Canadian Collaboration to Identify a Minimum Dataset for Antimicrobial Use Surveillance for Policy and Intervention Development across Food Animal Sectors
Abstract
:1. Introduction
2. Results
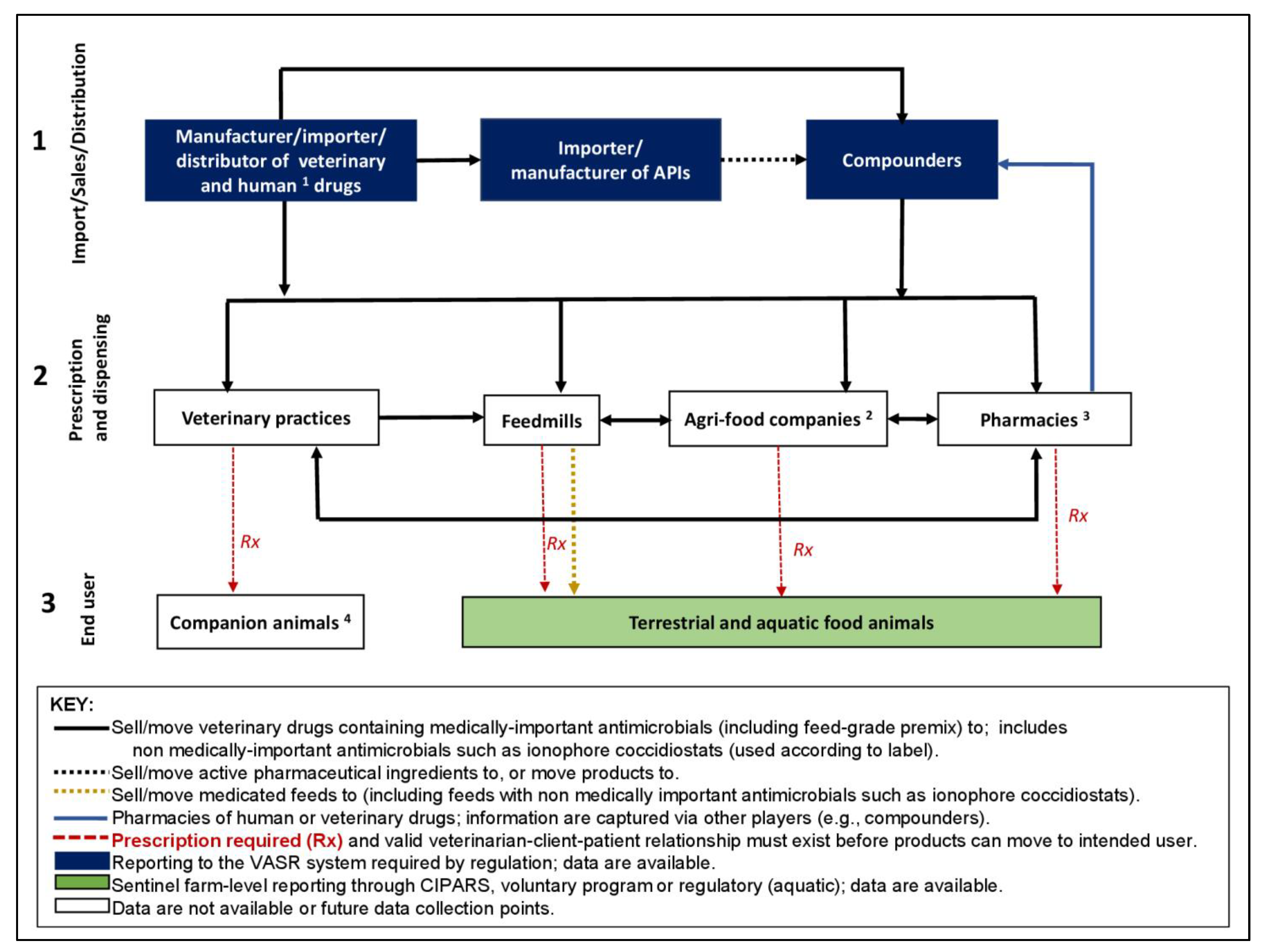
2.1. Contextual Review of Existing AMU Surveillance Infrastructures and the Antimicrobial Supply Distribution Pathway in Canada
- (1)
- Manufacturers, importers, and distributors of veterinary drugs (finished products), importers, manufacturers, and compounders of active pharmaceutical ingredients (APIs) (i.e., those who obtain antimicrobials from pharmacies/dispensers of veterinary drugs and human drugs for animal use (blue line in Figure 1). Reporting to VASR is required by regulation;
- (2)
- Prescription and dispensing facilities, including veterinary clinics, feed mills, agri-food companies who employ veterinarians (i.e., corporate veterinarians who prescribe antimicrobials for use in relevant production phases as part of the coordinated supply chain within their network including hatcheries, breeders, commercial growing farms-various commodities, and feedmills), and pharmacies/dispensers of veterinary drugs and human drugs for animal use). Data from this level are not yet available or targeted for a future data collection point;
