A Systematic Review on Clinical Safety and Efficacy of Vancomycin Loading Dose in Critically Ill Patients
Abstract
1. Introduction
2. Results
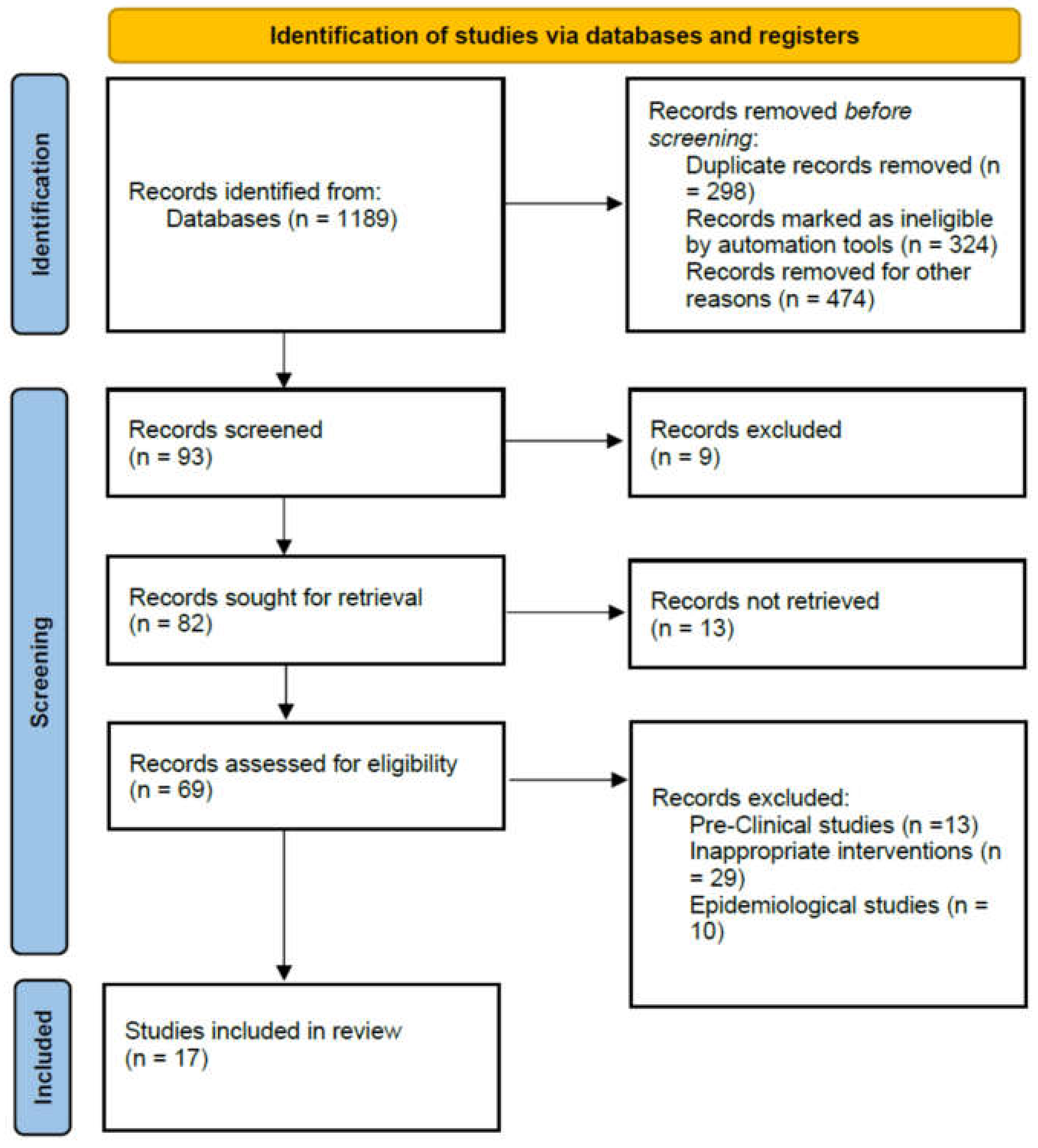
2.1. Literature Search
2.2. Study Characteristics
2.3. Attainment of Target Therapeutic Concentration and Clinical Response
2.4. Nephrotoxicity and Other Adverse Events
3. Discussion
4. Materials and Methods
4.1. Literature Search
4.2. Study Selection
4.3. Data Extraction
4.4. Article Quality Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takesue, Y.; Ohmagari, N.; Mochizuki, T.; Mikamo, H.; Seki, M.; Takakura, S.; Tokimatsu, I.; Takahashi, Y.; Kasahara, K. Practice guidelines for therapeutic drug monitoring of vancomycin: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013, 19, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-K.; Chen, Y.-L.; Chen, K.; Zhang, X.-L.; Du, G.-H.; He, B.; Li, D.-K.; Liu, Y.-N.; Yang, K.-H.; Zhang, Y.-Y. Therapeutic drug monitoring of vancomycin: A guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. J. Antimicrob. Chemother. 2016, 71, 3020–3025. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Haseeb, A.; Faidah, H.S.; Algethamy, M.; Alghamdi, S.; Alhazmi, G.A.; Alshomrani, A.O.; Alqethami, B.R.; Alotibi, H.S.; Almutiri, M.Z.; Almuqati, K.S. Antimicrobial Usage and Resistance in Makkah Region Hospitals: A Regional Point Prevalence Survey of Public Hospitals. Int. J. Environ. Res. Public Health 2022, 19, 254. [Google Scholar] [CrossRef]
- Saleem, Z.; Saeed, H.; Hassali, M.A.; Godman, B.; Asif, U.; Yousaf, M.; Ahmed, Z.; Riaz, H.; Raza, S.A. Pattern of inappropriate antibiotic use among hospitalized patients in Pakistan: A longitudinal surveillance and implications. Antimicrob. Resist. Infect. Control. 2019, 8, 188. [Google Scholar] [CrossRef]
- Haseeb, A.; Faidah, H.S.; Alghamdi, S.; Alotaibi, A.F.; Elrggal, M.E.; Mahrous, A.J.; Almarzoky Abuhussain, S.S.; Obaid, N.A.; Algethamy, M.; AlQarni, A. Dose Optimization of Colistin: A Systematic Review. Antibiotics 2021, 10, 1454. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C., Jr.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic Monitoring of Vancomycin in Adults: Summary of Consensus Recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 1275–1279. [Google Scholar] [CrossRef]
- Simor, A.E.; Gilbert, N.L.; Gravel, D.; Mulvey, M.R.; Bryce, E.; Loeb, M.; Matlow, A.; McGeer, A.; Louie, L.; Campbell, J. Methicillin-resistant Staphylococcus aureus colonization or infection in Canada: National surveillance and changing epidemiology, 1995–2007. Infect. Control. Hosp. Epidemiol. 2010, 31, 348–356. [Google Scholar] [CrossRef]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef]
