Therapy of Subclinical Mastitis during Lactation
Abstract
:1. Introduction
2. Results
2.1. Pretreatment Bacteriology
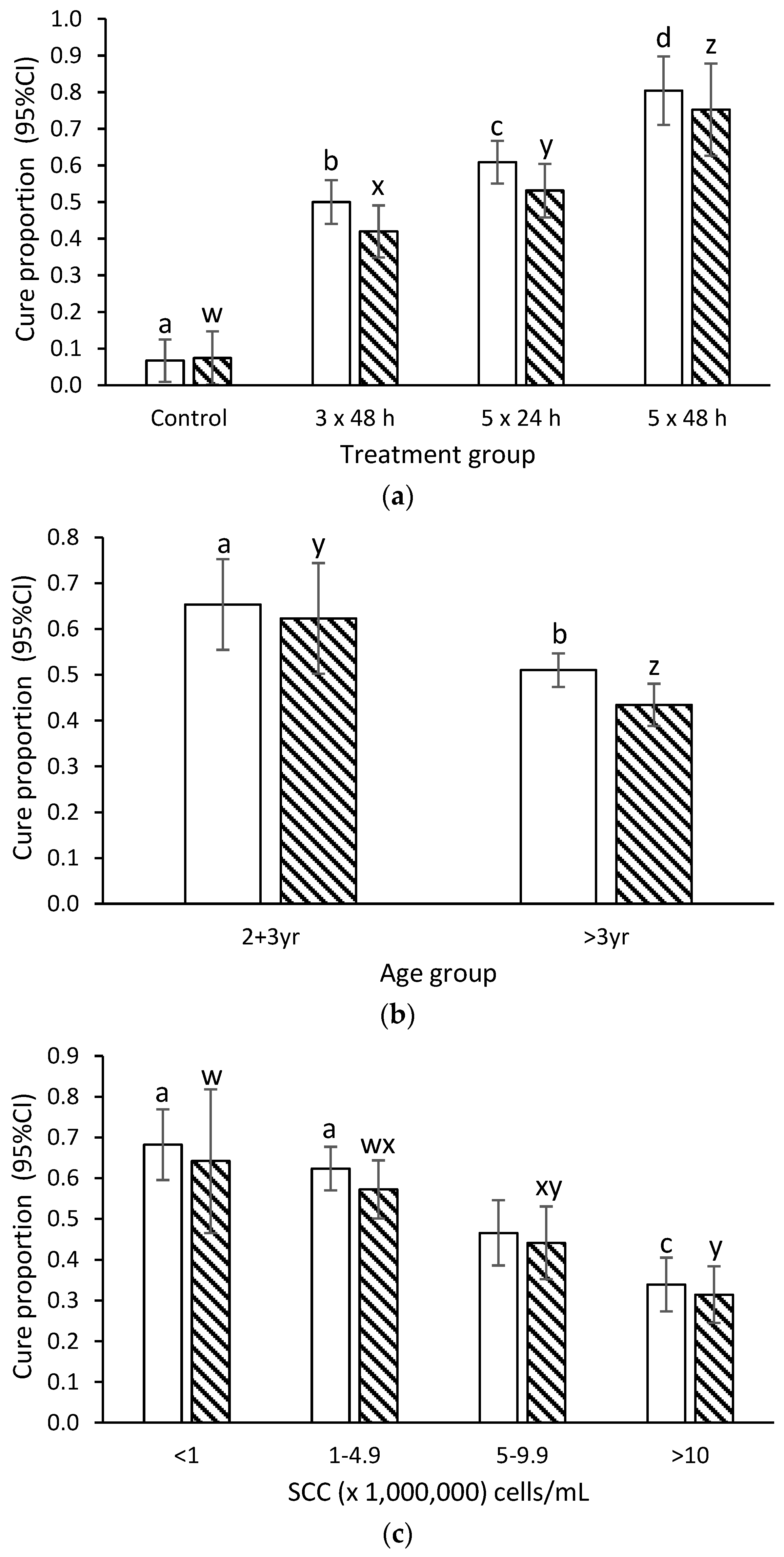
2.2. Bacteriological Cure
2.3. New Infection Rate
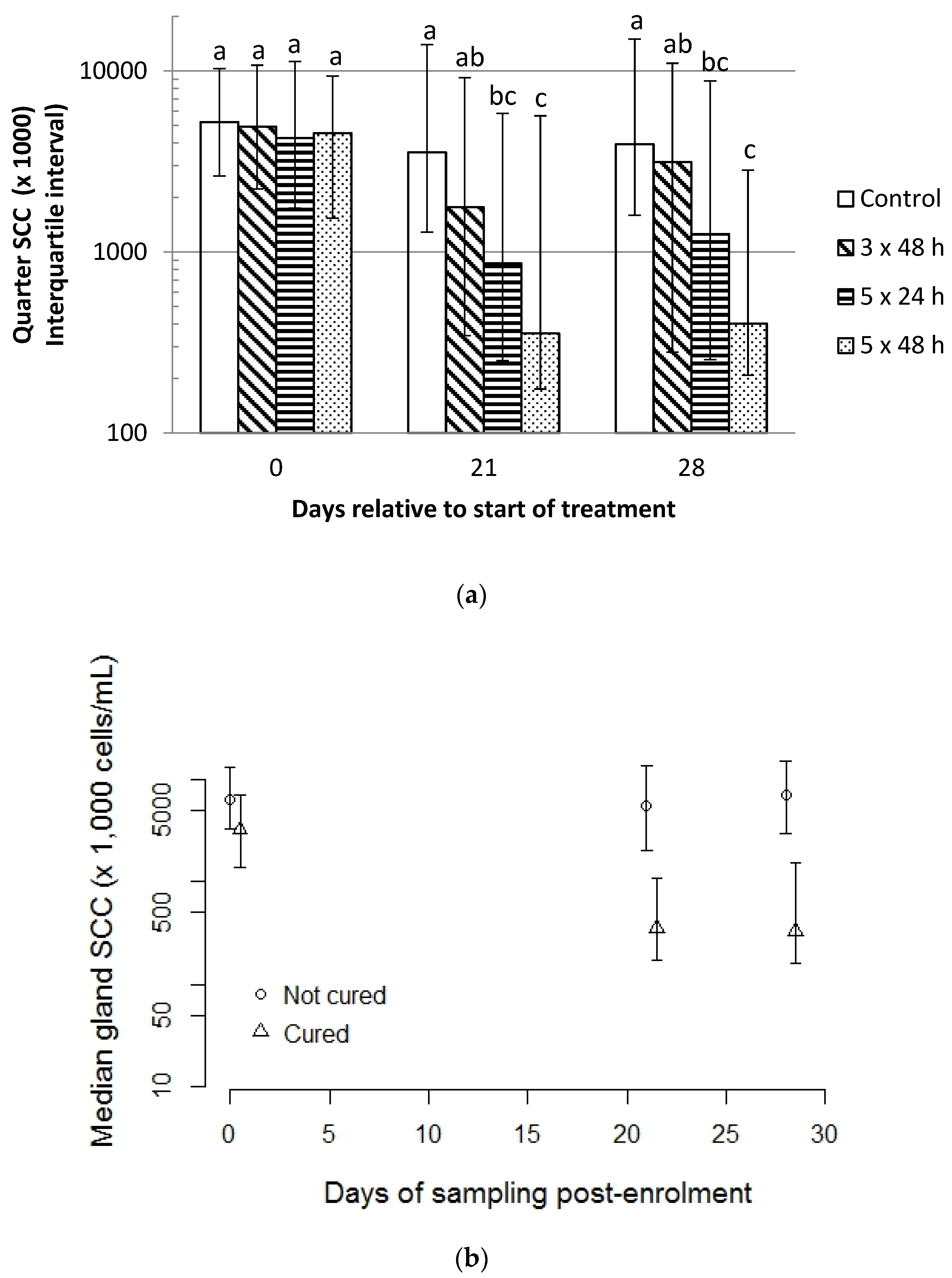
2.4. Quarter-Level Somatic Cell Count
2.5. Incidence of Clinical Mastitis within 28 Days of Enrolment
3. Discussion
3.1. Effect of Duration and Frequency of Treatment on Cure Rate
3.2. Effect of Age and SCC Duration on Cure Rate
3.3. Effect of Penicillin Resistance amongst Staph. aureus Isolates on Cure Rate
3.4. Effect of Treatment on Gland-Level SCC
4. Materials and Methods
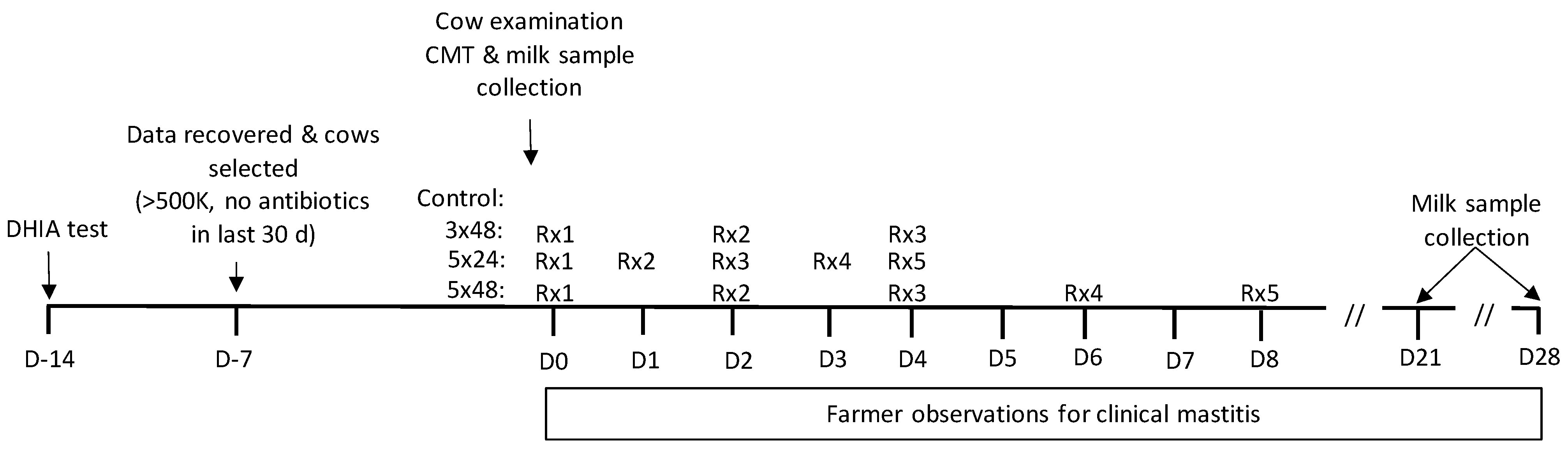
4.1. Cow Procedures
4.2. Laboratory Techniques
4.2.1. Microbiology
4.2.2. Somatic Cell-Count Determination
4.3. Data Handling and Analysis
4.4. Power Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruegg, P.L. Making antibiotic treatment decisions for clinical mastitis. Vet. Clin. Food Anim. Pract. 2018, 34, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Huijps, K.; Osterás, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- St.Rose, S.G.; Swinkels, J.M.; Kremer, W.D.J.; Kruitwagen, C.L.J.J.; Zadoks, R.N. Effect of penethamate hydriodide treatment on bacteriological cure, somatic cell count and milk production of cows and quarters with chronic subclinical Streptococcus uberis or Streptococcus dysgalactiae infection. J. Dairy Res. 2003, 70, 387–394. [Google Scholar] [CrossRef]
- Deluyker, H.A.; Van Oye, S.N.; Boucher, J.F. Factors affecting cure and somatic cell count after pirlimycin treatment of subclinical mastitis in lactating cows. J. Dairy Sci. 2005, 88, 604–614. [Google Scholar] [CrossRef]
- Steele, N.; McDougall, S. Effect of prolonged duration therapy of subclinical mastitis in lactating dairy cows using penethamate hydriodide. N. Z. Vet. J. 2014, 62, 38–46. [Google Scholar] [CrossRef]
- Barlow, J.W.; Zadoks, R.N.; Schukken, Y.H. Effect of lactation therapy on Staphylococcus aureus transmission dynamics in two commercial dairy herds. BMC Vet. Res. 2013, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Sol, S.; Sampimon, O.C.; Barkema, H.W.; Schukken, Y.H. Factors associated with cure after therapy of clinical mastitis caused by Staphylococcus aureus. J. Dairy Sci. 2000, 83, 278–284. [Google Scholar] [CrossRef]