- (3)
- End users of antimicrobials including pet owners and producers of terrestrial and aquatic food animals. Information on specific terrestrial food animal species is captured through the CIPARS Farm AMU/AMR Surveillance program (voluntary) and aquatic animals via the DFO (by regulation).
2.2. Stakeholders’ Need for AMU Data
- “To garner public trust, to demonstrate social responsibility”;
- “To understand AMU (in my sector)”;
- “To provide AMU benchmarks (reference or targets) and trends that will inform decision making/policy and demonstrate the impacts of interventions”;
- “To provide oversight of AMU”;
- “To reduce reliance on antimicrobials to preserve antimicrobial efficacy”;
- “To improve/inform/demonstrate antimicrobial stewardship and prudent use”;
- “To provide international comparisons”;
- “To guide research priorities”;
- “To educate the public, veterinarians and producers about AMU/AMR”.
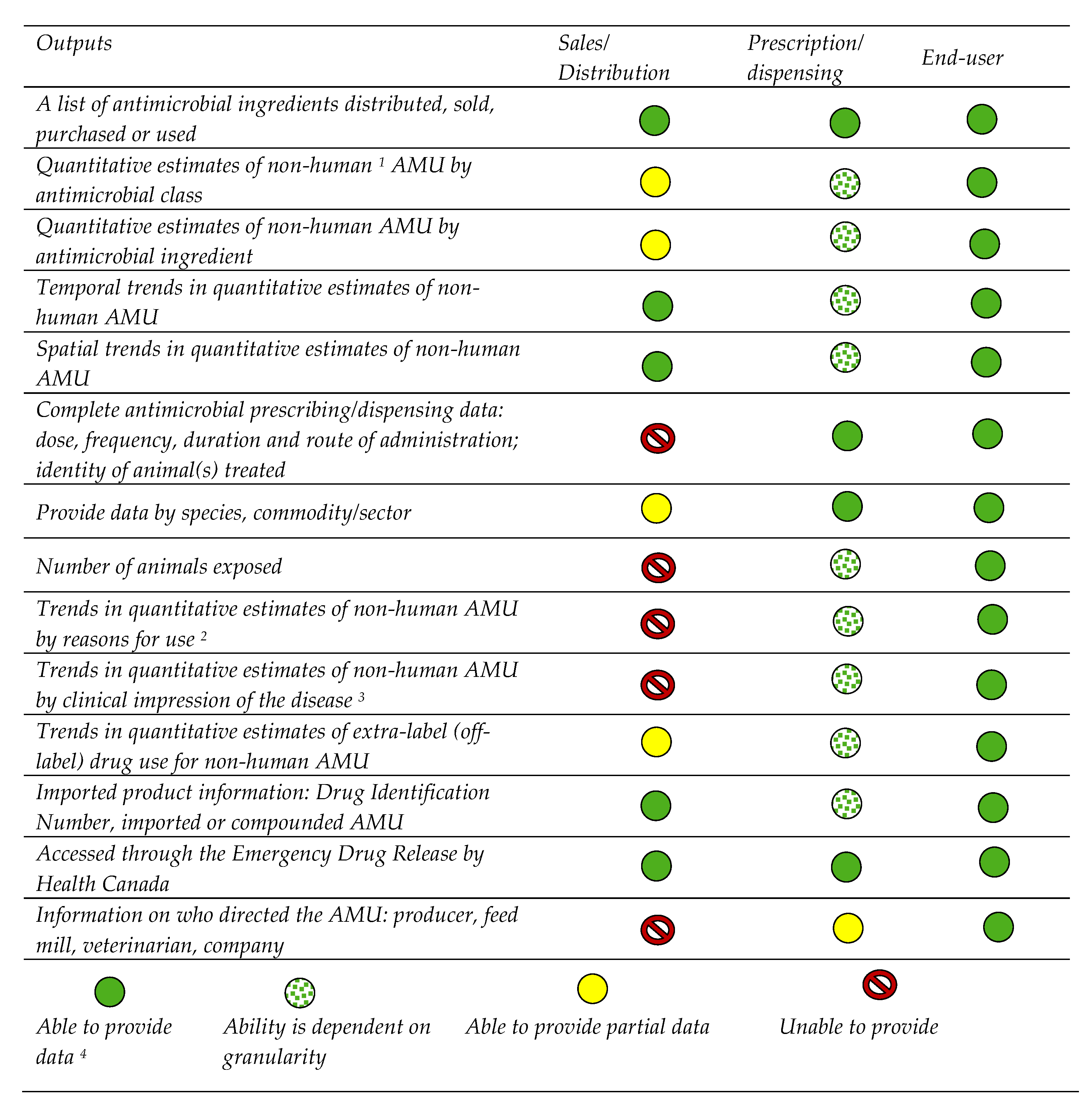
2.3. Outputs Desired and Important Considerations When Reporting AMU Data
2.4. The MDS-AMU-Surv Development, Framework for Data Collection, and Data Sources
2.4.1. Core MDS-AMU-Surv Data Elements
2.4.2. Data Source
2.4.3. AMU Surveillance Framework
- Frequency: An annual frequency of data collection was perceived as being sufficient for tracking AMU over time by the participants in most cases.
- Number of farms: depending on the surveillance objectives and capacity/resources, the sampling frame could be a census of farms across Canada, regional census (e.g., all farms in a given area), random sample (i.e., targets a certain percentage of farms across the area of interest), stratified sample, convenience/voluntary sample, those with electronic records, and those over a certain farm size.
- Who collects the data: existing platforms (government such as CIPARS or other organizations), provincial government, veterinary associations, producer associations, and processor associations were identified as options. These parties have direct access to some data or have a role in the AMU distribution chain (Figure 1).
- Incentives: existing data collection is either voluntary (e.g., CIPARS Farm) or regulatory (e.g., VASR, DFO) in nature. In private sector/industry initiatives, these are industry requirements as part of their on-farm food safety program, but there are no other incentives provided to the producer.
