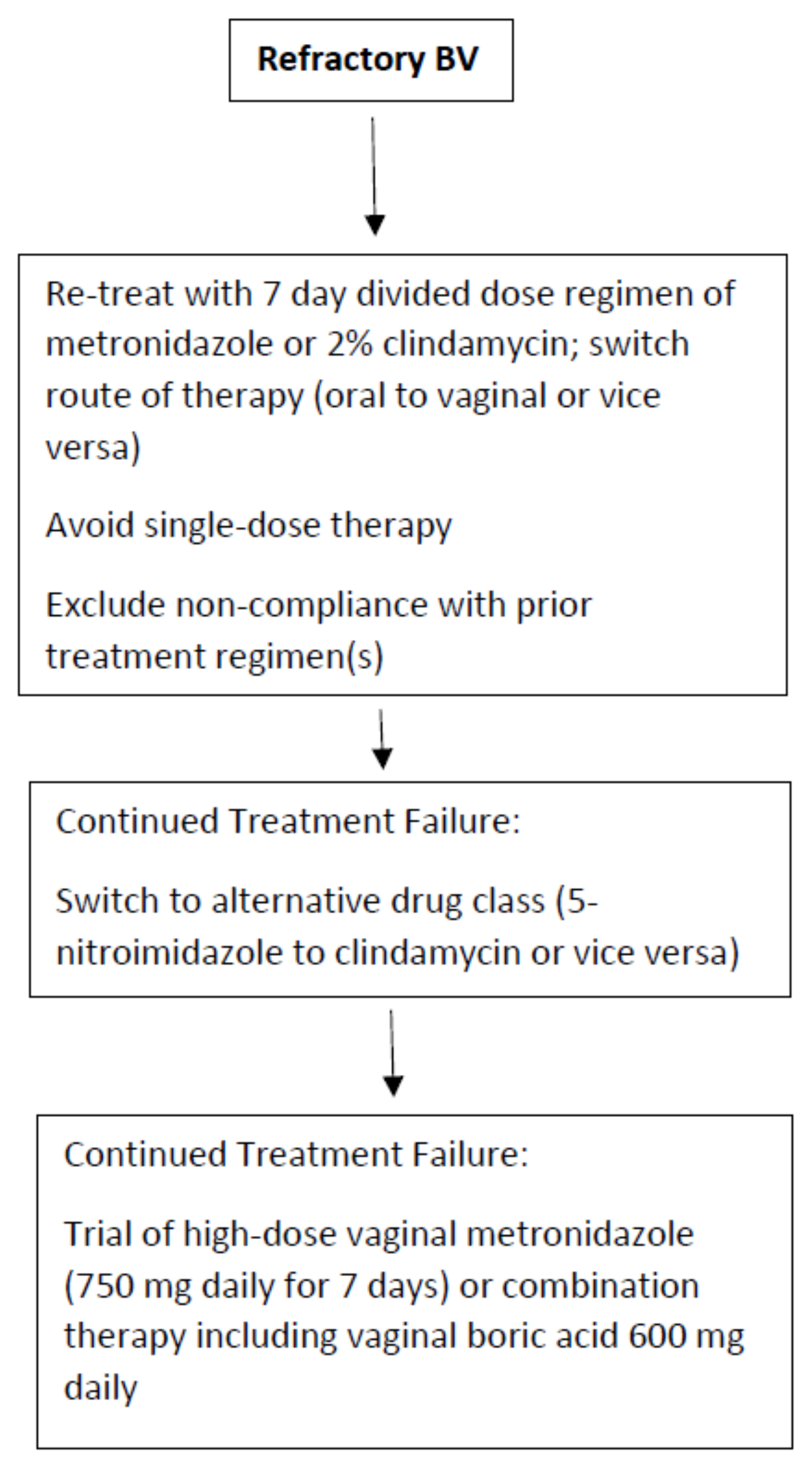
4.1. Refractory BV Treatment
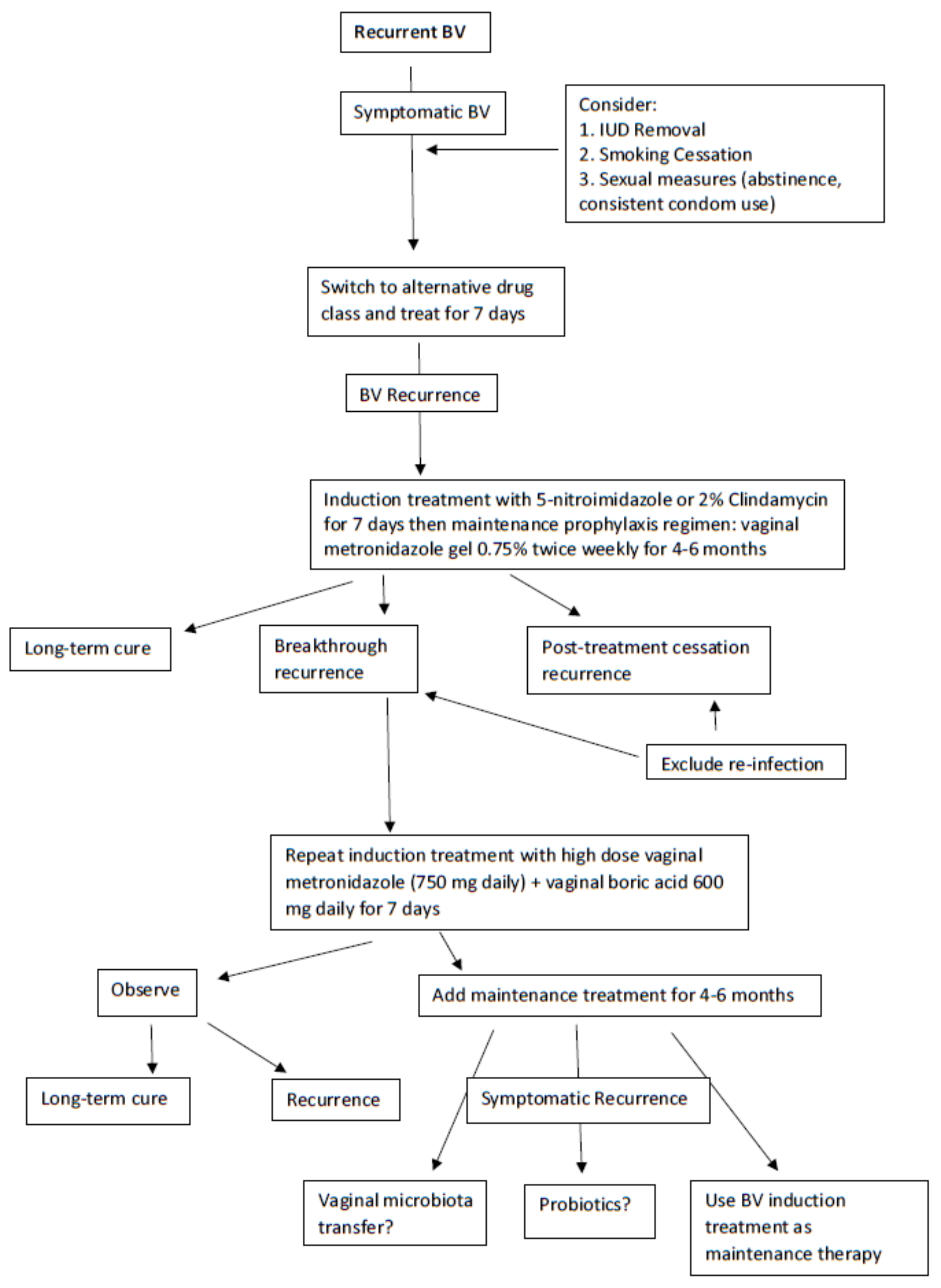
4.2. Recurrent BV (RBV) Treatment
5. Challenges in Conducting Research Studies of Women with Recurrent and Refractory BV and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peebles, K.; Velloza, J.; Balkus, J.E.; McClelland, R.S.; Barnabas, R.V. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2019, 46, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Leitich, H.; Bodner-Adler, B.; Brunbauer, M.; Kaider, A.; Egarter, C.; Husslein, P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am. J. Obstet. Gynecol. 2003, 189, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Kip, K.E.; Hillier, S.L.; Soper, D.E.; Stamm, C.A.; Sweet, R.L.; Rice, P.; Richter, H.E. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am. J. Epidemiol. 2005, 162, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, H.L., Jr.; Richardson, B.A.; Nyange, P.M.; Lavreys, L.; Hillier, S.L.; Chohan, B.; Mandaliya, K.; Ndinya-Achola, J.O.; Bwayo, J.; Kreiss, J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 1999, 180, 1863–1868. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005, 106 Pt 1, 1013–1023. [Google Scholar] [CrossRef] [Green Version]
- Schwebke, J.R.; Muzny, C.A.; Josey, W.E. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: A conceptual model. J. Infect. Dis. 2014, 210, 338–343. [Google Scholar] [CrossRef]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef]
- Muzny, C.A.; Laniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R. Gardnerella vaginalis: Still a Prime Suspect in the Pathogenesis of Bacterial Vaginosis. Curr. Infect. Dis. Rep. 2013, 15, 130–135. [Google Scholar] [CrossRef]
- Forcey, D.S.; Vodstrcil, L.A.; Hocking, J.S.; Fairley, C.K.; Law, M.; McNair, R.P.; Bradshaw, C.S. Factors Associated with Bacterial Vaginosis among Women Who Have Sex with Women: A Systematic Review. PLoS ONE 2015, 10, e0141905. [Google Scholar] [CrossRef] [Green Version]
- Swidsinski, A.; Doerffel, Y.; Loening-Baucke, V.; Swidsinski, S.; Verstraelen, H.; Vaneechoutte, M.; Lemm, V.; Schilling, J.; Mendling, W. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol. Obstet. Investig. 2010, 70, 256–263. [Google Scholar] [CrossRef]
- Mehta, S.D. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex. Transm. Dis. 2012, 39, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Schwebke, J.R.; Lensing, S.Y.; Lee, J.; Muzny, C.A.; Pontius, A.; Woznicki, N.; Aguin, T.; Sobel, J.D. Treatment of Male Sexual Partners of Women With Bacterial Vaginosis: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2021, 73, e672–e679. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Sobel, J.D.; Ferris, D.; Schwebke, J.; Nyirjesy, P.; Wiesenfeld, H.C.; Peipert, J.; Soper, D.; Ohmit, S.E.; Hillier, S.L. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am. J. Obstet. Gynecol. 2006, 194, 1283–1289. [Google Scholar] [CrossRef]