- Sandholm, M.; Kaartinen, L.; Pyorala, S. Bovine mastitis—why does therapy not always work? An overview. J. Vet. Pharmacol. Therap. 1990, 13, 248–260. [Google Scholar] [CrossRef]
- Hillerton, J.E.; Semmens, J.E. Comparsion of treatment of mastitis by oxytocin or antibiotics following detection according to changes in milk electrical conductivity prior to visible signs. J. Dairy Sci. 1999, 82, 93–98. [Google Scholar] [CrossRef]
- Davis, W.T.; Maplesden, D.C.; Natzke, R.P.; Philpot, W.N.; Garrett, P.; Card, C.S. Benzathine cloxacillin intramammary infusion for treatment of mastitis in dry cows. Vet. Med. Small Anim. Clin. 1975, 70, 287–289. [Google Scholar]
- Ziv, G.; Storper, M. Intramuscular treatment of subclinical staphylococcal mastitis in lactating cows with penicillin G, methicillin and their esters. J. Vet. Pharmacol. Therap. 1985, 8, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Owens, W.E.; Ray, C.H.; Watts, J.L.; Yancey, R.J. Comparison of success of antibiotic therapy during lactation and results of antimicrobial susceptibility tests for bovine mastitis. J. Dairy Sci. 1997, 80, 313–317. [Google Scholar] [CrossRef]
- Craig, W.A. Choosing an Antibiotic on the basis of Pharmacodynamics. Ear, Nose, Throat J. 1998, 77, 7. [Google Scholar]
- McKellar, Q.A.; Sanchez Bruni, S.F.; Jones, D.G. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J. Vet. Pharmacol. Therap. 2004, 27, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.P.; Gillespie, B.E.; Headrick, S.J.; Moorehead, H.; Lunn, P.; Dowlen, H.H.; Johnson, D.L.; Lamar, K.C.; Chester, S.T.; Moseley, W.M. Efficacy of extended ceftiofur intramammary therapy for treatment of subclinical mastitis in lactating dairy cows. J. Dairy Sci. 2004, 87, 2393–2400. [Google Scholar] [CrossRef]
- Gillespie, B.E.; Moorehead, H.; Lun, P.; Dowlen, H.; Johnson, D.L.; Lamar, K.C.; Lewis, M.J.; Ivey, S.J.; Halberg, J.; Chester, S.; et al. Efficacy of extended pirlimycin therapy for treatment of environmental Streptococcus spp and Staphylococcus aureus intramammary infections in lactating dairy cows. Vet. Therap. 2002, 3, 373–380. [Google Scholar]