- Tsutsuura, M.; Moriyama, H.; Kojima, N.; Mizukami, Y.; Tashiro, S.; Osa, S.; Enoki, Y.; Taguchi, K.; Oda, K.; Fujii, S. The monitoring of vancomycin: A systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect. Dis. 2021, 21, 153. [Google Scholar] [CrossRef]
- Dilworth, T.J.; Schulz, L.T.; Rose, W.E. Vancomycin advanced therapeutic drug monitoring: Exercise in futility or virtuous endeavor to improve drug efficacy and safety? Clin. Infect. Dis. 2021, 72, e675–e681. [Google Scholar] [CrossRef]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Rybak, M.J. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: Support for consensus guidelines suggested targets. Clin. Infect. Dis. 2011, 52, 975–981. [Google Scholar] [CrossRef]
- Holmes, N.E.; Turnidge, J.D.; Munckhof, W.J.; Robinson, J.O.; Korman, T.M.; O’Sullivan, M.V.; Anderson, T.L.; Roberts, S.A.; Warren, S.J.; Gao, W. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2013, 57, 1654–1663. [Google Scholar] [CrossRef]
- Gawronski, K.M.; Goff, D.A.; Brown, J.; Khadem, T.M.; Bauer, K.A. A stewardship program’s retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin. Ther. 2013, 35, 772–779. [Google Scholar] [CrossRef]
- Haseeb, A.; Abourehab, M.A.; Almalki, W.A.; Almontashri, A.M.; Bajawi, S.A.; Aljoaid, A.M.; Alsahabi, B.M.; Algethamy, M.; AlQarni, A.; Iqbal, M.S. Trimethoprim-Sulfamethoxazole (Bactrim) Dose Optimization in Pneumocystis jirovecii Pneumonia (PCP) Management: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2833. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotscahfer, J.C.; Moellering, R.C., Jr.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Alhifany, A.A.; Alqurashi, A.F.; Al-Agamy, M.H.; Alkhushaym, N.; Alhomoud, F.; Alhomoud, F.K.; Almangour, T.A. Employment of mapping technology in antimicrobial resistance reporting in Saudi Arabia. Geospat. Health 2020, 15, 868. [Google Scholar] [CrossRef]
- Almeleebia, T.M.; Alhifany, A.A.; Almutairi, F.; Alshibani, M.; Alhossan, A.M. Regulating antimicrobial sales in Saudi Arabia: Achievements and challenges. Int. J. Clin. Pract. 2021, 75, e13833. [Google Scholar] [CrossRef]
- Saleem, Z.; Godman, B.; Azhar, F.; Kalungia, A.C.; Fadare, J.; Opanga, S.; Markovic-Pekovic, V.; Hoxha, I.; Saeed, A.; Al-Gethamy, M. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti Infect. Ther. 2022, 20, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, A.; Finkelstein, Y.; Nava-Ocampo, A.; Arnold, A.; Jones, S.; Monuteaux, M.; Sandora, T.J.; Patterson, A.; Harper, M.B. A randomized controlled trial of a vancomycin loading dose in children. Pediatr. Infect. Dis. J. 2013, 32, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Rosini, J.M.; Laughner, J.; Levine, B.J.; Papas, M.A.; Reinhardt, J.F.; Jasani, N.B. A randomized trial of loading vancomycin in the emergency department. Ann. Pharmacother. 