- Surapaneni, S.; Akins, R.; Sobel, J.D. Recurrent Bacterial Vaginosis: An Unmet Therapeutic Challenge. Experience with a Combination Pharmacotherapy Long-Term Suppressive Regimen. Sex. Transm. Dis. 2021, 48, 761–765. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Swidsinski, S.; Dörffel, Y.; Scholze, J.; Lochs, H.; Verstraelen, H. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 2008, 198, 97.e1–97.e6. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins—Gynecology. Vaginitis in Nonpregnant Patients: ACOG Practice Bulletin, Number 215. Obstet. Gynecol. 2020, 135, e1–e17. [Google Scholar] [CrossRef]
- Nagaraja, P. Antibiotic resistance of Gardnerella vaginalis in recurrent bacterial vaginosis. Indian J. Med. Microbiol. 2008, 26, 155–157. [Google Scholar]
- Li, T.; Zhang, Z.; Wang, F.; He, Y.; Zong, X.; Bai, H.; Liu, Z. Antimicrobial Susceptibility Testing of Metronidazole and Clindamycin against Gardnerella vaginalis in Planktonic and Biofilm Formation. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 1361825. [Google Scholar] [CrossRef]
- Petrina, M.A.B.; Cosentino, L.A.; Rabe, L.K.; Hillier, S.L. Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin. Anaerobe 2017, 47, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haggoud, A.; M’Hand, R.A.; Reysset, G.; El M’Daghri, N.; Benbachir, M.; Moumni, M. Prevalence and characteristics of nim genes encoding 5-nitroimidazole resistance among Bacteroides strains isolated in Morocco. Microb. Drug. Resist. 2001, 7, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.P.; Sellier, N.; Rager, M.N.; Reysset, G. Metabolism of a 5-nitroimidazole in susceptible and resistant isogenic strains of Bacteroides fragilis. Antimicrob. Agents Chemother. 1997, 41, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Bannatyne, R.M.; Smith, A.M. Recurrent bacterial vaginosis and metronidazole resistance in Gardnerella vaginalis. Sex. Transm. Infect. 1998, 74, 455–456. [Google Scholar]
- Aroutcheva, A.A.; Simoes, J.A.; Behbakht, K.; Faro, S. Gardnerella vaginalis isolated from patients with bacterial vaginosis and from patients with healthy vaginal ecosystems. Clin. Infect. Dis. 2001, 33, 1022–1027. [Google Scholar] [CrossRef] [Green Version]
- Hill, G.B. The microbiology of bacterial vaginosis. Am. J. Obstet. Gynecol. 1993, 169 Pt 2, 450–454. [Google Scholar] [CrossRef]