- McDougall, S.; Clausen, L.; Hintukainen, J.; Hunnam, J. Randomized, controlled, superiority study of extended duration of therapy with an intramammary antibiotic for treatment of clinical mastitis. J. Dairy Sci. 2019, 102, 4376–4386. [Google Scholar] [CrossRef]
- Tomazi, T.; Sumnicht, M.; Tomazi, A.C.C.H.; Silva, J.C.C.; Bringhenti, L.; Duarte, L.M.; Silva, M.M.M.; Rodrigues, M.X.; Bicalho, R.C. Negatively controlled, randomized clinical trial comparing different antimicrobial interventions for treatment of clinical mastitis caused by gram-positive pathogens. J. Dairy Sci. 2021, 104, 3364–3385. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Mevius, D.J.; Schroeter, A.; Teale, C.; Meunier, D.; Butaye, P.; Franco, A.; Utinane, A.; Amado, A.; Moreno, M.; et al. Prevalence of antimicrobial resistance among bacterial pathogens isolated from cattle in different European countries: 2002–2004. Acta Vet. Scand. 2008, 50, 28. [Google Scholar] [CrossRef] [Green Version]
- Kalmus, P.; Aasmäe, B.; Kärssin, A.; Orro, T.; Kask, K. Udder pathogens and their resistance to antimicrobial agents in dairy cows in Estonia. Acta Vet. Scand. 2011, 53, 4. [Google Scholar] [CrossRef] [Green Version]
- McDougall, S.; Hussein, H.; Petrovski, K. Antimicrobial resistance in Staphylococcus aureus, Streptococcus uberis and Streptococcus dysgalactiae from dairy cows with mastitis. N. Z. Vet. J. 2014, 62, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bagcigil, A.F.; Taponen, S.; Koort, J.; Bengtsson, B.; Myllyniemi, A.-L.; Pyörälä, S. Genetic basis of penicillin resistance of S. aureus isolated in bovine mastitis. Acta Vet. Scand. 2012, 54, 69. [Google Scholar] [CrossRef] [Green Version]
- Nayler, J.H.C.; Long, A.A.W.; Brown, D.M.; Acred, P.; Rolinson, G.N.; Batchelor, F.R.; Stevens, S.; Sutherland, R. Chemistry, toxicology, pharmacology and microbiology of a new acid-stable penicillin, resistant to penicillinase (BRL 1621,). Nature 1962, 195, 1264–1267. [Google Scholar] [CrossRef]
- Wilson, C.D.; Westgarth, D.R.; Kingwill, R.G.; Griffin, T.K.; Neave, F.K.; Dodd, F.H. The effect of infusion of sodium cloxacillin in all infected quarters of lactating cows in sixteen herds. Br Vet J. 1972, 128, 71–86. [Google Scholar] [CrossRef]