2015, 49, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wesolek, J.L.; McNorton, K.; Delgado, G., Jr.; Giuliano, C.A. Effect of vancomycin initial dosing on time to systemic inflammatory response syndrome resolution in patients with methicillin-resistant Staphylococcus aureus bacteremia. J. Chemother. 2018, 30, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Takesue, Y.; Nakajima, K.; Ichiki, K.; Ishikawa, K.; Takai, Y.; Yamada, K.; Wada, Y.; Tsuchida, T.; Otani, N. Vancomycin loading dose is associated with increased early clinical response without attainment of initial target trough concentration at a steady state in patients with methicillin-resistant Staphylococcus aureus infections. J. Clin. Pharm. Ther. 2020, 45, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.G.; Huh, K.; Sohn, Y.M.; Park, H.J.; Na, S.J.; Jeon, K. Effect of vancomycin loading dose on clinical outcome in critically ill patients with methicillin-resistant Staphylococcus aureus pneumonia. J. Thorac. Dis. 2021, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Ortwine, J.K.; Zasowski, E.J.; Pogue, J.M.; Hanni, C.; Giuliano, C.; Casapao, A.M.; Mynatt, R.; Rybak, M.J. Relationship status between vancomycin loading dose and treatment failure in patients with MRSA bacteremia: It’s complicated. Infect. Dis. Ther. 2019, 8, 627–640. [Google Scholar] [CrossRef]
- Flannery, A.H.; Wallace, K.L.; Rhudy, C.N.; Olmsted, A.S.; Minrath, R.C.; Pope, S.M.; Cook, A.M.; Burgess, D.S.; Morris, P.E. Efficacy and safety of vancomycin loading doses in critically ill patients with methicillin-resistant Staphylococcus aureus infection. Ther. Adv. Infect. Dis. 2021, 8, 20499361211005965. [Google Scholar] [CrossRef]
- Cheong, J.; Makmor-Bakry, M.; Lau, C.; Rahman, R.A. The relationship between trough concentration of vancomycin and effect on methicillin-resistant Staphylococcus aureus in critically ill patients. S. Afr. Med. J. 2012, 102, 616–619. [Google Scholar] [CrossRef]
- Marvin, J.L.; Levine, B.J.; Papas, M.; Rosini, J.M. An evaluation of the incidence of nephrotoxicity after a loading dose of vancomycin in patients with severe renal impairment. J. Emerg. Med. 2019, 56, 701–708. [Google Scholar] [CrossRef]
- Dolan, E.; Hellinga, R.; London, M.; Ryan, K.; Dehority, W. Effect of Vancomycin Loading Doses on the Attainment of Target Trough Concentrations in Hospitalized Children. J. Pediatr. Pharmacol. Ther. 2020, 25, 423–430. [Google Scholar] [CrossRef]