- Patterson, J.L.; Stull-Lane, A.; Girerd, P.H.; Jefferson, K.K. Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes. Microbiology 2010, 156 Pt 2, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Beigi, R.H.; Austin, M.N.; Meyn, L.A.; Krohn, M.A.; Hillier, S.L. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am. J. Obstet. Gynecol. 2004, 191, 1124–1129. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Woody, J.; Hunt, C.; Budd, W. Antimicrobial resistance genes and modelling of treatment failure in bacterial vaginosis: Clinical study of 289 symptomatic women. J. Med. Microbiol. 2016, 65, 377–386. [Google Scholar] [CrossRef]
- Deng, Z.-L.; Gottschick, C.; Bhuju, S.; Masur, C.; Abels, C.; Wagner-Döbler, I. Metatranscriptome Analysis of the Vaginal Microbiota Reveals Potential Mechanisms for Protection against Metronidazole in Bacterial Vaginosis. mSphere 2018, 3, e00262-18. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Perez, D.; Coudray, M.S.; Colbert, B.; Krupp, K.; Kumari, H.; Stebliankin, V.; Mathee, K.; Cook, R.L.; Schwebke, J.; Narasimhan, G.; et al. Effect of metronidazole on vaginal microbiota associated with asymptomatic bacterial vaginosis. Access Microbiol. 2021, 3, 000226. [Google Scholar] [CrossRef]
- Sobel, J.D.; Sobel, R. Current and emerging pharmacotherapy for recurrent bacterial vaginosis. Expert Opin. Pharmacother. 2021, 22, 1593–1600. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Desmond, R.A. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin. Infect. Dis. 2007, 44, 213–219. [Google Scholar] [CrossRef]
- Sanchez, S.; Garcia, P.J.; Thomas, K.K.; Catlin, M.; Holmes, K.K. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: A randomized controlled trial. Am. J. Obstet. Gynecol. 2004, 191, 1898–1906. [Google Scholar] [CrossRef]
- Aguin, T.J.; Akins, R.A.; Sobel, J.D. High-dose vaginal metronidazole for recurrent bacterial vaginosis—A pilot study. J. Low Genit. Tract Dis. 2014, 18, 156–161. [Google Scholar] [CrossRef]
- Sobel, J.D.; Kaur, N.; A Woznicki, N.; Boikov, D.; Aguin, T.; Gill, G.; Akins, R.A. Conventional oral and secondary high dose vaginal metronidazole therapy for recurrent bacterial vaginosis: Clinical outcomes, impacts of sex and menses. Infect. Drug Resist. 2019, 12, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- Vodstrcil, L.A.; Muzny, C.A.; Plummer, E.L.; Sobel, J.D.; Bradshaw, C.S. Bacterial vaginosis: Drivers of recurrence and challenges and opportunities in partner treatment. BMC Med. 2021, 19, 194. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent bacterial vaginosis, relapse or reinfection: The role of sexual transmission. BJOG 2021, 128, 768. [Google Scholar] [CrossRef]
