Anti-Infective Treatment and Resistance Is Rarely Problematic with Eye Infections
Abstract
1. Introduction
2. Eye Infections
3. Diagnostics of Eye Infections
4. Anti-Infective Treatment of Ocular Infections
4.1. In Vitro Antibiotic Susceptibility Testing Batteries
4.1.1. Endophthalmitis
4.1.2. Keratitis
4.1.3. Conjunctivitis
5. Anti-Infective Resistance of Ocular Bacterial Isolates
6. Alternative Anti-Infective Delivery and Therapy
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basic and Clinical Science Course 8. Infectious diseases of the cornea, and external eye. In External Disease and Cornea: Bacterial, Fungal, and Parasitic Infections; American Academy of Ophthalmology: San Francisco, CA, USA, 2021; pp. 317–351. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Kowalski, R.P.; Karenchak, L.M.; Gordon, Y.J. Comparison of ciprofloxacin and ofloxacin using human corneal susceptibility levels. Cornea 1998, 17, 282–287. [Google Scholar] [CrossRef]
- Constantinou, M.; Daniell, M.; Snibson, G.R.; Vu, H.T.; Taylor, H.R. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis. Ophthalmology 2007, 114, 1622–1629. [Google Scholar] [CrossRef]
- Mah, F.S.; Sanfilippo, C.M. Besifloxacin: Efficacy and safety in treatment and prevention of ocular bacterial infections. Ophthalmol. Ther. 2016, 5, 1–20. [Google Scholar] [CrossRef]
- Kowalski, R.P. Perspective: Is antibiotic resistance a problem in the treatment of ophthalmic infections? Exp. Rev. Ophthalmol. 2013, 8, 119–226. [Google Scholar] [CrossRef]
- Callegan, M.C.; Engelbert, M.; Parke, D.W.; Jett, B.D.; Gilmore, M.S. Bacterial endophthalmitis: Epidemiology, therapeutics, and bacterium-host interactions. Clin. Microbiol. Rev. 2002, 15, 111–124. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Nayyar, S.V.; Romanowski, E.G.; Shanks, R.M.Q.; Mammen, A.; Dhaliwal, D.K.; Jhanji, V. The prevalence of bacteria, fungi, viruses, and acanthamoeba from 3004 cases of keratitis, endophthalmitis, and conjunctivitis. Eye Contact Lens 2020, 46, 265–268. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Roat, M.I.; Thompson, P.P. Normal Flora of the Conjunctiva and Eyelids. In Duane’s Foundations of Clinical Ophthalmology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Kowalski, R.P.; Harwick, J.C. Incidence of moraxella conjunctival infection. Am. J. Ophthalmol. 1986, 101, 437–440. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Thompson, P.P.; Kinchington, P.R.; Gordon, Y.J. The evaluation of the SmartCycler® II System for the real-time detection of viruses and chlamydia from ocular specimens. Arch. Ophthalmol. 2006, 124, 1135–1139. [Google Scholar] [CrossRef][Green Version]
- Thompson, P.P.; Shanks, R.M.Q.; Gordon, Y.J.; Kowalski, R.P. Laboratory diagnosis of acanthamoeba keratitis using the Cepheid SmartCycler II in the presence of topical ophthalmic drugs. J. Clin. Microbiol. 2008, 46, 3232–3236. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Melan, M.A.; Karenchak, L.M.; Mammen, A. The comparison of validated PCR to culture isolation for detecting acanthamoeba from ocular samples. Eye Contact Lens 2015, 41, 341–343. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Karenchak, L.M.; Raju, L.V.; Ismail, N. The validation of nucleic acid amplification testing (NAAT) (Gen-Probe® Aptima®) for Chlamydia trachomatis from ocular samples. Ophthalmology 2014, 122, 244–247. [Google Scholar] [CrossRef]
- Doft, B.H. Treatment of postcataract extraction endophthalmitis: A summary of the results from the Endophthalmitis Vitrectomy Study. Arch. Ophthalmol. 2008, 126, 554–556. [Google Scholar] [CrossRef]
- Radhika, M.; Mithal, K.; Bawdekar, A.; Dave, V.; Jindal, A.; Relhan, N.; Albini, T.; Pathengay, A.; Flynn, H.W. Pharmacokinetics of intravitreal antibiotics in endophthalmitis. J. Ophthalmic Inflamm. Infect. 2014, 4, 22. [Google Scholar] [CrossRef]
- Azhdam, A.M.; Goldberg, R.A.; Ugradar, S. In vivo measurement of the human vitreous chamber volume using computed tomography imaging of 100 eyes. Transl. Vis. Sci. Technol. 2020, 9, 2–5. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Kowalski, T.A.; Shanks, R.M.Q.; Romanowski, E.G.; Karenchak, L.M.; Mah, F.S. In Vitro comparison of combination- and mono-therapy for the empiric and optimal coverage of bacterial keratitis based on incidence of infection. Cornea 2013, 32, 830–834. [Google Scholar] [CrossRef]