- Al-Mazraawy, B.O.; Girotto, J.E. Comparing Vancomycin Area Under the Curve with a Pharmacist Protocol that Incorporates Trough and Maximum Doses at a Children’s Hospital. J. Pediatr. Pharmacol. Ther. 2021, 26, 740–745. [Google Scholar] [CrossRef]
- Rosini, J.M.; Davis, J.J.; Muenzer, J.; Levine, B.J.; Papas, M.A.; Comer, D.; Arnold, R. High single-dose vancomycin loading is not associated with increased nephrotoxicity in emergency department sepsis patients. Acad. Emerg. Med. 2016, 23, 744–746. [Google Scholar] [CrossRef][Green Version]
- Truong, J.; Levkovich, B.; Padiglione, A. Simple approach to improving vancomycin dosing in intensive care: A standardised loading dose results in earlier therapeutic levels. Intern. Med. J. 2012, 42, 23–29. [Google Scholar] [CrossRef]
- Golenia, B.S.; Levine, A.R.; Moawad, I.M.; Yeh, D.D.; Arpino, P.A. Evaluation of a vancomycin dosing nomogram based on the modification of diet in renal disease equation in intensive care unit patients. J. Crit. Care 2013, 28, 710–716. [Google Scholar] [CrossRef]
- Álvarez, O.; Plaza-Plaza, J.C.; Ramirez, M.; Peralta, A.; Amador, C.A.; Amador, R. Pharmacokinetic assessment of vancomycin loading dose in critically ill patients. Antimicrob. Agents Chemother. 2017, 61, e00280-17. [Google Scholar] [CrossRef]
- Hodiamont, C.; Juffermans, N.; Berends, S.; van Vessem, D.; Hakkens, N.; Mathôt, R.; de Jong, M.; van Hest, R. Impact of a vancomycin loading dose on the achievement of target vancomycin exposure in the first 24 h and on the accompanying risk of nephrotoxicity in critically ill patients. J. Antimicrob. Chemother. 2021, 76, 2941–2949. [Google Scholar] [CrossRef]
- Denetclaw, T.H.; Dowling, T.C.; Steinke, D. Performance of a divided-load intravenous vancomycin dosing strategy for critically ill patients. Ann. Pharmacother. 2013, 47, 1611–1617. [Google Scholar] [CrossRef]
- Mei, H.; Wang, J.; Che, H.; Wang, R.; Cai, Y. The clinical efficacy and safety of vancomycin loading dose: A systematic review and meta-analysis. Medicine 2019, 98, e17639. [Google Scholar] [CrossRef]
- Soto, J.; Sacristan, J.; Alsar, M. Necessity of a loading dose when using vancomycin in critical-ill patients. J. Antimicrob. Chemother. 1991, 27, 875. [Google Scholar] [CrossRef] [PubMed]
- Mohammedi, I.; Descloux, E.; Argaud, L.; Le Scanff, J.; Robert, D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int. J. Antimicrob. Agents 2006, 27, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Vuagnat, A.; Stern, R.; Lotthe, A.; Schuhmacher, H.; Duong, M.; Hoffmeyer, P.; Bernard, L. High dose vancomycin for osteomyelitis: Continuous vs. intermittent infusion. J. Clin. Pharm. Ther. 2004, 29, 351–357. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies. 2014. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 September 2021).