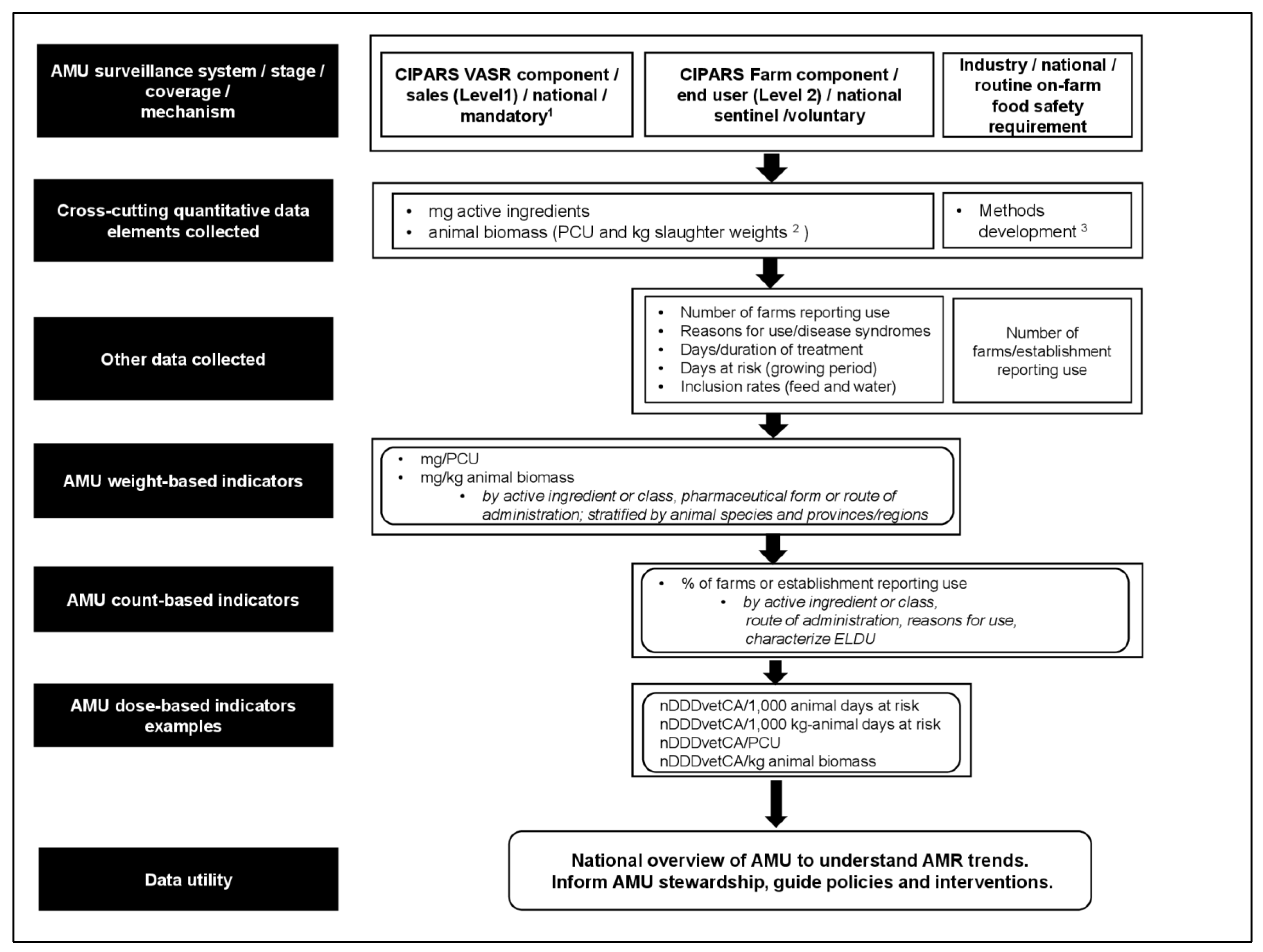
2.5. Application of MDS-AMU-Surv in Analysis and Reporting of Data
3. Discussion
4. Materials and Methods
4.1. The CAHSS Working Group
4.2. Review of Existing AMU Surveillance Infrastructures and the Antimicrobial Supply Distribution Pathway
4.3. Survey of CAHSS AMU/AMR WG to Understand the AMU Data Needs
- Data inclusion scores: 0—not required, 1—nice to have, and 2—must be included.
- Data collection feasibility scores: 0—not feasible, 1—could start collecting with significant effort, 2—could start collecting with minimal effort, and 3—currently being collected by your organization.
