Creating Robust Antimicrobial Materials with Sticky Tyrocidines
Abstract
1. Introduction
2. Results and Discussion
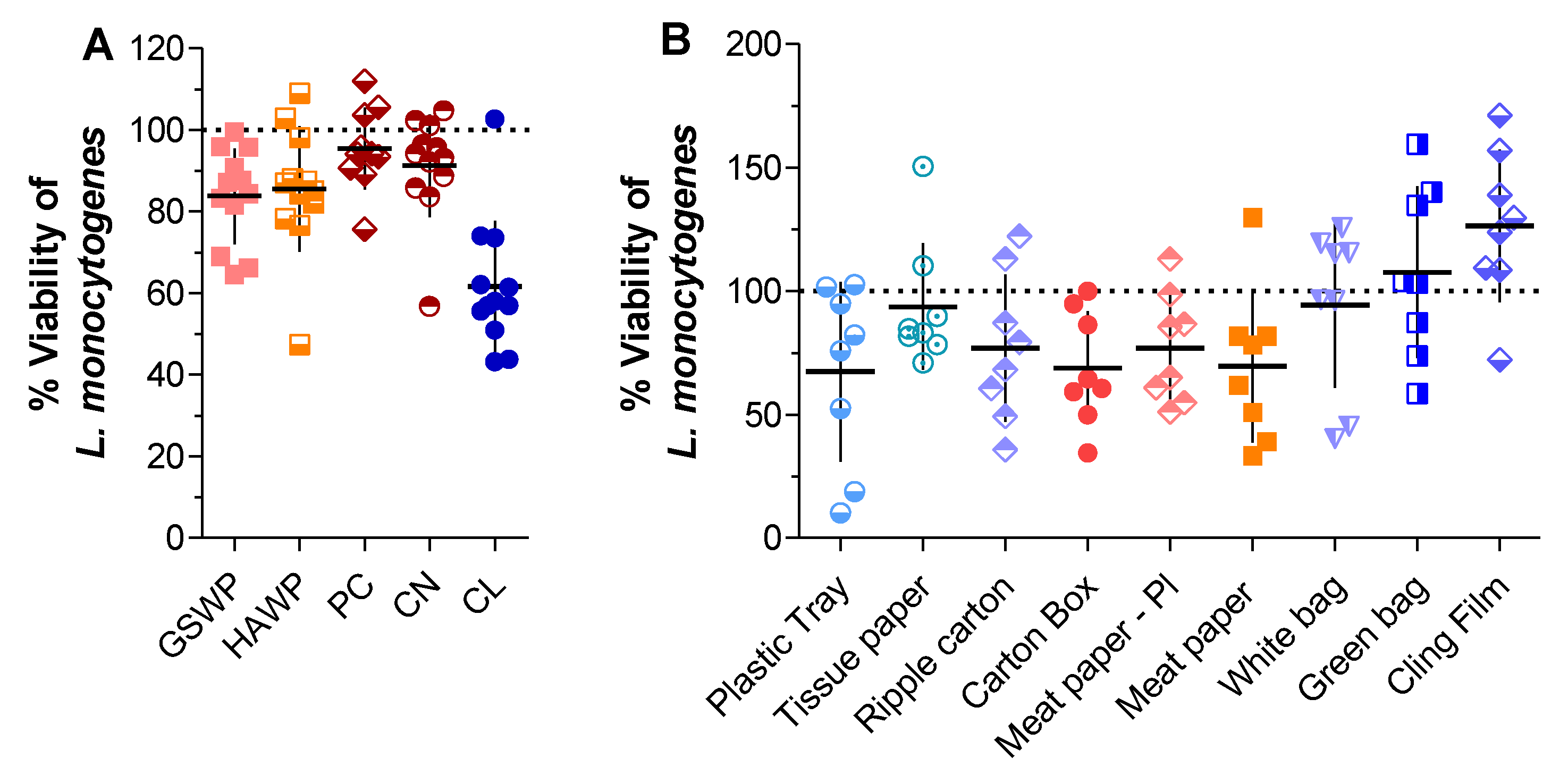
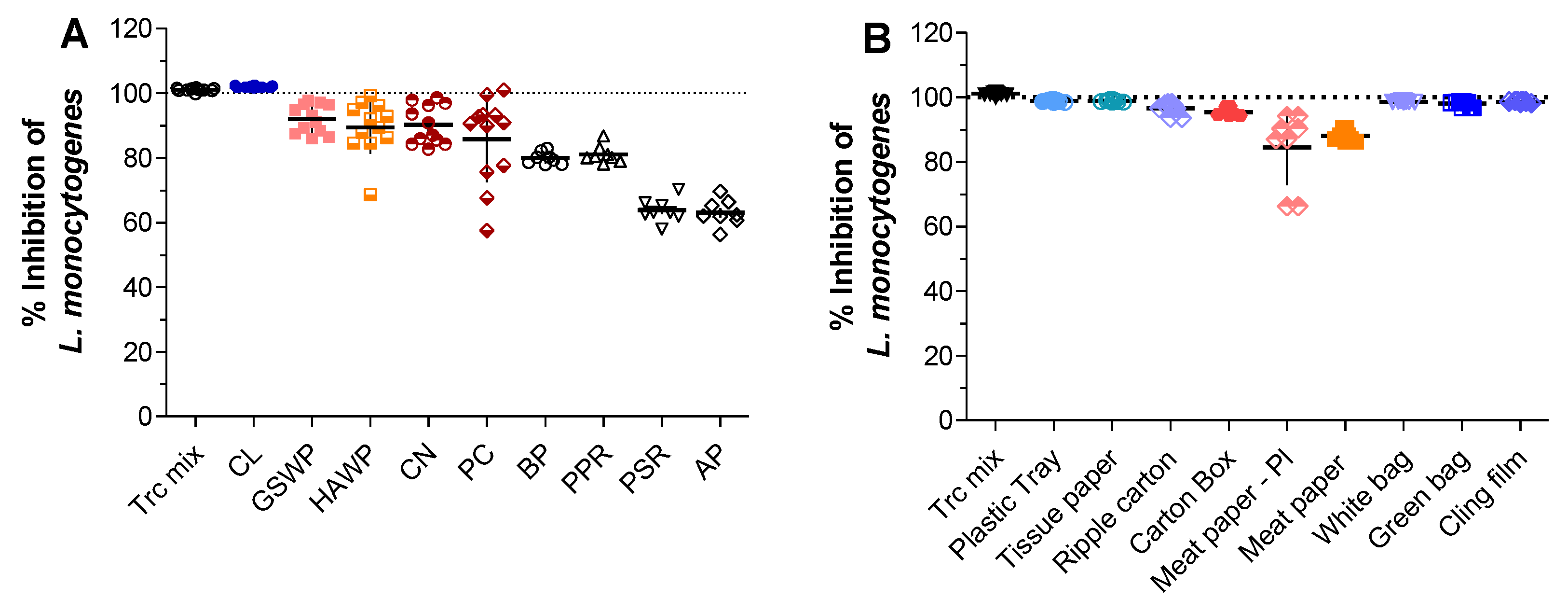
2.1. Activity of Trc-Mix Treated Material against L. monocytogenes
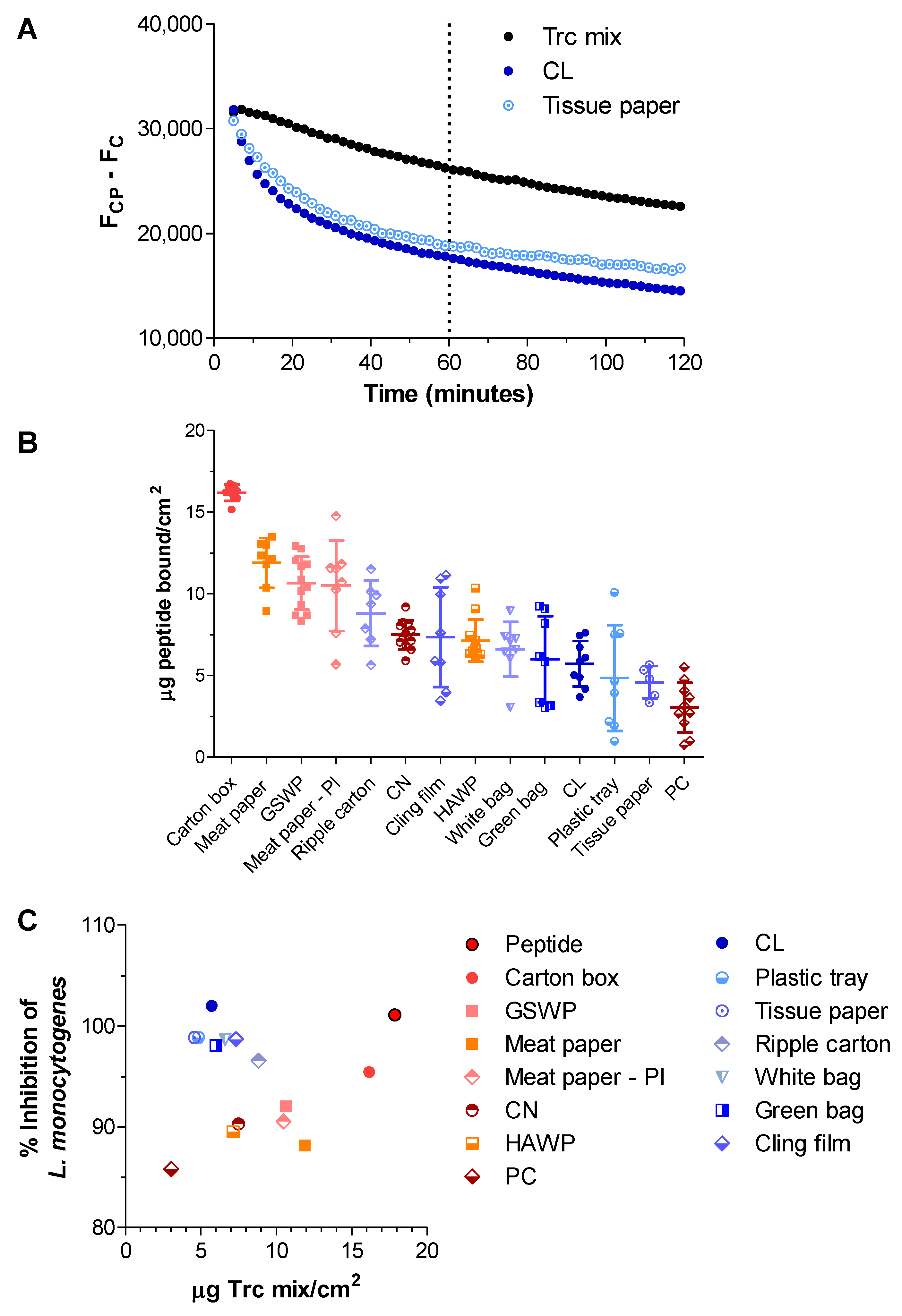
2.2. Determining the Amount of Trcs in Antimicrobial Materials
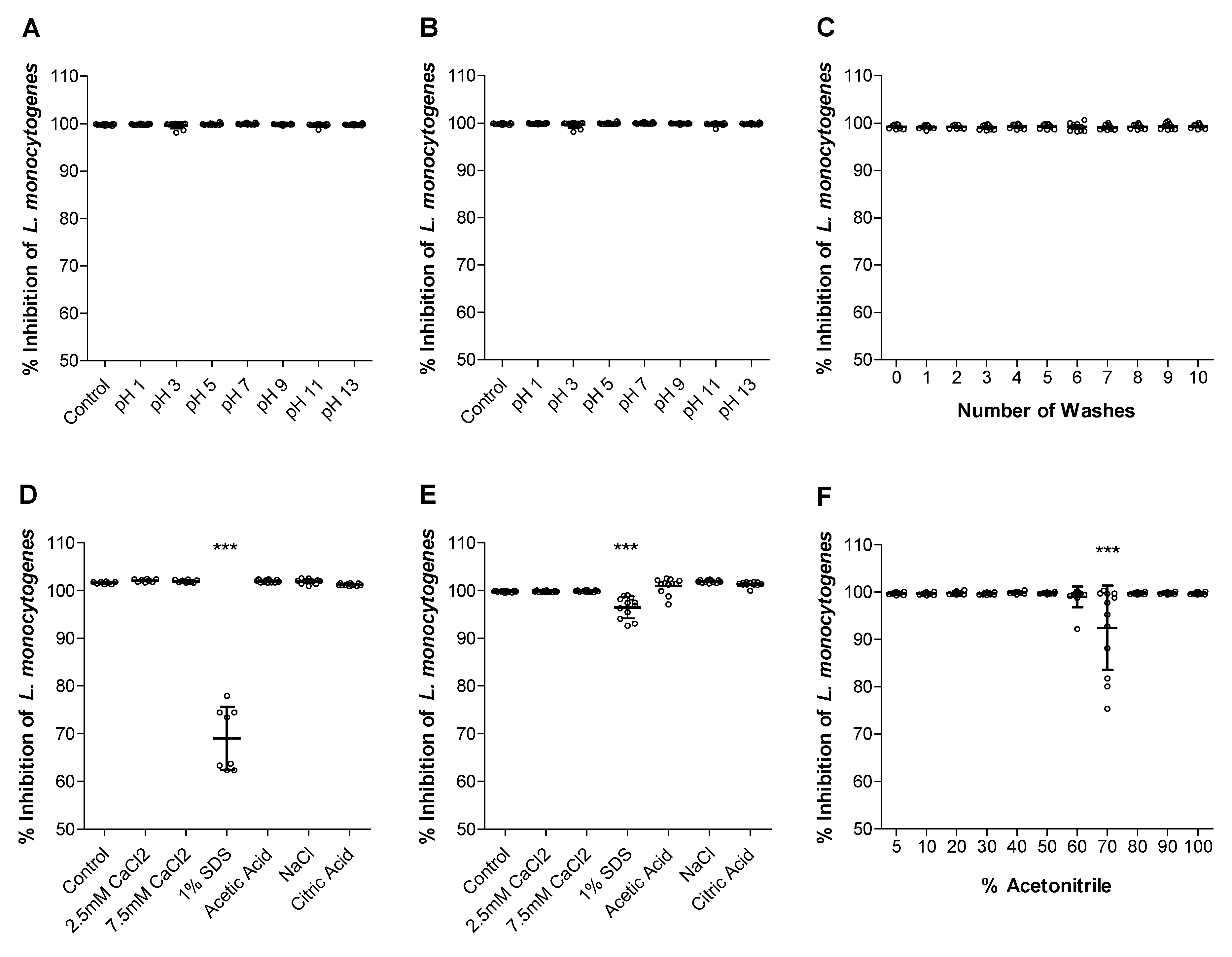
2.3. How Robust Is the Activity of the Trc-Cellulose?
