In Vitro Screening of a 1280 FDA-Approved Drugs Library against Multidrug-Resistant and Extensively Drug-Resistant Bacteria
Abstract
1. Introduction
2. Results
2.1. Antibiotic Resistance Profile and Genomic Support of Selected Strains Panel
2.2. General High-Throughput Screening Results
2.3. Main Hits by Species
2.4. Comparison with Data from the Literature
3. Discussion
3.1. What Are the Potential Therapeutic Options for Treating These MDR Bacteria?
3.2. To Find a Common Hit
3.3. To Repurpose a Molecule
3.4. Using Drug Associations
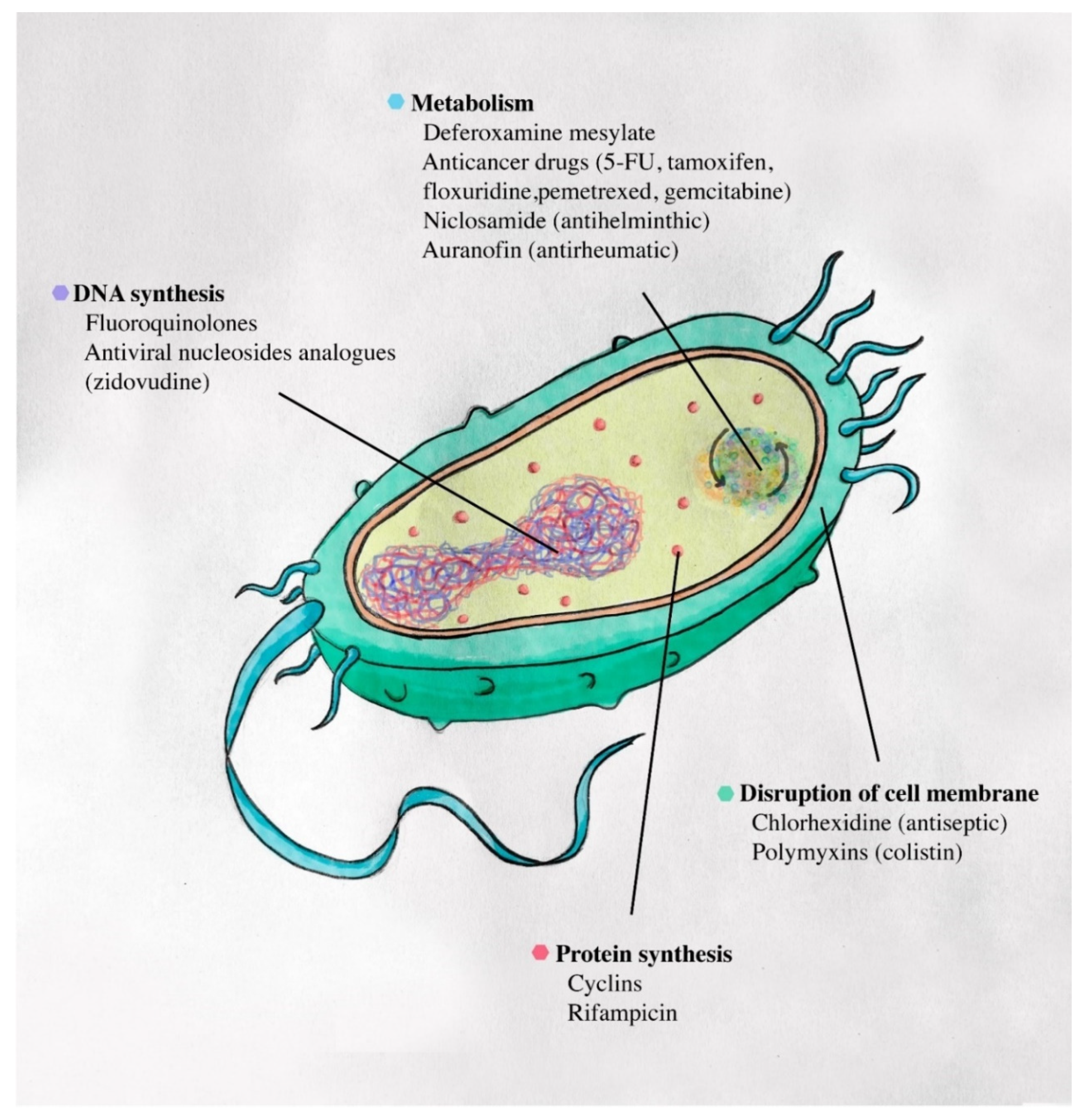
3.5. Damaging Basic Mechanisms of Bacteria
3.6. Personalizing the Treatment in a Compassionnal Approach
4. Materials and Methods
4.1. Collection and Susceptibility of Bacterial Strains
4.2. Preparation of Isolates
4.3. High-Throughput Screening Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bassetti, M.; Righi, E.; Carnelutti, A.; Graziano, E.; Russo, A. Multidrug-resistant Klebsiella pneumoniae: Challenges for treatment, prevention and infection control. Expert Rev. Anti. Infect. Ther. 2018, 16, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Musso, M.; Mosti, S.; Gualano, G.; Mencarini, P.; Urso, R.; Ghirga, P.; Rianda, A.; Del Nonno, F.; Goletti, D.; Palmieri, F. Hepatitis C virus infection: A challenge in the complex management of two cases of multidrug-resistant tuberculosis. BMC Infect. Dis. 2019, 19, 882. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Sherry, N.; Howden, B. Emerging Gram negative resistance to last-line antimicrobial agents fosfomycin, colistin and ceftazidime-avibactam–epidemiology, laboratory detection and treatment implications. Expert Rev. Anti-Infect. Ther. 2018, 16, 289–306. [Google Scholar] [CrossRef]
- Sirijan, S.; Nitaya, I. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- El Chakhtoura, N.G.; Saade, E.; Iovleva, A.; Yasmin, M.; Wilson, B.; Perez, F.; Bonomo, R.A. Therapies for multidrug resistant and extensively drug-resistant non-fermenting Gram-negative bacteria causing nosocomial infections: A perilous journey toward ‘molecularly targeted’ therapy. Expert Rev. Anti. Infect. Ther. 2018, 16, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Song, A.; Ellingboe, A.; Abdul-Aziz, M.H.; Harris, P.; Gavey, E.; Lipman, J. Infections by multidrug-resistant Gram-negative Bacteria: What’s new in our arsenal and what’s in the pipeline? Int. J. Antimicrob. Agents 2019, 53, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Peyclit, L.; Baron, S.A.; Rolain, J.-M. Drug repurposing to fight colistin and carbapenem-resistant bacteria. Front. Cell. Infect. Microbiol. 2019, 9, 193. [Google Scholar] [CrossRef]
- OMS (Organisation Mondiale de la Santé) Résistance Aux Antibiotiques. Available online: http://www.who.int/mediacentre/factsheets/antibiotic-resistance/fr/ (accessed on 9 February 2018).