- Thareja, T.; Kowalski, R.P.; Jhanji, V.; Kamyar, R.; Dhaliwal, D.K. MRSA keratitis and conjunctivitis: What does it mean practically? Curr. Ophthalmol. Rep. 2019, 7, 110–117. [Google Scholar] [CrossRef]
- Durrani, A.; Atta, S.; Bhat, A.K.; Mammen, A.; Dhaliwal, D.K.; Kowalski, R.P.; Jhanji, V. Methicillin-resistant Staphylococci aureus keratitis: Initial treatment, risk factors, clinical features, and treatment outcomes. Am. J. Ophthalmol. 2020, 214, 119–126. [Google Scholar] [CrossRef]
- Chang, V.S.; Dhaliwal, D.K.; Raju, L.V.; Kowalski, R.P. Is antibiotic resistance a major problem in the treatment of Staphylococcus aureus keratitis? A 20-year review. Cornea 2015, 34, 698–703. [Google Scholar] [CrossRef]
- Mather, R.; Karenchak, L.M.; Romanowski, E.G.; Kowalski, R.P. 4th generation fluoroquinolones: New weapons in the arsenal of ophthalmic antibiotics. Am. J. Ophthalmol. 2002, 133, 463–466. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Dhaliwal, D.K.; Karenchak, L.M.; Romanowski, E.G.; Mah, F.S.; Ritterband, R.C.; Gordon, Y.J. Gatifloxacin and moxifloxacin: An in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am. J. Ophthalmol. 2003, 136, 500–505. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Yates, K.A.; Romanowski, E.G.; Karenchak, L.M.; Mah, F.S.; Gordon, J.S. An ophthalmologist’s guide to understanding antibiotic susceptibility and minimum inhibitory concentration (MIC) data. Ophthalmology 2005, 112, 1987–1991. [Google Scholar] [CrossRef]
- Schechter, B.A.; Sheppard, J.D.; Sanfilippo, C.M.; DeCory, H.H.; Asbell, P.A. An evaluation of staphylococci from ocular surface infections treated empirically with topical besifloxacin: Antibiotic resistance, molecular characteristics, and clinical outcomes. Ophthalmol. Ther. 2020, 9, 159–173. [Google Scholar] [CrossRef]
- Wu, E.C.; Kowalski, R.P.; Romanowski, E.G.; Mah, F.S.; Gordon, Y.J.; Shanks, R.M.Q. AzaSite® inhibits Staphylococcus aureus and coagulase negative staphylococcus biofilm formation in vitro. J. Ocul. Pharmacol. Ther. 2010, 26, 557–562. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Romanowski, E.G.; Yates, K.A.; Romanowski, J.E.; Grewal, A. Is there a role for the topical penicillin treatment of Staphylococcus aureus keratitis based on elevated corneal concentrations? J. Clin. Ophthalmol. Optom. 2018, 2, 103. [Google Scholar]