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
| Author and Year | Reference | Study Design | Sample Size | Characteristics of Patients | Dosing Practice | Clinical Outcomes | Inference/Recommendation |
|---|---|---|---|---|---|---|---|
| Patients with MRSA infections | |||||||
| Wesolek et al. (2018) | [25] | Single-center retrospective cohort study | 124 | Sepsis patients due to MRSA infection | LD: >20 mg/kg, Non-LD: <20 mg/kg | LD versus non-LD median time to SIRS resolution (h): 67 versus 109; clinical responder (improvement or culture negative): 30/37 versus 73/87 | LD versus non-LD mortality: 7/37 versus 20/87; time to negative blood culture (h): 102.25 ± 71.23 versus 99.60 ± 71.06. Length of stay (h): 14.07 ± 10.03 versus 15.33 ± 8.60 |
| Ueda et al. (2020) | [26] | Retrospective cohort | 55 | MRSA, MR-CoNS or Enterococcus faecium infected patients with normal kidney function | LD: of 25 mg/kg vancomycin followed by 15 mg/kg twice daily was compared with traditional dosing | When compared to usual dosage, an LD yielded early clinical results. Cmin did not differ significantly between the regimens with and without an LD | In patients with a normal renal function, an LD of 25 mg/kg followed by 15 mg/kg twice per day did not attain the ideal Cmin at steady state |
| Yoon et al. (2021) | [27] | Retrospective cohorts | 81 | Critically ill patients with MRSA pneumonia | LDG of 25 mg/kg followed by 15–20 mg/kg every 12 h, and non-LDG | Initial LD was not linked to a better clinical outcome or rapid pharmacological target achievement | More research is needed to provide more evidence for this widely recommended practice |
| Ortwine et al. (2019) | [28] | Retrospective cohort | 316 | Patients with MRSA Bacteremia | LD ≥ 20 mg/kg and non-LD. | Initial vancomycin doses above 1750 mg were independently protective against failure without increasing the risk for nephrotoxicity | Weight-based dosing might not be the optimal strategy |
| Flannery et al. (2021) | [29] | Retrospective cohorts | 449 | Critically ill patients with MRSA infection | LD ≥20 mg/kg actual body weight and non-LD | LD was not linked to better clinical outcomes without an increased risk of AKI. Trough 10–15 mg/L: 13/469 versus 37/458 LD versus non-LD trough 15–20 mg/L: 236/469 versus 235/458. Mortality: 34/469 versus 63/458 | At 12 and 24 h, LDs of 30 mg/kg versus 15 mg/kg resulted in higher trough values, but not at 36 h |
| Cheong et al. (2012) | [30] | Retrospective study | 58 | Critically ill adult patients in ICU with MRSA infections | No details provided | LD versus non-LD clinical responder (improvement or culture negative): 9/10 versus 34/48 | Level II evidence |
| Patients with other infections | |||||||
| Marvin et al. (2019) | [31] | Retrospective cohort | 927 | Severe renally impaired patients | High Ld (>20 mg/kg) vs. low dose (≤20 mg/kg) of vancomycin | LD did not increase nephrotoxicity when compared to the lower dose | Future studies on vancomycin LD should include these patients |
| Dolan et al. (2020) | [32] | Retrospective cohort | 151 | Children | LD 20 to 25 mg/kg and without a LD | More likely to attain a target TC quicker than non-LD with no significant differences in the frequency of serum creatinine or oliguria | Despite receiving vancomycin LD, the majority of children had subtherapeutic TC. A larger prospective investigation is needed to determine the impact of LD |
| Al-Mazraawy et al. (2021) | [33] | Retrospective cohort | 223 | Children | AUC24 goals were 400 to 600 mg·h/L, that incorporated trough and maximum doses | To achieve the AUC24, an increased initial dose is required. No clinical failures were detected | Only one patient had an AUC24 greater than 600 mg·h/L, and none had an AUC24 greater than 620 mg·h/L |