- Law, G.L.; Tisoncik-Go, J.; Korth, M.J.; Katze, M.G. Drug repurposing: A better approach for infectious disease drug discovery? Curr. Opin. Immunol. 2013, 25, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, H.; Cassagne, C.; Ranque, S.; Rolain, J.M.; Bittar, F. Repurposing of ribavirin as an adjunct therapy against invasive Candida strains in an in vitro study. Antimicrob. Agents Chemother. 2019, 63, e00263-19. [Google Scholar] [CrossRef]
- Peyclit, L.; Baron, S.A.; Yousfi, H.; Rolain, J.M. Zidovudine: A salvage therapy for mcr-1 plasmid-mediated colistin-resistant bacterial infections? Int. J. Antimicrob. Agents 2018, 52, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Rajamuthiah, R.; Fuchs, B.B.; Conery, A.L.; Kim, W.; Jayamani, E.; Kwon, B.; Ausubel, F.M.; Mylonakis, E. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS ONE 2015, 10, e0124595. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Baquero, F. The refusal of the Society to accept antibiotic toxicity: Missing opportunities for therapy of severe infections. Clin. Microbiol. Infect. 2016, 22, 423–427. [Google Scholar] [CrossRef]
- Huang, H.C.; Weigt, S.S.; Derhovanessian, A.; Palchevskiy, V.; Ardehali, A.; Saggar, R.; Saggar, R.; Kubak, B.; Gregson, A.; Ross, D.J.; et al. Non-tuberculous mycobacterium infection after lung transplantation is associated with increased mortality. J. Hear. Lung Transplant. 2011, 30, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Snell, G.; Reed, A.; Stern, M.; Hadjiliadis, D. The evolution of lung transplantation for cystic fibrosis: A 2017 update. J. Cyst. Fibros. 2017, 16, 553–564. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Chiarelli, L.R.; Trespidi, G.; Mentasti, M.; Riccardi, G.; Buroni, S. Burkholderia cenocepacia infections in cystic fibrosis patients: Drug resistance and therapeutic approaches. Front. Microbiol. 2017, 8, 1592. [Google Scholar] [CrossRef]
- Rolain, J.-M.; Francois, P.; Hernandez, D.; Bittar, F.; Richet, H.; Fournous, G.; Mattenberger, Y.; Bosdure, E.; Stremler, N.; Dubus, J.-C.; et al. Genomic analysis of an emerging multiresistant Staphylococcus aureus strain rapidly spreading in cystic fibrosis patients revealed the presence of an antibiotic inducible bacteriophage. Biol. Direct 2009, 4, 1. [Google Scholar] [CrossRef]
- Fonseca e Silva, D.; Silva-Dias, A.; Gomes, R.; Martins-Oliveira, I.; Ramos, M.H.; Rodrigues, A.G.; Cantón, R.; Pina-Vaz, C. Evaluation of rapid colistin susceptibility directly from positive blood cultures using a flow cytometry assay. Int. J. Antimicrob. Agents 2019, 54, 820–823. [Google Scholar] [CrossRef]