- Peebles, K.; Kiweewa, F.M.; Palanee-Phillips, T.; Chappell, C.; Singh, D.; Bunge, K.E.; Naidoo, L.; Makanani, B.; Jeenarain, N.; Reynolds, D.; et al. Elevated Risk of Bacterial Vaginosis Among Users of the Copper Intrauterine Device: A Prospective Longitudinal Cohort Study. Clin. Infect. Dis. 2021, 73, 513–520. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; Dombrowski, J.C.; Wierzbicki, M.R.; Perlowski, C.; Pontius, A.; Dithmer, D.; Schwebke, J. Safety and Efficacy of a Novel Vaginal Anti-infective, TOL-463, in the Treatment of Bacterial Vaginosis and Vulvovaginal Candidiasis: A Randomized, Single-blind, Phase 2, Controlled Trial. Clin. Infect. Dis. 2019, 68, 803–809. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Carter, B.A.; Waldbaum, A.S.; Agnew, K.J.; Paull, J.R.; Price, C.F.; Castellarnau, A.; McCloud, P.; Kinghorn, G.R. A phase 3, randomized, controlled trial of Astodrimer 1% Gel for preventing recurrent bacterial vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 10, 100121. [Google Scholar] [CrossRef] [PubMed]
- Van de Wijgert, J.; Verwijs, M.C. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG 2020, 127, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Senok, A.C.; Verstraelen, H.; Temmerman, M.; Botta, G.A. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst. Rev. 2009, 4, CD006289. [Google Scholar] [CrossRef]
- Cohen, C.R.; Wierzbicki, M.R.; French, A.L.; Morris, S.; Newmann, S.; Reno, H.; Green, L.; Miller, S.; Powell, J.; Parks, T.; et al. Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis. N. Engl. J. Med. 2020, 382, 1906–1915. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year | Bacterial Species, Number of Isolates Tested | Antibiotics Used in Susceptibility Testing | Results | Conclusions |
|---|---|---|---|---|
| Nagaraja, 2008 [19] | 50 clinical isolates of G. vaginalis | MTZ, clindamycin | 34 (68%) of isolates resistant to MTZ; 38 (76%) of isolates sensitive to clindamycin | Clindamycin is better in eradicating G. vaginalis than MTZ in vitro |
| Petrina, 2017 [21] | 605 BVAB | MTZ, TDZ, SEC, clindamycin | MIC90 for SEC was similar to MTZ and TDZ for A. tetradius, A. vaginae, Bacteroides spp., F. magna, G. vaginalis, M. indolicus, Megasphaera-like bacteria, M. curtisii, M. mulieris, P. lacrimalis, P. harei, Porphyromonas spp., P. bivia, P. amnii, and P. timonensis. A proportion of P. bivia (40%), P. amnii (14%), and P. timonensis (58%) isolates were resistant to clindamycin with MIC values > 128 µg/mL. MTZ and SEC were superior to clindamycin for Prevotella spp., Bacteroides spp., A. tetradius, and F. magna. In contrast, clindamycin had greater activity against A. vaginae, G. vaginalis, and Mobiluncus spp. compared to 5-nitroimidazoles | More than a third of the Prevotella spp. were resistant to clindamycin SEC has similar in vitro activity against a range of BVAB compared to MTZ or TDZ. It also spares vaginal lactobacilli (data not shown) |