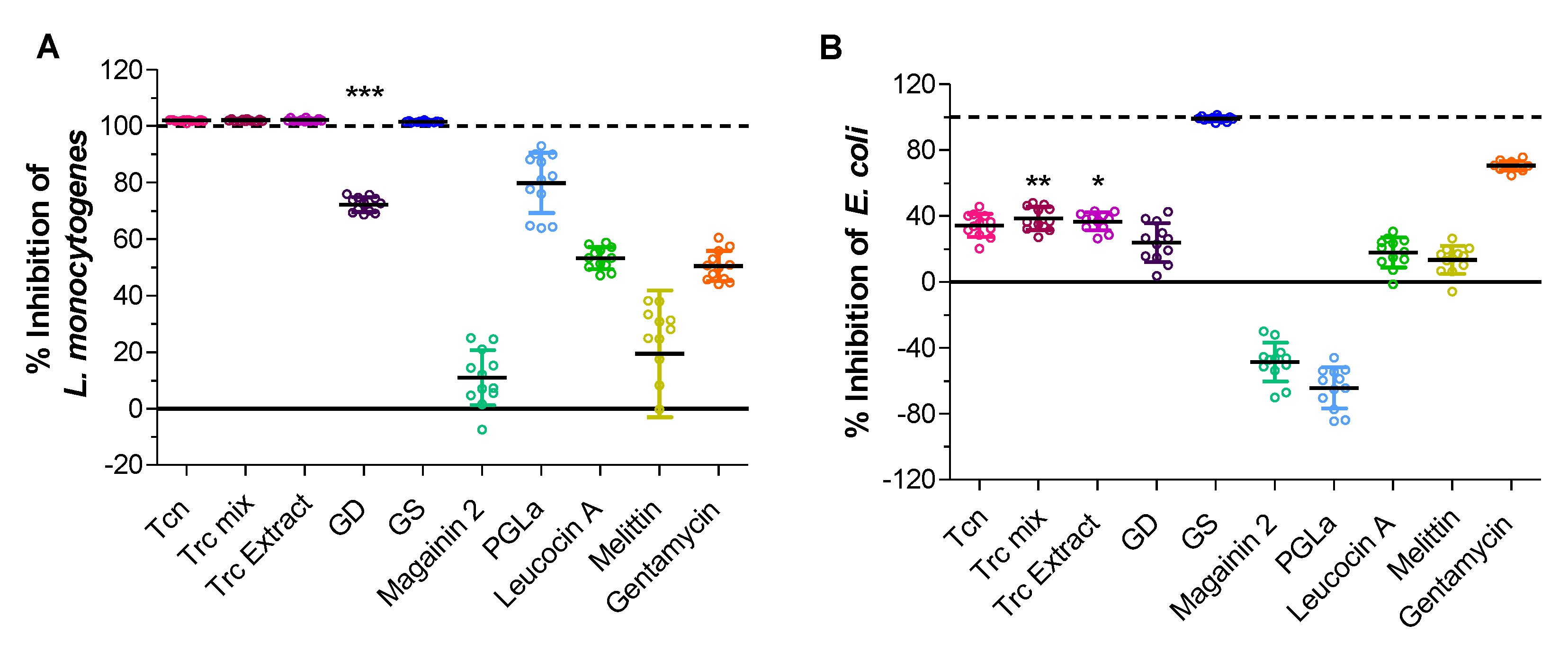
2.4. Role of Type of the Active Compound on Surface Activity—Are Trcs Unique Sticky Peptides?
3. Materials and Methods
3.1. Materials
3.2. Selecting Base Materials
3.3. Creation of Antimicrobial Materials
3.4. Antimicrobial Activity of Trc-Containing Materials
3.5. Determining the Amount of Trcs in Antimicrobial Materials
3.6. Heat Exposure of Trc-Cellulose