- Bakour, S.; Touati, A.; Bachiri, T.; Sahli, F.; Tiouit, D.; Naim, M.; Azouaou, M.; Rolain, J.M. First report of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and rapid spread of metallo-β-lactamase NDM-1 in Algerian hospitals. J. Infect. Chemother. 2014, 20, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Maria, T.; Ioannis, B. Dronedarone pharmacokinetics. Front. Pharmacol. 2010, 1. [Google Scholar] [CrossRef]
- Mankad, P.; Kalahasty, G. Antiarrhythmic Drugs: Risks and Benefits. Med. Clin. N. Am. 2019, 103, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.F.; Kratzke, R.A.; D’Cunha, J.; Maddaus, M.A.; Sanghavi, K.; Kirstein, M.N. Gemcitabine and metabolite pharmacokinetics in advanced NSCLC patients after bronchial artery infusion and intravenous infusion. Cancer Chemother. Pharmacol. 2019, 83, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Danesi, R.; De Braud, F.; De Pas, T.; Curigliano, G.; Giovannetti, E.; Del Tacca, M. Drug distribution and pharmacokinetic/pharmacodynamic relationship of paclitaxel and gemcitabine in patients with non-small-cell lung cancer. Ann. Oncol. 2001, 12, 1553–1559. [Google Scholar] [CrossRef]
- Porter, J.B. Deferoxamine pharmacokinetics. Semin. Hematol. 2001, 38, 63–68. [Google Scholar] [CrossRef]
- Mcdonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Abbas, S.; Sastry, S. Chlorhexidine: Patient Bathing and Infection Prevention. Curr. Infect. Dis. Rep. 2016, 18, 25. [Google Scholar] [CrossRef]
- Cassir, N.; Thomas, G.; Hraiech, S.; Brunet, J.; Fournier, P.E.; La Scola, B.; Papazian, L. Chlorhexidine daily bathing: Impact on health care-associated infections caused by gram-negative bacteria. Am. J. Infect. Control 2015, 43, 640–643. [Google Scholar] [CrossRef]
- Shah, A.; Pasrija, C.; Boulos, F.; Pham, S.; Griffith, B.P.; Amoroso, A.; Sanchez, P.G.; Kon, Z.N. Decontamination and lung transplantation of a patient with cystic fibrosis with resistant infections. Ann. Thorac. Surg. 2019, 107, e239–e241. [Google Scholar] [CrossRef]
- Reyes, K.; Bardossy, A.C.; Zervos, M. Vancomycin-Resistant Enterococci: Epidemiology, Infection Prevention, and Control. Infect. Dis. Clin. N. Am. 2016, 30, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Singh, R.; McKinnell, J.A.; Park, S.; Gombosev, A.; Eells, S.J.; Gillen, D.L.; Kim, D.; Rashid, S.; Macias-Gil, R.; et al. Decolonization to reduce postdischarge infection risk among MRSA carriers. N. Engl. J. Med. 2019, 380, 638–650. [Google Scholar] [CrossRef]
- Hayden, M.K.; Lin, M.Y.; Lolans, K.; Weiner, S.; Blom, D.; Moore, N.M.; Fogg, L.; Henry, D.; Lyles, R.; Thurlow, C.; et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin. Infect. Dis. 2015, 60, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Messika, J.; La Combe, B.; Ricard, J.-D. Oropharyngeal colonization: Epidemiology, treatment and ventilator-associated pneumonia prevention. Ann. Transl. Med. 2018, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Ayerbe-Algaba, R.; Gil-Marqués, M.L.; Jiménez-Mejías, M.E.; Sánchez-Encinales, V.; Parra-Millán, R.; Pachón-Ibáñez, M.E.; Pachón, J.; Smani, Y. Synergistic Activity of Niclosamide in Combination with Colistin Against Colistin-Susceptible and Colistin-Resistant Acinetobacter baumannii and Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 348. [Google Scholar] [CrossRef]