- Davis, W.T.; Maplesdon, D.C.; Natzke, R.P.; Philpot, W.N. Sodium cloxacillin for treatment of mastitis in lactating cows. J. Dairy Sci. 1975, 58, 1822–1827. [Google Scholar] [CrossRef]
- Browning, J.W.; Mein, G.A.; Barton, M.; Nicholls, T.J.; Brightling, P. Effects of antibiotic therapy at drying off on mastitis in the dry period and early lactation. Aust. Vet. J. 1990, 67, 440–442. [Google Scholar] [CrossRef]
- Halasa, T.; Nielen, M.; Whist, A.C.; Østerås, O. Meta-analysis of dry cow management for dairy cattle. Part 2. Cure of existing intramammary infections. J. Dairy Sci. 2009, 92, 3150–3157. [Google Scholar] [CrossRef]
- Guerin-Faublee, V.; Carret, G.; Houffschmitt, P. In vitro activity of 10 antimicrobial agents against bacteria isolated from cows with clinical mastitis. Vet. Rec. 2003, 152, 466–471. [Google Scholar] [CrossRef]
- Tenhagen, B.; Köster, G.; Wallman, J.; Heuwieser, W. Prevalence of mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Brandenburg, Germany. J. Dairy Sci. 2006, 89, 2542–2551. [Google Scholar] [CrossRef] [Green Version]
- Haenni, M.; Galofaro, L.; Ythier, M.; Giddey, M.; Majcherczyk, P.; Moreillon, P.; Madec, J.-Y. Penicillin-binding protein gene alterations in Streptococcus uberis isolates presenting decreased susceptibility to penicillin? Antimicrob. Agents Chemother. 2010, 54, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- McDougall, S.; Clausen, L.; Ha, H.-J.; Gibson, I.; Bryan, M.; Hadjirin, N.; Lay, E.; Raisen, C.; Ba, X.; Restif, O.; et al. Mechanisms of beta-lactam resistance of Streptococcus uberis isolated from bovine mastitis cases. Vet. Micro. 2020, 242, 108592. [Google Scholar] [CrossRef] [PubMed]
- Sol, J.; Sampimon, O.C.; Snoep, J.J.; Schukken, Y.H. Factors associated with bacteriological cure during lactation after therapy for subclinical mastitis caused by Staphylococcus aureus. J. Dairy Sci. 1997, 80, 2803–2808. [Google Scholar] [CrossRef]