- Romanowski, J.E.; Romanowski, E.G.; Yates, K.A.; Kowalski, R.P. The successful treatment of experimental methicillin-resistant Staphylococcus aureus keratitis with topical penicillin. Ophthalmol Res. Rep. 2018, 59, 3658. [Google Scholar] [CrossRef]
- Romanowski, J.E.; Nayyar, S.V.; Romanowski, E.G.; Jhanji, V.; Shanks, R.M.Q.; Kowalski, R.P. Speciation and antibiotic susceptibilities of coagulase negative staphylococci isolated from ocular infections. Antibiotics 2021, 10, 721. [Google Scholar] [CrossRef]
- Asbell, P.A.; Sanfillippo, C.M.; Sahm, D.F.; DeCory, H.H. Trends in Antibiotic resistance among ocular microorganisms in the United States from 2009 and 2018. JAMA Ophthalmol. 2020, 138, 439–450. [Google Scholar] [CrossRef]
- Block, S.L.; Hedrick, J.; Tyler, R.; Smith, A.; Findlay, R.; Keegan, E.; Stroman, D.W. Increasing bacterial resistance in pediatric acute conjunctivitis (1997–1998). Antimicrob. Agents Chemother. 2000, 44, 1650–1654. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of fluoroquinolone resistance. Drug Resist. Updates 1999, 2, 38–55. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–444. [Google Scholar] [CrossRef]
- Chaudhry, N.A.; Tabandeh, H.; Rosenfeld, P.J.; Miller, D.; Davis, J. Scleral buckle infection with ciprofloxacin-resistant Pseudomonas aeruginosa. Arch. Ophthalmol. 1998, 116, 1251. [Google Scholar]
- Garg, P.; Sharma, S.; Rao, G.N. Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology 1999, 106, 1319–1323. [Google Scholar] [CrossRef]
- Chaudhry, N.A.; Flynn, H.W.; Murray, T.G.; Tabandeh, H.; Mello, M.O.; Miller, D. Emerging ciprofloxacin-resistant Pseudomonas aeruginosa. Am. J. Ophthalmol. 1999, 128, 509–510. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Mah, F.S.; Yates, K.A.; Kowalski, R.P.; Gordon, Y.J. The successful treatment of gatifloxacin-resistant Staphylococcus aureus keratitis with Zymar (gatifloxacin 0.3%) in a NZW rabbit model. Am. J. Ophthalmol. 2005, 139, 867–877. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Kowalski, R.P.; Gordon, Y.J. Emerging fluoroquinolone resistance in bacterial keratitis: A five year review. Ophthalmology 1999, 106, 1313–1318. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Romanowski, E.G.; Mah, F.S.; Shanks, R.M.Q.; Gordon, Y.J. Topical Levofloxacin 1.5% (IQUIX) overcomes in vitro resistance in rabbit keratitis models. Acta Opthalmol. 2010, 88, e120–e125. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Romanowski, J.E.; Shanks, R.M.Q.; Mammen, A.; Dhaliwal, D.K.; Jhanji, V.; Kowalski, R.P. Topical vancomycin 5% is more efficacious than 2.5% and 1.25% for reducing viable MRSA in infectious keratitis. Cornea 2020, 39, 250–253. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.M.; Torkildsen, G.L.; Brubaker, K.; Zink, R.C.; Kowalski, R.P.; Mah, F.S.; Pflugfelder, S.C. Multi-Center, open-label study evaluating the efficacy of azithromycin ophthalmic solution 1% on the signs and symptoms of subjects with blepharitis. Cornea 2010, 29, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Tauber, S.; Cupp, G.; Garber, R.; Bartell, J.; Vohra, F.; Stroman, D. Microbiological efficacy of a new ophthalmic formulation of moxifloxacin dosed twice-daily for bacterial conjunctivitis. Adv. Ther. 2010, 28, 566–574. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Chethana, S.R.; Mohammed, G.A. Hydrogel contact lense for extended delivery of an antibiotic in combination with anti-inflammatory drug for ophthalmic application. Asian J. Biomed. Pharm. Sci. 2015, 5, 16–21. [Google Scholar]
- Hui, A.; Willcox, M.; Jones, L. In vitro and in vivo evaluation of novel ciprofloxacin-releasing silicone hydrogel contact lenses. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4896–4899. [Google Scholar] [CrossRef] [PubMed]
- Liangju, K.; Ross, A.E.; Kanu, L.N.; Romanowski, E.G.; Kowalski, R.P.; Kohane, D.S.; Ciolino, J.B. A novel, widely-accessible besifloxacin quantification method: Application to ocular pharmacokinetic study in rabbits. J. Chromatogr. B 2021, 1185, 123010. [Google Scholar]