- Domalaon, R.; Malaka De Silva, P.; Kumar, A.; Zhanel, G.G.; Schweizer, F. The anthelmintic drug niclosamide synergizes with colistin and reverses colistin resistance in Gram-negative bacilli. Antimicrob. Agents Chemother. 2019, 63, e02574-18. [Google Scholar] [CrossRef]
- Mohammad, H.; AbdelKhalek, A.; Abutaleb, N.S.; Seleem, M.N. Repurposing niclosamide for intestinal decolonization of vancomycin-resistant enterococci. Int. J. Antimicrob. Agents 2018, 51, 897–904. [Google Scholar] [CrossRef]
- Torres, N.S.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2016, 60, 5663–5672. [Google Scholar] [CrossRef]
- Montoya, M.C.; Krysan, D.J. Repurposing estrogen receptor antagonists for the treatment of infectious disease. MBio 2018, 9, e02272-18. [Google Scholar] [CrossRef]
- DeGraw, J.I.; Kisliuk, R.L.; Gaumont, Y.; Baugh, C.M. Antimicrobial Activity of 8-Deazafolic Acid. J. Med. Chem. 1974, 17, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.T. Antimicrobial activity of 21 anti-neoplastic agents. Br. J. Cancer 1984, 49, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Elwell, L.P.; Ferone, R.; Freeman, G.A.; Fyfe, J.A.; Hill, J.A.; Ray, P.H.; Richards, C.A.; Singer, S.C.; Knick, V.B.; Rideout, J.L.; et al. Antibacterial activity and mechanism of action of 3′-azido-3′-deoxythymidine (BW A509U). Antimicrob. Agents Chemother. 1987, 31, 274–280. [Google Scholar] [CrossRef]
- Serrano, L.; Lees, P. The applied pharmacology of azaperone in ponies. Res. Vet. Sci. 1976, 20, 316–323. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, T.; Song, G.; Hu, Y.; Liang, J. Determination of lomerizine in human plasma by liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 947–948, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, L.; Zhang, X.; Feng, Y.; Zong, Z. In vitro activity of neomycin, streptomycin, paromomycin and apramycin against carbapenem-resistant Enterobacteriaceae clinical strains. Front. Microbiol. 2017, 8, 2275. [Google Scholar] [CrossRef]
- Schneider, E.K.; Reyes-Ortega, F.; Velkov, T.; Li, J. Antibiotic–non-antibiotic combinations for combating extremely drug-resistant Gram-negative ‘superbugs’. Essays Biochem. 2017, 61, 115–125. [Google Scholar] [CrossRef]
- Raad, I.; Darouiche, R.; Hachem, R.; Mansouri, M.; Bodey, G.P. The Broad-Spectrum Activity and Efficacy of Catheters Coated With Minocycline and Rifampin. J. Infect. Dis. 1996, 173, 418–424. [Google Scholar] [CrossRef]