| Li, 2020 | 10 clinical isolates of G. vaginalis | MTZ, clindamycin at planktonic and biofilm levels | Planktonic isolates had greater susceptibility (76.7% vs. 38.2%) and lower resistance (23.3% vs. 58.5%) to clindamycin vs. MTZ (p < 0.05 for both) In comparison to planktonic isolates, the MIC of MTZ was higher for biofilm-forming isolates, the resistance rate was 27.3%, and the MBEC was >128 µg/mL. The MIC of clindamycin was also higher for biofilm-forming isolates compared to planktonic isolates, the resistance rate was 27.3%, and the MBEC was 28.4 ± 6.50 µg/mL | Clindamycin may be a better treatment option than MTZ for G. vaginalis, as it exhibits relatively higher susceptibility and lower resistance rates in vitro |
| First Author, Year | Patient Population | Bacterial Species Tested, Antibiotics Used | Results | Conclusions |
|---|---|---|---|---|
| Bannatyne, 1998 [25] | 80 women with single or multiple episodes of symptomatic BV pre- and post-treatment with 2 g oral MTZ daily for 2–5 days | G. vaginalis isolates; MTZ | 88–100% pre-treatment isolates were susceptible to MTZ, based on the number of BV episodes The number of susceptible isolates after first (76–82%), second (53–82%), third (36%), and fourth (0%) rounds of treatment, respectively, declined | Recurrent BV infections were more likely due to relapse than re-infection |
| Aroutcheva, 2001 [26] | 117 women, 27.4% of whom had BV | G. vaginalis isolates; MTZ | G. vaginalis biotypes 5 and 7 were most resistant to MTZ although biotype 5 was predominantly associated with a healthy vaginal microbiota (p = 0.0004) | No specific phenotype or genotype of G. vaginalis causes BV |
| Beigi, 2004 [29] | 95 non-pregnant women with BV pre- and post-treatment (47 received vaginal MTZ for 5 days and 48 received vaginal clindamycin ovules for 3 days) | 1059 BVAB; MTZ, clindamycin | Pre-treatment: <1% and 17% of BVAB were resistant to MTZ and clindamycin, respectively Post-treatment: no increase in MTZ resistance in BVAB although 53% were resistant to clindamycin | Treatment of BV with clindamycin is associated with marked evidence of antimicrobial resistance among BVAB |
| Bostwick, 2016 [30] | 326 age-matched nongravid women of reproductive age with and without BV | Next-generation sequencing used to describe the complete vaginal microbiota and identify bacterial genes associated with resistance to a wide range of antibiotics | AMR genes were identified in all drug classes tested: macrolides 35.2%; lincosamides, 35.6%; tetracyclines, 21.8%; aminoglycosides (streptomycin, gentamicin and tobramycin), 5.2% each; 5-nitroimidazoles, 0.3%;triazoles, 18.7% There was more than a fourfold-higher frequency of AMR genes in pathogens from BV than from non-BV patients for macrolides (58.2 versus 12.3%), lincosamides (58.9 versus 12.3%) and tetracyclines (35.6 versus 8.0%), respectively | AMR genes were present in the majority of vaginal microbiomes of women with symptomatic BV |