- McDougall, S.; Arthur, D.G.; Bryan, M.A.; Vermunt, J.J.; Weir, A.M. Clinical and bacteriological response to treatment of clinical mastitis with one of three intramammary antibiotics. N. Z. Vet. J. 2007, 55, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.J.; Green, M.J. Factors affecting cure when treating bovine clinical mastitis with cephalosporin-based intramammary preparations. J. Dairy Sci. 2009, 92, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinberg, A.; Lopez-Villalobos, N.; Lawrence, K.; Nulsen, M. Prediction of penicillin resistance in Staphylococcus aureus isolates from dairy cows with mastitis, based on prior test results. N. Z. Vet. J. 2005, 53, 332–335. [Google Scholar] [CrossRef]
- Petrovski, K.R.; Grinberg, A.; Williamson, N.B.; Abdalla, M.E.; Lopez-Villalobos, N.; Parkinson, T.J.; Tucker, I.G.; Rapnicki, P. Susceptibility to antimicrobials of mastitis-causing Staphylococcus aureus, Streptococcus uberis and Str. dysgalactiae from New Zealand and the USA as assessed by the disk diffusion test. Aust. Vet. J. 2015, 93, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Taponen, S.; Jantunen, A.; Pyorala, E.; Pyorala, S. Efficacy of targeted 5-day combined parenteral and intramammary treatment of clinical mastitis caused by penicillin-susceptible or penicillin-resistant Staphylococcus aureus. Acta Vet. Scand. 2003, 44, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef] [Green Version]
- Mein, G.A.; Neijenhuis, F.; Morgan, W.F.; Reinemann, D.J.; Hillerton, J.E.; Baines, J.R.; Ohnstad, I.; Rasmussen, M.D.; Timms, L.; Britt, J.S.; et al. Evaluation of bovine teat conditions in commercial dairy herds 1. Non-infectious factors. In Proceedings of the 2nd International Symposium on Mastitis and Milk Quality, Vancouver, BC, Canada, 12–14 September 2001; NMC Inc.: Madison, WI, USA, 2001; pp. 347–351. [Google Scholar]
- Hogan, J.S.; Gonzalez, R.N.; Harmon, R.J.; Nickerson, S.C.; Oliver, S.P.; Pankey, J.W.; Smith, K.L. (Eds.) Laboratory Handbook on Bovine Mastitis; NMC Inc.: Madison, WI, USA, 1999. [Google Scholar]
- CLSI (Ed.) Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard, 4th ed.; CLSI: Wayne, PA, USA, 2013. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing, 2.14.1; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Bailey, D.; Alimadhi, F. Logit.mixed: Mixed Effects Logistic Model. In Zelig: Everyone’s Statistical Software; Imai, K., King, G., Lau, O., Eds.; R Foundation for Statistical Computing: Vienna, Austria, 2007; Available online: http://gking.harvard.edu/ (accessed on 29 April 2012).