- Isenberg, H.D.; Alperstein, P.; France, K. In vitro activity of ciprofloxacin, levofloxacin, and trovafloxacin, alone and in combination with β-lactams, against clinical isolates of Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Burkholderia cepacia. Diagn. Microbiol. Infect. Dis. 1999, 33, 81–86. [Google Scholar] [CrossRef]
- AI-Hasan, M.N.; Wilson, J.W.; Lahr, B.D.; Thomsen, K.M.; Eckel-Passow, J.E.; Vetter, E.A.; Tleyjeh, I.M.; Baddour, L.M. Beta-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob. Agents Chemother. 2009, 53, 1386–1394. [Google Scholar] [CrossRef]
- Lee, H.J.; Bergen, P.J.; Bulitta, J.B.; Tsuji, B.; Forrest, A.; Nation, R.L.; Li, J. Synergistic activity of colistin and rifampin combination against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacokinetic/ pharmacodynamic model. Antimicrob. Agents Chemother. 2013, 57, 3738–3745. [Google Scholar] [CrossRef]
- Hu, Y.F.; Liu, C.P.; Wang, N.Y.; Shih, S.C. In vitro antibacterial activity of rifampicin in combination with imipenem, meropenem and doripenem against multidrug-resistant clinical isolates of Pseudomonas aeruginosa. BMC Infect. Dis. 2016, 16, 444. [Google Scholar] [CrossRef]
- Bergen, P.J.; Forrest, A.; Bulitta, J.B.; Tsuji, B.T.; Sidjabat, H.E.; Paterson, D.L.; Li, J.; Nation, R.L. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob. Agents Chemother. 2011, 55, 5134–5142. [Google Scholar] [CrossRef]
- Torres, N.S.; Montelongo-Jauregui, D.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Antimicrobial and antibiofilm activity of synergistic combinations of a commercially available small compound library with colistin against Pseudomonas aeruginosa. Front. Microbiol. 2018, 9, 2541. [Google Scholar] [CrossRef]
- Sun, W.; Weingarten, R.A.; Xu, M.; Southall, N.; Dai, S.; Shinn, P.; Sanderson, P.E.; Williamson, P.R.; Frank, K.M.; Zheng, W. Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerg. Microbes Infect. 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.M.; Rosen, L.S.; Yoshida, K.; Rasco, D.; Shapiro, G.I.; Sun, W. A phase 1 study of the pharmacokinetics of nucleoside analog trifluridine and thymidine phosphorylase inhibitor tipiracil (components of TAS-102) vs trifluridine alone. Investig. New Drugs 2017, 35, 189–197. [Google Scholar] [CrossRef]
- Huayhuaz, J.A.A.; Vitorino, H.A.; Campos, O.S.; Serrano, S.H.P.; Kaneko, T.M.; Espósito, B.P. Desferrioxamine and desferrioxamine-caffeine as carriers of aluminum and gallium to microbes via the Trojan Horse Effect. J. Trace Elem. Med. Biol. 2017, 41, 16–22. [Google Scholar] [CrossRef]