4.4. Development of the MDS-AMU-Surv
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Acronyms or Descriptions: | |
|---|---|
| AMU | antimicrobial use |
| AMR | antimicrobial resistance |
| API | active pharmaceutical ingredient |
| CAHI | Canadian Animal Health Institute |
| CAHSS | Canadian Animal Health Surveillance System |
| CIPARS | Canadian Integrated Program for Antimicrobial Resistance Surveillance: CIPARS VASR component/data from Level 1 in the AMU distribution chain—pertains to the CIPARS surveillance component, which conducts the data analysis and reporting for the antimicrobial sales data collected. CIPARS Farm component/data from Level 3 in the AMU distribution chain (end user)—also known as CIPARS Farm AMU/AMR surveillance program or CIPARS Farm program and pertains to the farm component of CIPARS, which coordinates the voluntary data and sample collection from a network of sentinel veterinary practices and their producers across Canada. |
| DDDvetCA and nDDDvetCA | defined daily doses in animals using Canadian standards and number of defined daily doses in animals using Canadian standards (milligrams of antimicrobials adjusted by the species-specific DDDvetCA standard for each antimicrobial active ingredient). |
| MDS-AMU-surv | minimum dataset for antimicrobial use surveillance. |
| PCU | population correction unit |
| SAVI | Stewardship of Antimicrobials by Veterinarians Initiative |
| TI100 or TI1000 | Treatment Incidence 100 or 1000; CIPARS reports the TI1000 antimicrobial use indicator as nDDDvetCA/1000 animal days at risk. |
| VASR | Veterinary Antimicrobial Sales Reporting; the data collection platform for the reporting of sales data (Level 1 of the AMU distribution chain). |
| VCPR | veterinarian–client–patient relationship |
References
- WHO. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 18 November 2021).
- FAO. The FAO Action Plan on Antimicrobial Resistance 2021–2025. Available online: https://www.fao.org/documents/card/en/c/cb5545en (accessed on 19 November 2021).
- FAO. The FAO Action Plan on Antimicrobial Resistance, 2016–2020. Available online: http://www.fao.org/antimicrobial-resistance/en/ (accessed on 2 July 2018).
- OIE. The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. Available online: http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf (accessed on 23 September 2018).
- Government of Canada. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. Available online: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-framework-action.html (accessed on 28 September 2018).
- Government of Canada. Veterinary Antimicrobial Sales Reporting. Available online: https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/veterinary-antimicrobial-sales-reporting.html (accessed on 18 November 2021).
- Government of Canada. List A: List of Certain Antimicrobial Active Pharmaceutical Ingredients. 2020. Available online: https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/veterinary-antimicrobial-sales-reporting/list-a.html (accessed on 19 December 2021).
- Government of Canada. Government of Canada Food and Drugs Act. Regulations Amending the Food and Drug Regulations (Veterinary Drugs—Antimicrobial Resistance). 2017; Volume 151, No. 10. Available online: http://www.gazette.gc.ca/rp-pr/p2/2017/2017-05-17/html/sor-dors76-eng.php (accessed on 25 May 2017).
- Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2018: Integrated Findings. Available online: https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillance-cipars/cipars-reports/2018-annual-report-integrated-findings.html (accessed on 16 February 2021).
- Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2018: Design and Methods. Available online: https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillance-cipars/cipars-reports/2018-annual-report-design-methods.html (accessed on 21 December 2021).
- Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance. 2016 Annual Report. Available online: http://publications.gc.ca/collections/collection_2018/aspc-phac/HP2-4-2016-eng.pdf (accessed on 16 July 2019).
- Government of Canada. Aquaculture Activities Regulations. Available online: https://www.dfo-mpo.gc.ca/aquaculture/management-gestion/aar-raa-eng.htm (accessed on 11 December 2021).
- FAO; OIE; WHO. Tripartite Zoonoses Guide. Available online: https://www.who.int/initiatives/tripartite-zoonosis-guide (accessed on 2 March 2021).
- CAHSS. Non-Human Antimicrobial Use Surveillance in Canada: Surveillance Objectives and Options. Available online: https://cahss.ca/cahss-tools/document-library/Non-human-antimicrobial-use-surveillance-in-Canada-Surveillance-Objectives-and-Options? (accessed on 18 November 2021).