| Control | 3 × 48 h | 5 × 24 h | 5 × 48 h | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % Cured | No. | % Cured | No. | % Cured | No. | % Cured | No. | % Cured | ||
| Minor | NAS 1 | 13 | 0.0 | 33 | 78.8 | 37 | 83.8 | 15 | 86.7 | 98 | 71.4 |
| Corynebacterium spp. | 15 | 6.7 | 36 | 58.3 | 59 | 76.3 | 9 | 100.0 | 119 | 63.9 | |
| All minor | 28 | 3.6 | 69 | 68.1 | 96 | 79.2 | 24 | 91.7 | 217 | 67.3 | |
| Major | Staph. aureus | 26 | 0.0 | 115 | 33.0 | 98 | 41.8 | 26 | 80.8 | 265 | 37.7 |
| Strep. agalactiae | 1 | 100.0 | 1 | 100.0 | 2 | 100.0 | |||||
| Strep. dysgalactiae | 6 | 16.7 | 15 | 93.3 | 11 | 90.9 | 1 | 100.0 | 33 | 78.8 | |
| Strep. uberis | 8 | 0.0 | 36 | 61.1 | 42 | 59.5 | 11 | 54.5 | 97 | 54.6 | |
| All Streptococci | 15 | 13.3 | 52 | 71.2 | 53 | 66.0 | 12 | 58.3 | 132 | 61.4 | |
| E. coli | 2 | 100.0 | 2 | 100.0 | 4 | 100.0 | |||||
| Mixed 2 | 11 | 18.2 | 33 | 33.3 | 26 | 69.2 | 9 | 88.9 | 79 | 49.4 | |
| Gram -ve rods | 1 | 100.0 | 1 | 100.0 | |||||||
| Nocardia spp. | 1 | 100.0 | 4 | 100.0 | 1 | 100.0 | 6 | 100.0 | |||
| All majors | 52 | 7.7 | 204 | 44.1 | 183 | 54.6 | 48 | 77.1 | 480 | 46.7 | |
| Total glands | 80 | 6.3 | 273 | 49.8 | 279 | 61.6 | 72 | 80.6 | 704 | 52.7 | |
| Total cows | 50 | 173 | 175 | 49 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDougall, S.; Clausen, L.M.; Hussein, H.M.; Compton, C.W.R. Therapy of Subclinical Mastitis during Lactation. Antibiotics 2022, 11, 209. https://doi.org/10.3390/antibiotics11020209
McDougall S, Clausen LM, Hussein HM, Compton CWR. Therapy of Subclinical Mastitis during Lactation. Antibiotics. 2022; 11(2):209. https://doi.org/10.3390/antibiotics11020209
Chicago/Turabian StyleMcDougall, Scott, Laura M. Clausen, Hassan M. Hussein, and Chris W. R. Compton. 2022. "Therapy of Subclinical Mastitis during Lactation" Antibiotics 11, no. 2: 209. https://doi.org/10.3390/antibiotics11020209
APA StyleMcDougall, S., Clausen, L. M., Hussein, H. M., & Compton, C. W. R. (2022). Therapy of Subclinical Mastitis during Lactation. Antibiotics, 11(2), 209. https://doi.org/10.3390/antibiotics11020209