- van Asbeck, B.S.; Marcelis, J.H.; Marx, J.J.M.; Struyvenberg, A.; van Kats, J.H.; Verhoef, J. Inhibition of bacterial multiplication by the iron chelator deferoxamine: Potentiating effect of ascorbic acid. Eur. J. Clin. Microbiol. 1983, 2, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Hartzen, S.H.; Frimodt-MØLler, N.; Thomsen, V.F. The antibacterial activity of a siderophore: The activity of deferoxamine in vitro and its influence on the effect of antibiotics against Escherichia coli, Proteus mirabilis and coagulase-negative staphylococci. APMIS 1994, 102, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Yssel, A.E.J.; Vanderleyden, J.; Steenackers, H.P. Repurposing of nucleoside-and nucleobase-derivative drugs as antibiotics and biofilm inhibitors. J. Antimicrob. Chemother. 2017, 72, 2156–2170. [Google Scholar] [CrossRef]
- Hussein, M.H.; Schneider, E.K.; Elliott, A.G.; Han, M.; Reyes-Ortega, F.; Morris, F.; Blastovich, M.A.T.; Jasim, R.; Currie, B.; Mayo, M.; et al. From Breast Cancer to Antimicrobial: Combating Extremely Resistant Gram-Negative Superbugs Using Novel Combinations of Polymyxin B with Selective Estrogen Receptor Modulators. Microb. Drug Resist. 2017, 23, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Miró-Canturri, A.; Ayerbe-Algaba, R.; Smani, Y. Drug Repurposing for the Treatment of Bacterial and Fungal Infections. Front. Microbiol. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Soo, V.; Kwan, B.; Quezada, H.; Castillo-Juárez, I.; Pérez-Eretza, B.; García-Contreras, S.; Martínez-Vázquez, M.; Wood, T.; García-Contreras, R. Repurposing of Anticancer Drugs for the Treatment of Bacterial Infections. Curr. Top. Med. Chem. 2017, 17, 1157–1176. [Google Scholar] [CrossRef] [PubMed]
- De Lastours, V.; Fantin, B. Résistance aux fluoroquinolones en 2013: Quel impact pour l’interniste? Rev. Med. Interne 2014, 35, 601–608. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-resistant Gram-negative Bacteria: A Systematic Review of Current Epidemiology, Prognosis and Treatment Options. J. Antimicrob Chemother 2020, 75, 271–282. [Google Scholar] [CrossRef]
- De Clercq, E. Antiviral drugs in current clinical use. J. Clin. Virol. 2004, 30, 115–133. [Google Scholar] [CrossRef]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug Resist. Updat. 2016, 28, 82–90. [Google Scholar] [CrossRef]
- Touret, F.; Gilles, M.; Barral, K.; Nougairède, A.; van Helden, J.; Decroly, E.; de Lamballerie, X.; Coutard, B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020, 10, 13093. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Zapata-Morales, J.R.; Escobedo-Moratilla, A.; Díaz de León-Cabrero, M.; Torres-Roque, I.; Pérez-Urizar, J. Determination of Pinaverium Bromide in Human Plasma by a Sensitive and Robust UPLC–MS-MS Method and Application to a Pharmacokinetic Study in Mexican Subjects. J. Chromatogr. Sci. 2015, 53, 1373–1378. [Google Scholar] [CrossRef][Green Version]
- De Mattos, A.C.; Altmeyer, C.; Tominaga, T.T.; Khalil, N.M.; Mainardes, R.M. Polymeric nanoparticles for oral delivery of 5-fluorouracil: Formulation optimization, cytotoxicity assay and pre-clinical pharmacokinetics study. Eur. J. Pharm. Sci. 2016, 84, 83–91. [Google Scholar] [CrossRef]