3.7. Solvent Exposure of Trc-Cellulose
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gol, N.B.; Patel, P.R.; Rao, T.R. Improvement of Quality and Shelf-Life of Strawberries with Edible Coatings Enriched with Chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. The Effect of Edible Coating Based on Arabic Gum, Sodium Caseinate and Essential Oil of Cinnamon and Lemon Grass on Guava. Food Chem. 2017, 245, 820–828. [Google Scholar] [CrossRef]
- Chhikara, S.; Kumar, D. Edible Coating and Edible Film as Food Packaging Material: A Review. J. Packag. Technol. Res. 2021, 1–10. [Google Scholar] [CrossRef]
- Kumarihami, H.M.P.C.; Kim, Y.-H.; Kwack, Y.-B.; Kim, J.; Kim, J.G. Application of Chitosan as Edible Coating to Enhance Storability and Fruit Quality of Kiwifruit: A Review. Sci. Hortic. 2022, 292, 110647. [Google Scholar] [CrossRef]
- Sung, S.Y.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.C.; Vikhraman, M. Antimicrobial Agents for Food Packaging Applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Opara, U.L.; Fadiji, T. Compression Damage Susceptibility of Apple Fruit Packed inside Ventilated Corrugated Paperboard Package. Sci. Hortic. 2018, 227, 154–161. [Google Scholar] [CrossRef]
- SHAFFE Ranking in Volume of Exports 2018. Available online: https://shaffe.net/ (accessed on 13 January 2020).