- Mammen, A.; Romanowski, E.G.; Fedorchak, M.V.; Dhaliwal, D.K.; Shanks, R.M.Q.; Kowalski, R.P. Endophthalmitis prophylaxis using a single drop of thermo-responsive controlled-release microspheres loaded with moxifloxacin in a rabbit model. Transl. Vis. Sci. Technol. 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Klarlund, J.K.; Callaghan, J.D.; Stella, N.A.; Kowalski, R.P.; McNamara, N.A.; Shanks, R.M.Q. Use of collagen binding domains to deliver molecules to the cornea. J. Ocul. Pharmacol. 2019, 35, 491–496. [Google Scholar] [CrossRef]
- Thakar, R.; Komanduri, N.; Dudhipala, N.; Tripathi, D.; Repka, M.A.; Majumdar, S. Development and optimization of hot-melt extruded moxifloxacin hydrochloride inserts, for ocular applications, using the design of experiments. Int. J. Pharm. 2021, 603, 120676. [Google Scholar] [CrossRef]
- Youssef, A.A.A.; Cai, C.; Dudhipala, N.; Majumdar, S. Design of topical nanoemusion for the management of bacterial keratitis. Pharmaceuticals 2021, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Shanks, R.M.Q.; Davra, V.R.; Romanowski, E.G.; Brothers, K.M.; Stella, N.A.; Godboley, D.; Kadouri, D.E. An eye to a kill: Using predatory bacteria to control gram-negative pathogens associated with ocular infections. PLoS ONE 2013, 8, e66723. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Gupta, S.; Pericleous, A.; Kadouri, D.E.; Shanks, R.M.Q. Clearance of Gram-negative bacterial pathogens from the ocular surface by predatory bacteria. Antibiotics 2021, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.A.; Bovelle, R.; Han, G.; Kwagyan, J. Corneal collagen cross-linking for bacterial infectious keratitis. Cochrane Database Syst. Rev. 2020, 6. [Google Scholar] [CrossRef]
- Narnjo, A.; Arboleda, A.; Martinez, J.D.; Durkee, H.; Aguillar, C.; Relhan, N.; Nikpoor, N.; Galor, A.; Dubovy, S.R.; Leblanc, R.; et al. Rose Bengal photodynamic antimicrobial therapy (RB-PDAT) for patients with progressive infectious keratitis: A pilot clinical study. Am. J. Ophthalmol. 2019, 208, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ghafoorianfar, S.; Ghorani-Azam, A.; Mohajeri, S.A.; Farzin, D. Efficiency of nanoparticles for treatment of ocular infections: Systemic literature review. J. Drug Deliv. Sci. Technol. 2020, 57, 101765. [Google Scholar] [CrossRef]