- Sawada, M.; Matsui, Y.; Okudaira, Y. Concentrations of a New Antitumor Agent, 1-hexylcarbamoyl-5-fluorouracil in Serum and Gynecologic Tumor Tissue. J. Jpn. Obstet. Gynecol. Soc. 1983, 35, 2421–2426. [Google Scholar]
- Gaete, L.E.; Ortiz, M.; Soto, J.; Saavedra, I. Amiodarone absorption and elimination after oral and intravenous administration in healthy individuals. Rev. Med. Chil. 1995, 123, 713–719. [Google Scholar] [PubMed]
- Ghobadi, C.; Mirhosseini, N.; Shiran, M.R.; Moghadamnia, A.; Lennard, M.S.; Ledger, W.L.; Rostami-Hodjegan, A. Single-dose pharmacokinetic study of clomiphene citrate isomers in anovular patients with polycystic ovary disease. J. Clin. Pharmacol. 2009, 49, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Binkhorst, L.; Kloth, J.S.L.; de Wit, A.S.; de Bruijn, P.; Lam, M.H.; Chaves, I.; Burger, H.; van Alphen, R.J.; Hamberg, P.; van Schaik, R.H.N.; et al. Circadian variation in tamoxifen pharmacokinetics in mice and breast cancer patients. Breast Cancer Res. Treat. 2015, 152, 119–128. [Google Scholar] [CrossRef]
- Kavathiya, K.; Gurjar, M.; Patil, A.; Naik, M.; Noronha, V.; Joshi, A.; Gota, V.; Prabhash, K. A Comparative Pharmacokinetic Study of 2 Pemetrexed Formulations in Indian Adult Chemonaive Patients With Adenocarcinoma Stage III/IV Non–Small Cell Lung Cancer. Clin. Pharmacol. Drug Dev. 2017, 6, 234–239. [Google Scholar] [CrossRef]
- Judson, I.; Maughan, T.; Beale, P.; Primrose, J.; Hoskin, P.; Hanwell, J.; Berry, C.; Walker, M.; Sutcliffe, F. Effects of impaired renal function on the pharmacokinetics of raltitrexed (Tomudex ZD1694). Br. J. Cancer 1998, 78, 1188–1193. [Google Scholar] [CrossRef]
- Inoue, K.; Yuasa, H. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab. Pharmacokinet. 2014, 29, 12–19. [Google Scholar] [CrossRef]
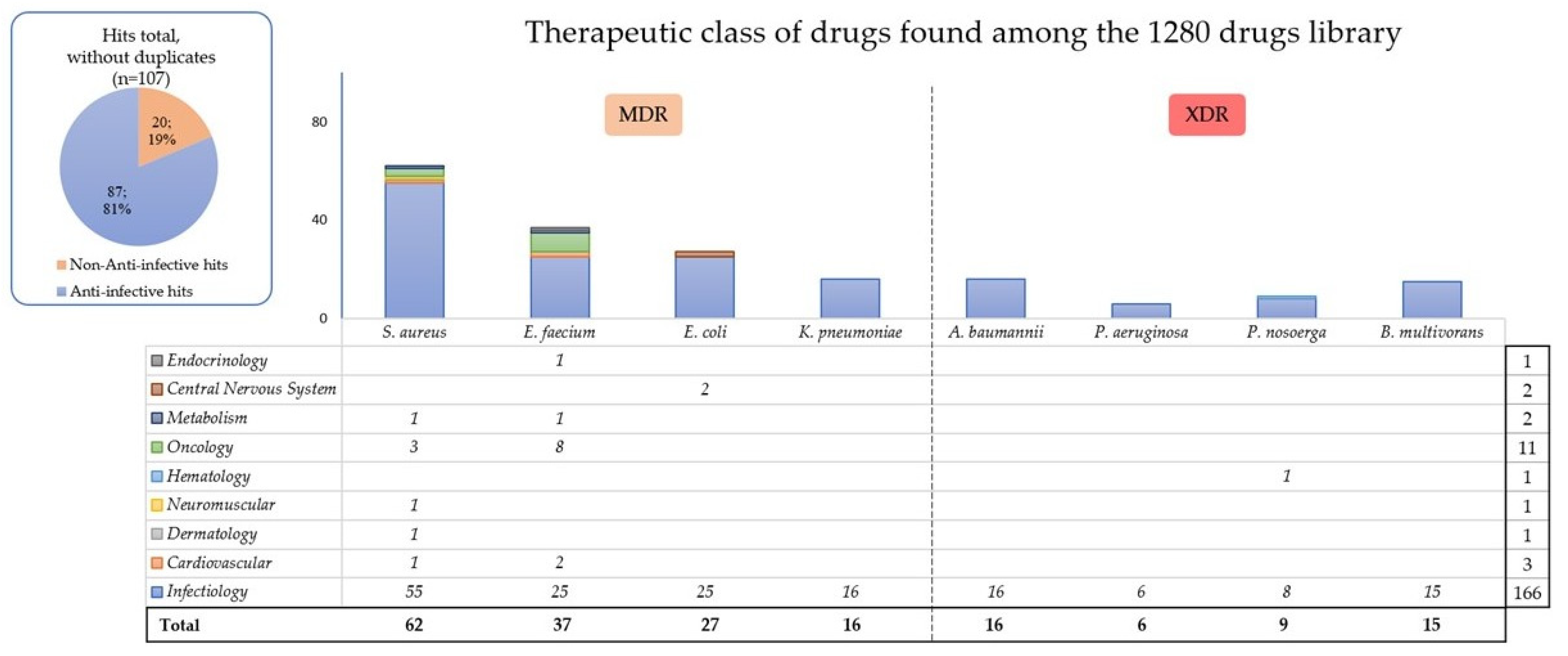

| Strain and No. | Characterization [3] | Non-Susceptibility to at Least One Agent in All Those Classes | Known Resistance Genes |
|---|---|---|---|
| S. aureus P1943 [20] | MDR | Fluoroquinolones, anti-staphylococcal β-lactams, glycopeptides, and macrolides | mecA, gyrA, aaD, bleO, and ermC |
| E. faecium P5015 | MDR | Aminoglycosides, glycopeptides, and tetracyclines | vanA |
| E. coli DSM 105182 [21] | MDR | Polymyxins, tetracyclins, and fluoroquinolones | mcr-1 |
| K. pneumoniae P9495 | MDR | Aminoglycosides, penicillins + β-lactamase inhibitors, carbapenems, extended-spectrum cephalosporins, fluoroquinolones, folate pathway inhibitors, monobactacms, and polymyxins | blaOXA-48 |
| A. baumannii P1887 [22] | MDR | Aminoglycosides, carbapenems, fluoroquinolones, penicillins + β-lactamase inhibitors, extended-spectrum cephalosporins, and folate pathway inhibitors | blaOXA-51, blaOXA-23, and blaNDM-1 |