- Rautenbach, M.; Vosloo, J.A.; van Rensburg, W.; Engelbrecht, Y. Natural Antimicrobial Peptides as Green Microbicides in Agriculture; Green Economy Research Report, Green Fund; Development Bank of Southern Africa: Midrand, South Africa, 2015. [Google Scholar]
- Haws, K.L.; Winterich, K.P.; Naylor, R.W. Seeing the World through GREEN-Tinted Glasses: Green Consumption Values and Responses to Environmentally Friendly Products. J. Consum. Psychol. 2014, 24, 336–354. [Google Scholar] [CrossRef]
- Kühn, S.; Rebolledo, E.L.B.; van Franeker, J.A. Deleterious Effects of Litter on Marine Life. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 75–116. ISBN 9783319165097. [Google Scholar]
- Gall, S.C.; Thompson, R.C. The Impact of Debris on Marine Life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Agamuthu, P.; Mehran, S.; Norkhairah, A.; Norkhairiyah, A. Marine Debris: A Review of Impacts and Global Initiatives. Waste Manag. Res. 2019, 37, 987–1002. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Novel Formulations for Antimicrobial Peptides. Int. J. Mol. Sci. 2014, 15, 18040–18083. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to The Use of Nisin (E 234) as a Food Additive. EFSA J. 2006, 4, 314. [Google Scholar] [CrossRef]
- US Food and Drug Administration, Nisin Preparation. Affirmation of GRAS Status as Direct Human Food Ingredients. Fed. Regist. 1988, 53, 11247–11251. [Google Scholar]
- Barbosa, A.A.T.; de Araújo, H.G.S.; Matos, P.N.; Carnelossi, M.A.G.; de Castro, A.A. Effects of Nisin-Incorporated Films on the Microbiological and Physicochemical Quality of Minimally Processed Mangoes. Int. J. Food Microbiol. 2013, 164, 135–140. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Gidley, M.J.; Dykes, G.A. Potential of a Nisin-Containing Bacterial Cellulose Film to Inhibit Listeria monocytogenes on Processed Meats. Food Microbiol. 2008, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; El-Fahmy, S.; Revol-Junelles, A.M.; Desobry, S. Cellulose Derivative Based Active Coatings: Effects of Nisin and Plasticizer on Physico-Chemical and Antimicrobial Properties of Hydroxypropyl Methylcellulose Films. Carbohydr. Polym. 2010, 81, 219–225. [Google Scholar] [CrossRef]
- Ercolini, D.; Ferrocino, I.; La Storia, A.; Mauriello, G.; Gigli, S.; Masi, P.; Villani, F. Development of Spoilage Microbiota in Beef Stored in Nisin Activated Packaging. Food Microbiol. 2010, 27, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, G.D.L.E.; De Luca, E.; La Storia, A.; Villani, F.; Ercolini, D. Antimicrobial Activity of a Nisin-activated Plastic Film for Food Packaging. Lett. Appl. Microbiol. 2005, 41, 464–469. [Google Scholar] [CrossRef]
- Wu, H.; Teng, C.; Liu, B.; Tian, H.; Wang, J. Characterization and Long Term Antimicrobial Activity of the Nisin Anchored Cellulose Films. Int. J. Biol. Macromol. 2018, 113, 487–493. [Google Scholar] [CrossRef]
- Gunes, O.C.; Albayrak, A.Z. Antibacterial Polypeptide Nisin Containing Cotton Modified Hydrogel Composite Wound Dressings. Polym. Bull. 2021, 78, 6409–6428. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Zimet, P.; Valadez, R.; Raffaelli, S.; Estevez, M.B.; Pardo, H.; Alborés, S. Biogenic Silver Nanoparticles Conjugated with Nisin: Improving the Antimicrobial and Antibiofilm Properties of Nanomaterials. Chemistry 2021, 3, 1271–1285. [Google Scholar] [CrossRef]
- Cutter, C.N.; Siragusa, G.R. Population Reductions of Gram-Negative Pathogens Following Treatments with Nisin and Chelators under Various Conditions. J. Food Prot. 1995, 58, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Delves-Broughton, J. The Use of EDTA to Enhance the Efficacy of Nisin towards Gram-Negative Bacteria. Int. Biodeterior. Biodegrad. 1993, 32, 87–97. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of Nisin Resistance in Gram-Positive Bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Rydlo, T.; Miltz, J.; Mor, A. Eukaryotic Antimicrobial Peptides: Promises and Premises in Food Safety. J. Food Sci. 2006, 71. [Google Scholar] [CrossRef]