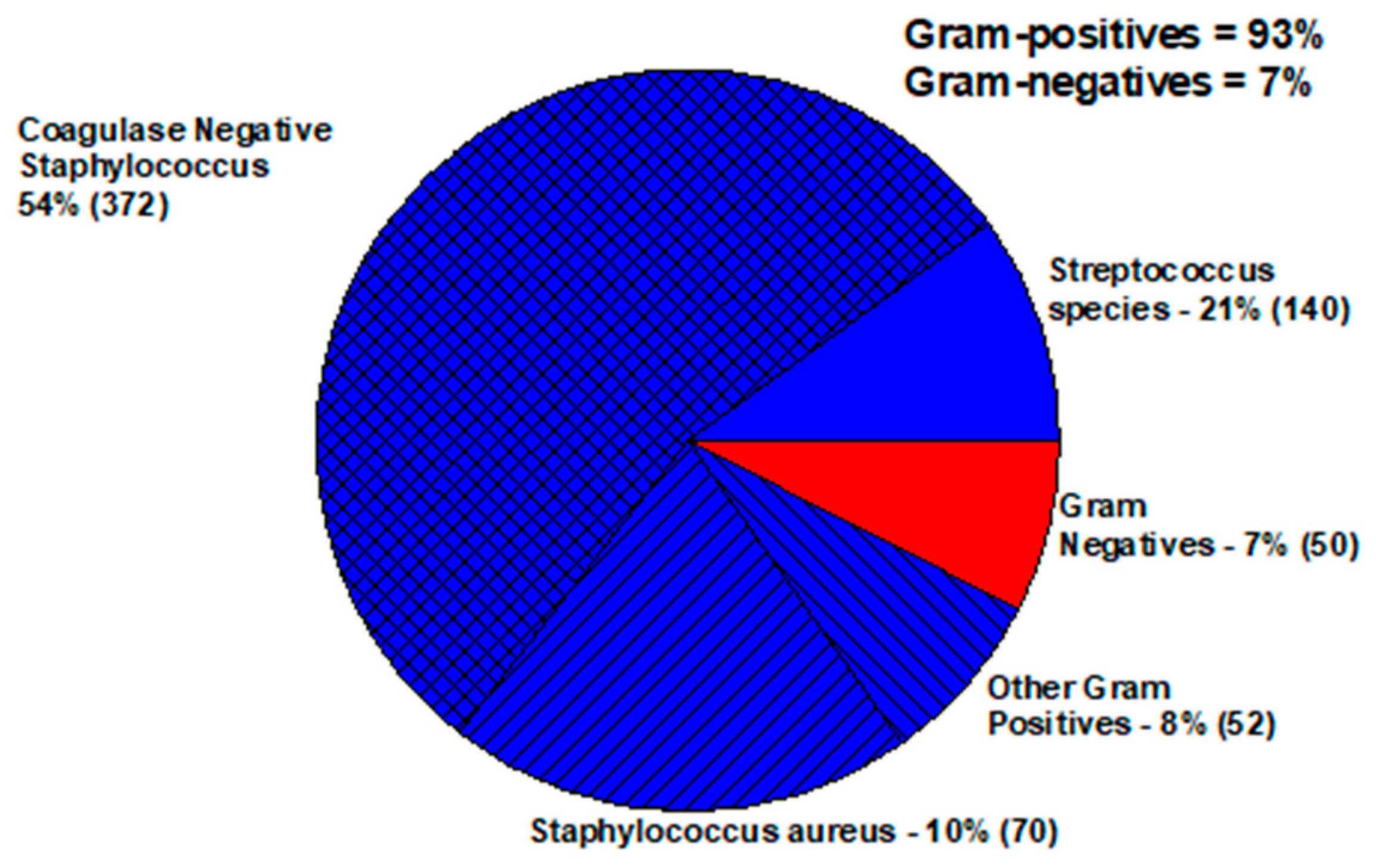
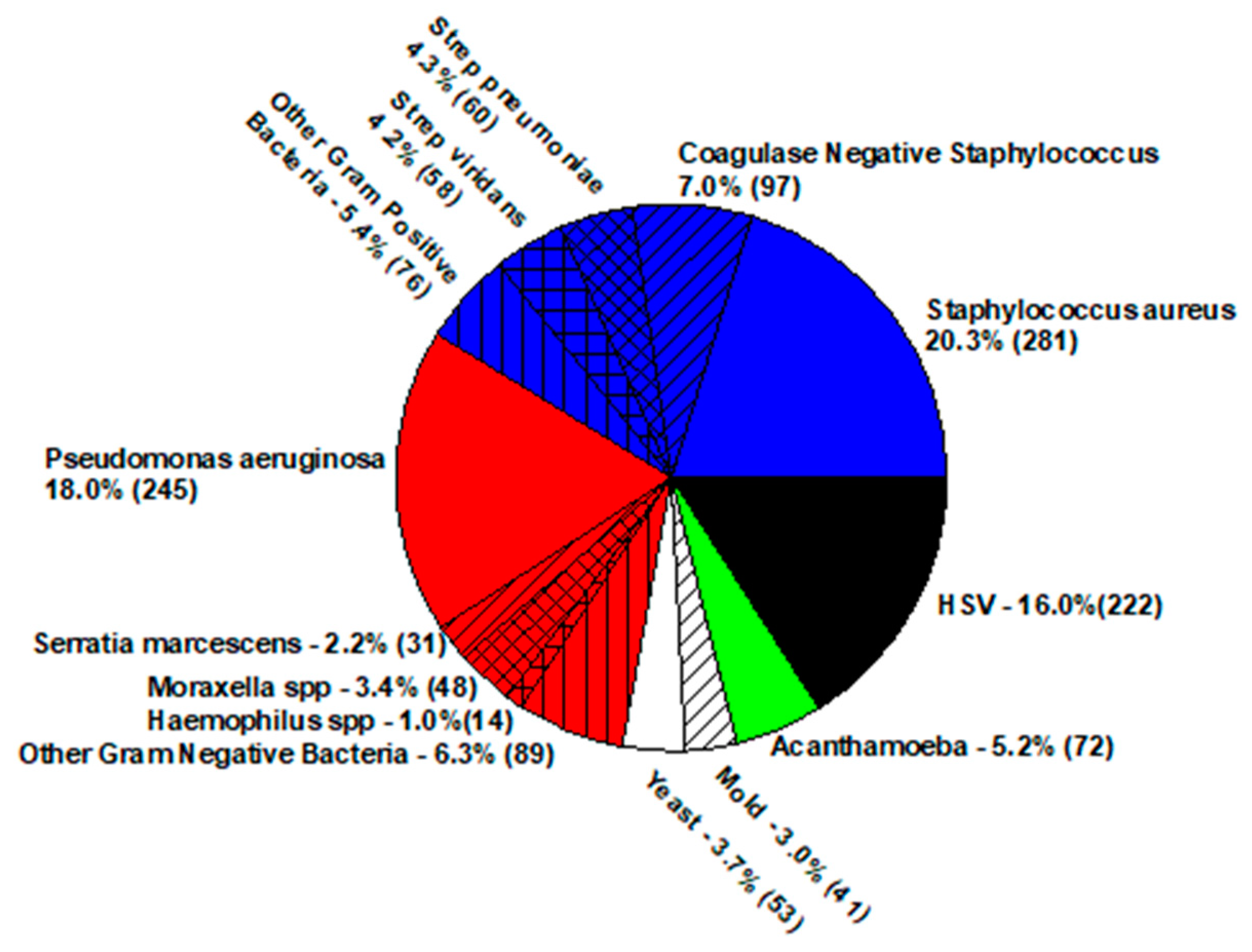
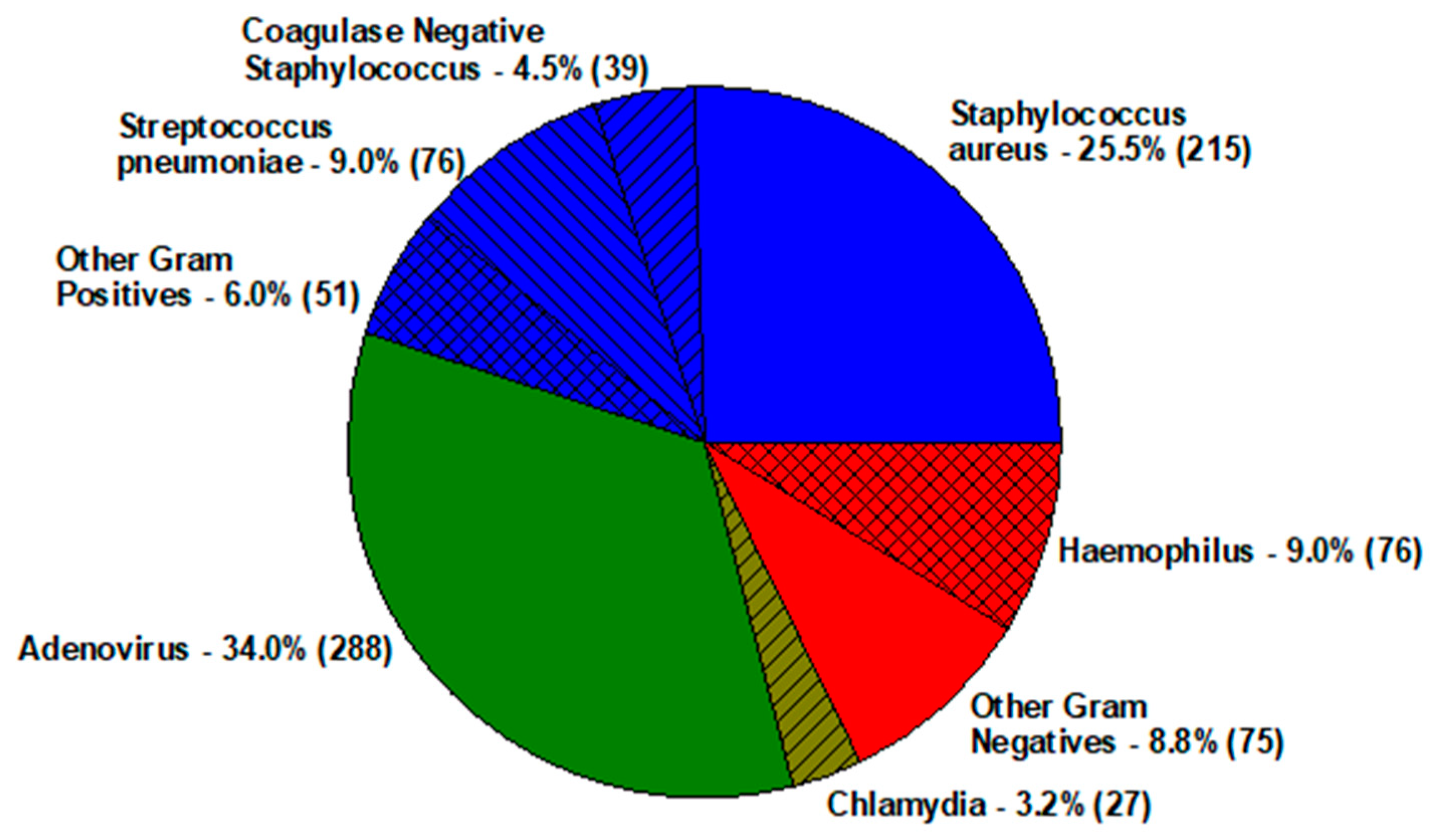


| Keratitis—Staphylococcus aureus | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Vancomycin | 48 | 0.38 | 0.75 | 0.75 | 1.0 | 1.5 | ≤2 | ≥16 |
| Gentamicin | 48 | 0.094 | 0.19 | 0.19 | 0.24 | 4.0 | ≤4 | ≥16 |
| Moxifloxacin | 45 | 0.032 | 0.064 | 0.094 | 1.5 | 12.0 | ≤0.5 | ≥2 |
| Cefoxitin | 46 | 3.0 | 4.0 | 4.0 | 24.0 | 48.0 | ≤4 | ≥8 |
| Cefazolin | 47 | 0.38 | 0.75 | 1.0 | 3.0 | 96.0 | No standard | No standard |
| Keratitis—Pseudomonas aeruginosa | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Tobramycin | 29 | 0.38 | 1.0 | 1.5 | 2.0 | 6.0 | ≤4 | ≥16 |
| Ceftazidime | 29 | 1.0 | 1.5 | 1.5 | 2.0 | 4.0 | ≤8 | ≥32 |
| Ciprofloxacin | 29 | 0.047 | 0.064 | 0.125 | 0.19 | 1.0 | ≤0.5 | ≥2 |
| Polymyxin B | 29 | 0.75 | 1.5 | 2.0 | 3.0 | 6.0 | ≤2 | 8 |
| Keratitis—Coagulase Negative Staphylococci | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Vancomycin | 17 | 0.75 | 1.0 | 1.5 | 1.5 | 3.0 | ≤4 | ≥32 |
| Gentamicin | 17 | 0.03 | 0.08 | 0.25 | 19.0 | 48 | ≤4 | ≥16 |
| Moxifloxacin | 17 | 0.06 | 0.88 | 3.0 | 64.0 | 64.0 | ≤0.5 | ≥2 |
| Cefazolin | 17 | 0.38 | 0.5 | 1.5 | 3.0 | 12.0 | No standard | No standard |
| Keratitis—Streptococcus viridans group | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Vancomycin | 13 | 0.25 | 0.44 | 0.5 | 0.88 | 2.00 | ≤1 | No standard |
| Gentamicin | 7 | 0.38 | 0.75 | 2.0 | 3.0 | 32.0 | ≤4 | ≥16 |
| Moxifloxacin | 13 | 0.064 | 0.094 | 0.125 | 0.19 | 0.038 | ≤1 | ≥4 |
| Cefazolin | 8 | 0.02 | 0.14 | 0.32 | 3.38 | 16 | No standard | No standard |
| Keratitis—Other Gram-positive bacteria | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Vancomycin | 8 | 0.38 | 0.41 | 0.625 | 1.0 | 1.5 | ≤1 | No standard |
| Gentamicin | 6 | 0.001 | 0.8 | 2.5 | 60 | 96.0 | ≤4 | ≥ 16 |
| Moxifloxacin | 8 | 0.014 | 0.211 | 0.365 | 1.28 | 4.0 | ≤1 | ≥ 4 |
| cefazolin | 8 | 0.001 | 0.1 | 8.1 | 4.2 | 128 | No standard | No standard |
| Keratitis—Other Gram-negative Bacteria | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Tobramycin | 14 | 0.25 | 0.348 | 1.0 | 3.0 | 3.0 | ≤4 | ≥16 |