| P. aeruginosa P6540 | XDR | Aminoglycosides, antipseudomonal cephalosporins, antipseudomonal fluoroquinolones, penicillins + β-lactamase inhibitors, monobactacms, and polymyxins | |
| B. multivorans P6539 | XDR | Aminoglycosides, carbapenems, cephalosporins, fluoroquinolones, penicillins + β-lactamase inhibitors, monobactacms, folate pathways inhibitors, glycylcyclines, and polymyxins | |
| P. nosoerga P8103 | XDR | Aminoglycosides, carbapenems, cephalosporins, fluoroquinolones, penicillins + β-lactamase inhibitors, rifamycins, folate pathways inhibitors, tetracyclins, phosphonic acids, and polymyxins | blaOXA-158 |
| Name of Strain | Hits Except “Infectiology” Class |
|---|---|
| S. aureus P1943 | Dronedarone hydrochloride (cardiovascular); thonzonium bromide (dermatology); auranofin (metabolism); pinaverium bromide (neuromuscular); and 5-fluorouracil, carmofur, and gemcitabine (oncology) |
| E. faecium P5015 | Amiodarone and dronedarone hydrochloride (cardiovascular); clomiphene citrate (Z and E) (endocrinology); auranofin (metabolism); tamoxifen citrate, gemcitabine, carmofur, floxuridine, pemetrexed disodium, raltitrexed, 5-fluorouracil, and methotrexate (oncology) |
| E. coli DSM 105182 | Azaperone and lomerizine hydrochloride (central nervous system) |
| P. nosoerga P8103 | Deferoxamine mesylate (hematology) |
| Combination Therapy | Drug Repurposing | Increased Dosages | |
|---|---|---|---|
| P. aeruginosa | Rifampicin + imipenem Colistin + imipenem | Auranofin (+ colistin ± ceftazidime) | Colistin |
| P. nosoerga | Rifampicin + minocyclin | Deferoxamine + ascorbic acid (+ gentamicin) | Rifampicin |
| B. multivorans | Fluoroquinolones + β-lactams | - | Fluoroquinolones |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peyclit, L.; Baron, S.A.; Hadjadj, L.; Rolain, J.-M. In Vitro Screening of a 1280 FDA-Approved Drugs Library against Multidrug-Resistant and Extensively Drug-Resistant Bacteria. Antibiotics 2022, 11, 291. https://doi.org/10.3390/antibiotics11030291
Peyclit L, Baron SA, Hadjadj L, Rolain J-M. In Vitro Screening of a 1280 FDA-Approved Drugs Library against Multidrug-Resistant and Extensively Drug-Resistant Bacteria. Antibiotics. 2022; 11(3):291. https://doi.org/10.3390/antibiotics11030291
Chicago/Turabian StylePeyclit, Lucie, Sophie Alexandra Baron, Linda Hadjadj, and Jean-Marc Rolain. 2022. "In Vitro Screening of a 1280 FDA-Approved Drugs Library against Multidrug-Resistant and Extensively Drug-Resistant Bacteria" Antibiotics 11, no. 3: 291. https://doi.org/10.3390/antibiotics11030291
APA StylePeyclit, L., Baron, S. A., Hadjadj, L., & Rolain, J.-M. (2022). In Vitro Screening of a 1280 FDA-Approved Drugs Library against Multidrug-Resistant and Extensively Drug-Resistant Bacteria. Antibiotics, 11(3), 291. https://doi.org/10.3390/antibiotics11030291