- Government of Canada. Oversight on the Quality of Active Pharmaceutical Ingredients for Veterinary Use in Canada. Available online: https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/oversight-quality-active-pharmaceutical-ingredients-veterinary-use.html (accessed on 18 November 2021).
- CAHI. About the Canadian Animal Health Institute. Available online: https://www.cahi-icsa.ca/about-us (accessed on 18 November 2021).
- Impact Vet. Available online: www.impactvet.com (accessed on 6 January 2022).
- Government of Canada. Canadian Network for Public Health Intelligence (CNPHI). Available online: https://science.gc.ca/eic/site/063.nsf/eng/98243.html (accessed on 18 November 2021).
- FAO; The Ministry of Environment and Food, Denmark. Tackling Antimicrobial Use and Resistance in Pig Production Lessons Learned in Denmark. Available online: http://www.fao.org/antimicrobial-resistance/resources/publications-archive/case-studies-series-denmark/en/ (accessed on 16 February 2021).
- Government of Canada. National Aquaculture Public Reporting Data. Available online: https://open.canada.ca/data/en/dataset/288b6dc4-16dc-43cc-80a4-2a45b1f93383 (accessed on 22 November 2021).
- Government of Canada. Compendium of Medicating Ingredient Brochure. Available online: https://inspection.canada.ca/animal-health/livestock-feeds/medicating-ingredients/eng/1300212600464/1320602461227 (accessed on 6 January 2022).
- Government of Canada. The Drug Product Database Online Query. Available online: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp (accessed on 3 January 2022).
- Animalytix, LLC. Compendium of Veterinary Products, Canada Edition. Available online: https://bam.cvpservice.com/ (accessed on 1 June 2021).
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, F.; Obritzhauser, W.; Agunos, A.; Carson, C.; Høg, B.B.; Andersen, V.D.; Chauvin, C.; et al. Monitoring of farm-level antimicrobial use to guide stewardship: Overview of existing systems and analysis of key components and processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- AACTING. AACTING Network on Quantification, Benchmarking and Reporting of Veterinary Antimicrobial Usage (AMU) at Farm Level. Monitoring Systems. Available online: https://aacting.org/monitoring-systems/ (accessed on 2 June 2018).
- Collineau, L.; Belloc, C.; Stark, K.D.; Hemonic, A.; Postma, M.; Dewulf, J.; Chauvin, C. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses Public Health 2016, 64, 165–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agunos, A.; Gow, S.P.; Deckert, A.E.; Kuiper, G.; Leger, D.F. Informing stewardship measures in Canadian food animal species through integrated reporting of antimicrobial use and antimicrobial resistance surveillance data-part I, methodology development. Pathogens 2021, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- OIE. Fifth OIE Annual Report on Antimicrobial Agents Intended for Use in Animals. Available online: https://www.oie.int/en/document/fifth-oie-annual-report-on-antimicrobial-agents-intended-for-use-in-animals/ (accessed on 1 June 2021).
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018. Trends from 2010 to 2018. Tenth ESVAC Report. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/european-surveillance-veterinary-antimicrobial-consumption-esvac (accessed on 1 June 2021).
- Government of Canada. Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html (accessed on 22 November 2021).
- Narbonne, J.A.; Radke, B.R.; Price, D.; Hanington, P.C.; Babujee, A.; Otto, S.J.G. Antimicrobial use surveillance indicators for finfish aquaculture production: A Review. Front. Vet. Sci. 2021, 8, 595152. [Google Scholar] [CrossRef] [PubMed]
- AMR Network. Available online: https://www.amrnetwork.ca/ (accessed on 22 November 2021).
- WHO. Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria. Application of a One Health Approach. Guidance from the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) In collaboration with Food and Agriculture Organization of the United Nations (FAO), and World Organisation. Available online: https://apps.who.int/iris/bitstream/handle/10665/255747/9789241512411-eng.pdf;jsessionid=C959E32A695A75232D9436A80F69A9D9?sequence=1 (accessed on 3 June 2019).