| Ceftazidime | 13 | 0.064 | 0.125 | 0.19 | 1.0 | 3.0 | ≤8 | ≥32 |
| Ciprofloxacin | 13 | 0.012 | 0.019 | 0.032 | 0.47 | 4.00 | ≤1 | ≥4 |
| Polymyxin B | 12 | 0.001 | 0.56 | 1.0 | 6.38 | 32.00 | ≤2 | ≥8 |
| Conjunctivitis—Staphylococcus aureus | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Moxifloxacin | 39 | 0.03 | 0.05 | 0.06 | 2.0 | 64.00 | ≤0.5 | ≥2 |
| Cefoxitin | 40 | 0.8 | 4.0 | 4.0 | 15.0 | 512 | ≤4 | ≥8 |
| Conjunctivitis—Other Gram-positive bacteria | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Moxifloxacin | 16 | 0.094 | 0.19 | 0.25 | 0.35 | 0.5 | ≤1 | ≥4 |
| Conjunctivitis—Other Gram-negative bacteria | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Tobramycin | 19 | 0.75 | 0.75 | 1.5 | 2.0 | 32 | ≤4 | ≥16 |
| Ciprofloxacin | 19 | 0.008 | 0.06 | 0.047 | 0.38 | 0.63 | ≤1 | ≥4 |
| Polymyxin B | 19 | 0.001 | 0.75 | 1.0 | 2.0 | 64 | ≤2 | ≥8 |
| Moxifloxacin | 13 | 0.047 | 0.094 | 0.19 | 0.94 | 6.0 | No standard | No standard |
| Endophthalmitis—Gram-positive bacteria | ||||||||
| Antibiotic | Number | Minimum | Quarter1 | Median | Quarter3 | Maximum | MIC Susceptible | MIC Resistant |
| Vancomycin | 25 | 0.5 | 1.0 | 1.5 | 2.0 | 2.0 | ≤4 | ≥32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, R.P.; Nayyar, S.V.; Romanowski, E.G.; Jhanji, V. Anti-Infective Treatment and Resistance Is Rarely Problematic with Eye Infections. Antibiotics 2022, 11, 204. https://doi.org/10.3390/antibiotics11020204
Kowalski RP, Nayyar SV, Romanowski EG, Jhanji V. Anti-Infective Treatment and Resistance Is Rarely Problematic with Eye Infections. Antibiotics. 2022; 11(2):204. https://doi.org/10.3390/antibiotics11020204
Chicago/Turabian StyleKowalski, Regis P., Shannon V. Nayyar, Eric G. Romanowski, and Vishal Jhanji. 2022. "Anti-Infective Treatment and Resistance Is Rarely Problematic with Eye Infections" Antibiotics 11, no. 2: 204. https://doi.org/10.3390/antibiotics11020204
APA StyleKowalski, R. P., Nayyar, S. V., Romanowski, E. G., & Jhanji, V. (2022). Anti-Infective Treatment and Resistance Is Rarely Problematic with Eye Infections. Antibiotics, 11(2), 204. https://doi.org/10.3390/antibiotics11020204