| Demirjian et al. (2013) | [23] | Single-center double- blind RCT | 59 | Children aged 2–18 years with different infections | LD: 30 mg/kg, infused over 2 h; non-LD: 20 mg/kg, infused over 2 h | Trough 15–20 mg/L and >20 mg/L at 8 h better attained with LD, but red man syndrome and nephrotoxicity also appeared in patients | This is level I evidence. Nephrotoxicity appeared in patients using concomitant nephrotoxins. However, the creatinine value became normal after 12 days |
| Rosini et al. (2015) | [24] | Single-center RCT | 99 | Adult ED patients with different infections | LD: 30 mg/kg (<3.6 g), MD: 15 mg/kg q12 h for three doses infused at a rate of <1000 mg/h; non-LD: 15 mg/kg (< 1–8g); MD: 15 mg/kg ql2h for three doses infused at a rate of < 1000 mg/h | LD versus non-LD; trough 15–20 mg/L at 12 h: 17/50 versus 1/49; trough 10–15 mg/L at 8 h: 23/50 versus 6/49; LD versus non-LD infusion reactions: 3/50 versus 2/49. Nephrotoxicity: 2/50 versus 3/49. Mortality: 1/50 versus 0/49 | This is also level I evidence. Nephrotoxicity appeared within 24 h in a few patients. No patient needed readmission or dialysis for nephrotoxicity within 30 days |
| Rosini et al. (2016) | [34] | Retrospective cohort study | 1330 | Adult ED patients | Non-LD: >20 mg/kg; MD: not mentioned; non-LD: <20 mg/kg; MD: not mentioned | LD versus non-LD nephrotoxicity: 49/851 versus 53/479 | Level II evidence |
| Truong et al. (2012) | [35] | Pre/postinterventionstudy | 82 | Adult ICU patients | LD: 2 g, infused over 4 h; MD: depend on patient clinical status; non-LD: standard therapy, MD: depend on patient clinical status | LD versus non-LD trough <15 mg/L: 18/39 versus 16/22; trough at 15–20 mg/L: 10/39 versus 4/22. Nephrotoxicity: total n = 4 (when trough >20 mg/L) | Level II evidence where both postintervention and preintervention groups had more nephrotoxicity |
| Golonia et al. (2013) | [36] | Pre/postobservational trial | 117 | Adult ICU patients | LD, post nomogram: 22.5–25 mg/kg (range 1000–2250 mg); non-LD: pre nomogram: standard therapy (1000 mg q12h) | LD versus non-LD trough <15 mg/L at initial pre-fourth dose: 17/60 versus 35/57. Trough 15–20 mg/L at initial pre-fourth dose n: 25/60 versus 11/57 trough >20 mg/L at initial pre-fourth dose n: 18/60 versus 11/57; LD versus non-LD nephrotoxicity: 11/60 versus 10/57 | Pharmacokinetic data based on eGFR via MDRD equation and actual body weight from preimplementation group were employed to develop nomogram. Nephrotoxicity appeared after 5 days in the preimplementation and postimplementation groups |
| Alvarez et al. (2017) | [37] | Concurrent cohort study | 41 | Adult critically ill patients with sepsis | LD: 25–30 mg/kg or LD based on population pharmacokinetic parameters of the critically ill patient; MD: not mentioned; non-LD: without LD (no details); MD: not mentioned | LD versus non-LD. Trough <15 mg/L within 24 h after first dose: 7/23 versus 16/18. Trough 15–20 mg/L within 24 h after first dose: 9/23 versus 1/18. Trough >20 mg/L within 24 h after first dose: 7/23 versus 1/18. | LD versus PPK-LD versus non-LD. Scr increased: 4/11 (36.3) versus 2/12 versus 6/18; no nephrotoxicity related with vancomycin was observed |
| Hodiamont et al. (2021) | [38] | Prospective observational | 82 | Critically ill patients | LDG: 25 mg/kg; conventional dose group: 1000 mg | Achieving AUC0–24 ≥ 400 mg·h/L was more significant in patients who received a weight-based LD of 25 mg/kg, without increased the risk of new-onset AKI | Patients with AUC0–24 > 400 mg·h/L had a considerably greater risk of AKI |