- AACTING. Guidelines for Collection, Analysis and Reporting of Farm-Level Antimicrobial Use, in the Scope of Antimicrobial stewardship. Available online: https://aacting.org/aacting-guidelines/ (accessed on 7 January 2022).
- United Kingdom Veterinary Medicines Directorate. UK-VARSS Veterinary Antimicrobial Resistance and Sales Surveillance 2020. Available online: https://www.gov.uk/government/publications/veterinary-antimicrobial-resistance-and-sales-surveillance-2020 (accessed on 18 November 2021).
- SDA. General Information about The Netherlands Veterinary Medicines Institute (SDa). Available online: https://www.autoriteitdiergeneesmiddelen.nl/en/about-sda/general (accessed on 16 February 2021).
- MARAN. MARAN 2019 Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in The Netherlands in 2018. Available online: https://rivm.openrepository.com/handle/10029/623134 (accessed on 4 August 2020).
- DANMAP. DANMAP 2020 Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Available online: https://www.danmap.org/Reports/2020 (accessed on 19 December 2021).
- Food and Drug Administration Thailand. Thailand’s National Strategic Plan on Antimicrobial Resistance 2017–2021 (NSP-AMR)—Goals by the year 2021. Available online: https://amrthailand.net/English (accessed on 1 February 2022).
- Québec Ministry of Agriculture, Fisheries and Food. Surveillance in Connection with the Use of Antibiotics. Available online: https://www.mapaq.gouv.qc.ca/fr/Productions/santeanimale/maladies/antibio/Pages/Surveillance.aspx (accessed on 19 December 2021).
- Canadian Veterinary Medical Association. Stewardship of Antimicrobials by Veterinarians Initiative. Available online: https://savi.canadianveterinarians.net/en/about/ (accessed on 18 November 2021).
- Chicken Farmers of Canada. AMU Strategy, a Prescription for Change. Available online: http://www.chickenfarmers.ca/wp-content/uploads/2018/01/AMU-Magazine_ENG_web-2.pdf (accessed on 7 February 2018).
- Turkey Farmers of Canada. Guidelines for Antimicrobial Use in the Turkey Industry. Available online: https://www.amstewardship.ca/guidelines-for-antimicrobial-use-in-the-turkey-industry-published-by-the-turkey-farmers-of-canada/ (accessed on 13 April 2020).
- Bosman, A.L.; Loest, D.; Carson, C.A.; Agunos, A.; Collineau, L.; Leger, D.F. Developing Canadian defined daily doses for animals: A metric to quantify antimicrobial use. Front. Vet. Sci. 2019, 6, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agunos, A.; Gow, S.P.; Deckert, A.E.; Leger, D.F. Informing stewardship measures in Canadian food animal species through integrated reporting of antimicrobial use and antimicrobial resistance surveillance data-part II, application. Pathogens 2021, 10, 1491. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Gow, S.P.; Leger, D.F.; Deckert, A.E.; Carson, C.A.; Bosman, A.L.; Kadykalo, S.; Reid-Smith, R.J. Antimicrobial use indices-the value of reporting antimicrobial use in multiple ways using data from Canadian broiler chicken and turkey farms. Front. Vet. Sci. 2020, 7, 567872. [Google Scholar] [CrossRef] [PubMed]
- SWEDRES-SVARM 2019. Sales of Antibiotics and Occurrence of Antibiotic Resistance in Sweden. Available online: https://www.sva.se/en/our-topics/antibiotics/svarm-resistance-monitoring/swedres-svarm-reports/ (accessed on 5 August 2020).