| Deng, 2018 [31] | 37 women with BV, of which 31 were successfully treated with MTZ | Meta-transcriptomic analysis of the vaginal microbiota was performed, comparing women who responded to BV treatment versus those who did not | 7 of 8 clustered regularly interspaced short palindromic repeat (CRISPR)-associated (Cas) genes of G. vaginalis were highly upregulated in women with persistent BV | The CRISPR-Cas system may protect the vaginal microbiota against the DNA damaging effect of MTZ; suppressing these genes may improve the antibiotic therapy of BV |
| Ruiz-Perez, 2021 [32] | 5 African American women ages 19–22 with asymptomatic BV at baseline followed over 1 year; women received oral MTZ for each BV episode during this timeframe | Whole-genome sequencing was used to determine changes in the vaginal microbiota among women with BV treated with MTZ | Despite treatment, none of the 5 women reverted to normal vaginal microbiota during the study; 2 were consistently positive for BV while 3 experienced intermittent infection Gardnerella spp. were the most highly abundant bacterial spp. associated with BV. After treatment with MTZ, there was a decline in the relative abundance of Lactobacillus and Prevotella spp. and an increase in the relative abundance of Gardnerella spp. over time The metagenome of all participants contained AMR genes | This study showed specific microbiota changes with treatment, presence of many AMR genes, and recurrence and persistence of BV despite use of MTZ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzny, C.A.; Sobel, J.D. The Role of Antimicrobial Resistance in Refractory and Recurrent Bacterial Vaginosis and Current Recommendations for Treatment. Antibiotics 2022, 11, 500. https://doi.org/10.3390/antibiotics11040500
Muzny CA, Sobel JD. The Role of Antimicrobial Resistance in Refractory and Recurrent Bacterial Vaginosis and Current Recommendations for Treatment. Antibiotics. 2022; 11(4):500. https://doi.org/10.3390/antibiotics11040500
Chicago/Turabian StyleMuzny, Christina A., and Jack D. Sobel. 2022. "The Role of Antimicrobial Resistance in Refractory and Recurrent Bacterial Vaginosis and Current Recommendations for Treatment" Antibiotics 11, no. 4: 500. https://doi.org/10.3390/antibiotics11040500
APA StyleMuzny, C. A., & Sobel, J. D. (2022). The Role of Antimicrobial Resistance in Refractory and Recurrent Bacterial Vaginosis and Current Recommendations for Treatment. Antibiotics, 11(4), 500. https://doi.org/10.3390/antibiotics11040500