| Denetclaw et al. (2013) | [39] | Retrospective observational trial | 69 | Adult ICU patient | Initial dose: two doses of 15 mg/kg | Average TC (mg/L): 15.1 ± 3.4 and TC ≥14.8 mg/L by second dose | Initial TC not significantly different in patients with severe sepsis vs. not severe sepsis |
| Author and Year | Reference | Representation of Exposed Cohort | Selection of Non exposed Cohort | Ascertainment of Exposure | Demonstration that Outcome of Interest Was not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Adequacy of Follow Up of Cohorts | Score |
|---|---|---|---|---|---|---|---|---|---|
| Hodiamont et al. (2021) | [38] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Ueda et al. (2020) | [26] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Yoon et al. (2021) | [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Ortwine et al. (2019) | [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Flannery et al. (2021) | [29] | Yes | Yes | Yes | Yes | Yes | Yes | No | 6 |
| Marvin et al. (2019) | [31] | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Dolan et al. (2020) | [32] | Yes | Yes | Yes | Yes | Yes | Yes | No | 6 |
| Al-Mazraawy et al. (2021) | [33] | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Cheong et al. (2012) | [30] | Yes | Yes | Yes | Yes | Yes | No | No | 6 |
| Truong et al. (2012) | [35] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Golonia et al. (2013) | [36] | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Rosini et al. (2016) | [34] | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Alvarez et al. (2017) | [37] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Wesolek et al. (2018) | [25] | Yes | Yes | Yes | Yes | Yes | No | Yes | 8 |
| Denetclaw et al. (2013) | [39] | Yes | Yes | Yes | Yes | No | Yes | Yes | 7 |
| Study | References | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|---|
| Demirjian et al. (2013) | [23] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Rosini et al. (2015) | [24] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haseeb, A.; Alqurashi, M.K.; Althaqafi, A.S.; Alsharif, J.M.; Faidah, H.S.; Bushyah, M.; Alotaibi, A.F.; Elrggal, M.E.; Mahrous, A.J.; Abuhussain, S.S.A.; et al. A Systematic Review on Clinical Safety and Efficacy of Vancomycin Loading Dose in Critically Ill Patients. Antibiotics 2022, 11, 409. https://doi.org/10.3390/antibiotics11030409
Haseeb A, Alqurashi MK, Althaqafi AS, Alsharif JM, Faidah HS, Bushyah M, Alotaibi AF, Elrggal ME, Mahrous AJ, Abuhussain SSA, et al. A Systematic Review on Clinical Safety and Efficacy of Vancomycin Loading Dose in Critically Ill Patients. Antibiotics. 2022; 11(3):409. https://doi.org/10.3390/antibiotics11030409
Chicago/Turabian StyleHaseeb, Abdul, Mayyasah Khalid Alqurashi, Areej Sultan Althaqafi, Jumana Majdi Alsharif, Hani Saleh Faidah, Mashael Bushyah, Amal F. Alotaibi, Mahmoud Essam Elrggal, Ahmad Jamal Mahrous, Safa S. Almarzoky Abuhussain, and et al. 2022. "A Systematic Review on Clinical Safety and Efficacy of Vancomycin Loading Dose in Critically Ill Patients" Antibiotics 11, no. 3: 409. https://doi.org/10.3390/antibiotics11030409
APA StyleHaseeb, A., Alqurashi, M. K., Althaqafi, A. S., Alsharif, J. M., Faidah, H. S., Bushyah, M., Alotaibi, A. F., Elrggal, M. E., Mahrous, A. J., Abuhussain, S. S. A., Obaid, N. A., Algethamy, M., AlQarni, A., Khogeer, A. A., Saleem, Z., Iqbal, M. S., Ashgar, S. S., & Sheikh, A. (2022). A Systematic Review on Clinical Safety and Efficacy of Vancomycin Loading Dose in Critically Ill Patients. Antibiotics, 11(3), 409. https://doi.org/10.3390/antibiotics11030409