| Core Data Elements | Examples of Data That Would Fulfil the Element | Considerations for Surveillance Communication and Reporting |
|---|---|---|
| Antimicrobial active ingredient | On-farm treatment records (e.g., flock sheets), Farm purchases, Feed sales, Veterinarian’s medical records/prescriptions, Veterinarian’s dispensing record, Veterinary purchases, Manufacturer sales | Available in a number of formats, including the product or trade name, or the antimicrobial active ingredient itself. These data can then be easily converted to amount of active ingredient using a glossary of products that provides the concentration of active ingredient (and Anatomical Taxonomic Index codes for veterinary antimicrobials (ATCvet code)). This glossary should be linked to current references (i.e., to be used as a common reference across surveillance programs): Compendium of Medicating Ingredients Brochures [21] and the Drug Products Database [22]; Pharmaceutical products (with DIN) and compounded products (without DIN) as reported in the VASR system; Compendium of Veterinary Products [23]. Weight of antimicrobials (i.e., numerator data in some indicator calculations): pertains to the total quantity of AMU across all routes of administration. This is important for analysis of trends and the basis for further quantification of AMU (dose-based indicators). |
| Biomass unit | On-farm records (e.g., flock sheets), Processor records, Census data (to geographic region), Expert consensus (e.g., proportion of total animals in each production level, multiplier values for biomass calculation (kg) 1) | Required for both numerator (animals treated) and denominator (total animals) estimates. Total population (including mortalities and number of animals introduced to the flock or herd) and weights are important to collect but can come from several different sources, including:
|
| Reasons for use | Expert consensus (by proportion of total AMU) 3, On-farm records, Veterinary medical records | The ability to capture the main indications for medical AMU, such as for disease prevention/prophylaxis, disease control/metaphylaxis and disease treatment, or growth promotion, was viewed as necessary. More specific reason for using data (e.g., respiratory vs. gastrointestinal vs. other disease treatment) was also desirable but not considered a core necessity. This is additional information for contextualizing AMU and AMR data and the impact of regulatory changes in AMU. |
| Geographical location | On-farm records, Veterinary records, Processor records, Hatchery delivery receipts | Collecting the province or the region where the animals are raised or antimicrobials sold (for the VASR system) is important to enable geographical comparisons of use. |
| Time component | Multiple years, Yearly, Monthly, Weekly, Daily, Real-time, Production cycle/s (specify) | Required to compare trends over time and gauge changes in AMU following interventions. There are various time elements that need to be captured for more advanced quantification and analysis [24,25,26,27]:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Léger, D.F.; Anderson, M.E.C.; Bédard, F.D.; Burns, T.; Carson, C.A.; Deckert, A.E.; Gow, S.P.; James, C.; Li, X.-Z.; Ott, M.; et al. Canadian Collaboration to Identify a Minimum Dataset for Antimicrobial Use Surveillance for Policy and Intervention Development across Food Animal Sectors. Antibiotics 2022, 11, 226. https://doi.org/10.3390/antibiotics11020226
Léger DF, Anderson MEC, Bédard FD, Burns T, Carson CA, Deckert AE, Gow SP, James C, Li X-Z, Ott M, et al. Canadian Collaboration to Identify a Minimum Dataset for Antimicrobial Use Surveillance for Policy and Intervention Development across Food Animal Sectors. Antibiotics. 2022; 11(2):226. https://doi.org/10.3390/antibiotics11020226
Chicago/Turabian StyleLéger, David F., Maureen E. C. Anderson, François D. Bédard, Theresa Burns, Carolee A. Carson, Anne E. Deckert, Sheryl P. Gow, Cheryl James, Xian-Zhi Li, Michael Ott, and et al. 2022. "Canadian Collaboration to Identify a Minimum Dataset for Antimicrobial Use Surveillance for Policy and Intervention Development across Food Animal Sectors" Antibiotics 11, no. 2: 226. https://doi.org/10.3390/antibiotics11020226
APA StyleLéger, D. F., Anderson, M. E. C., Bédard, F. D., Burns, T., Carson, C. A., Deckert, A. E., Gow, S. P., James, C., Li, X.-Z., Ott, M., & Agunos, A. (2022). Canadian Collaboration to Identify a Minimum Dataset for Antimicrobial Use Surveillance for Policy and Intervention Development across Food Animal Sectors. Antibiotics, 11(2), 226. https://doi.org/10.3390/antibiotics11020226