- Oyama, M.; Kubota, K. Suppression of Tyrocidine Production by Purine Nucleotides and Related Substances in Bacillus brevis. FEMS Microbiol. Lett. 1990, 66, 277–279. [Google Scholar] [CrossRef][Green Version]
- Fujikawa, K.; Suzuki, T.; Kurahashi, K. Biosynthesis of Tyrocidine by a Cell-Free Enzyme System of Bacillus brevis ATCC 8185. I. Preparation of Partially Purified Enzyme System and Its Properties. Biochim. Biophys. Acta 1968, 161, 232–246. [Google Scholar] [CrossRef]
- Okuda, K.; Edwards, G.C.; Winnick, T. Biosynthesis of Gramicidin and Tyrocidine in the Dubos Strain of Bacillus brevis. I. Experiments with Growing Cultures. J. Bacteriol. 1963, 85, 329–338. [Google Scholar] [CrossRef]
- Federn, H.; Ristow, H. The GTP Pool in Bacillus brevis and Its Significance for Sporulation. Eur. J. Biochem. / FEBS 1987, 165, 223–227. [Google Scholar] [CrossRef]
- Vosloo, J.A.; Rautenbach, M. Following Tyrothricin Peptide Production by Brevibacillus parabrevis with Electrospray Mass Spectrometry. Biochimie 2020, 179, 101–112. [Google Scholar] [CrossRef]
- Spathelf, B.M.; Rautenbach, M. Anti-Listerial Activity and Structure-Activity Relationships of the Six Major Tyrocidines, Cyclic Decapeptides from Bacillus aneurinolyticus. Bioorg. Med. Chem. 2009, 17, 5541–5548. [Google Scholar] [CrossRef] [PubMed]
- Leussa, A.N.N.; Rautenbach, M. Detailed SAR and PCA of the Tyrocidines and Analogues towards Leucocin A-sensitive and Leucocin A-resistant Listeria monocytogenes. Chem. Biol. Drug Des. 2014, 84, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Dubos, R.J.; Hotchkiss, R.D.; Coburn, A.F. The Effect of Gramicidin and Tyrocidine on Bacterial Metabolism. J. Biol. Chem. 1942, 146, 421–426. [Google Scholar] [CrossRef]
- Troskie, A.M.; Rautenbach, M.; Delattin, N.; Vosloo, J.A.; Dathe, M.; Cammue, B.P.A.; Thevissen, K. Synergistic Activity of the Tyrocidines, Antimicrobial Cyclodecapeptides from Bacillus aneurinolyticus, with Amphotericin B and Caspofungin against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2014, 58, 3697–3707. [Google Scholar] [CrossRef]
- Troskie, A.M.; de Beer, A.; Vosloo, J.A.; Jacobs, K.; Rautenbach, M. Inhibition of Agronomically Relevant Fungal Phytopathogens by Tyrocidines, Cyclic Antimicrobial Peptides Isolated from Bacillus aneurinolyticus. Microbiology 2014, 160, 2089–2101. [Google Scholar] [CrossRef]
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A.; Dathe, M.E. Antifungal Membranolytic Activity of the Tyrocidines against Filamentous Plant Fungi. Biochimie 2016, 130, 122–131. [Google Scholar] [CrossRef]
- Chopra, S.; Torres-Ortiz, M.; Hokama, L.; Madrid, P.; Tanga, M.; Mortelmans, K.; Kodukula, K.; Galande, A.K. Repurposing FDA-Approved Drugs to Combat Drug-Resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 2598–2601. [Google Scholar] [CrossRef]
- Dubos, R.J.; Hotchkiss, R.D. The Production of Bactericidal Substances by Aerobic Sporulating Bacilli. J. Exp. Med. 1941, 73, 629–640. [Google Scholar] [CrossRef]
- Lang, C.; Staiger, C. Tyrothricin—An Underrated Agent for the Treatment of Bacterial Skin Infections and Superficial Wounds? Pharm. Int. J. Pharm. Sci. 2016, 71, 299–305. [Google Scholar]
- Mach, B.; Slayman, C.W. Mode of Action of Tyrocidine on Neupospora. Biochim. Biophys. Acta 1966, 124, 351–361. [Google Scholar] [CrossRef]
- Dubos, R.J. Studies on a Bactericidal Agent Extracted from a Soil Bacillus. I. Preparation of the Agent. Its Activity in Vitro. J. Exp. Med. 1939, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Fynn, G.H. Energetics of Growth in a Tyrothricin-Producing Strain of Bacillus brevis. J. Gen. Microbiol. 1972, 74, 305–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wenzel, M.; Rautenbach, M.; Vosloo, J.A.; Siersma, T.; Aisenbrey, C.H.; Zaitseva, E.; Laubscher, W.E.; van Rensburg, W.; Behrends, J.C. The Multifaceted Antibacterial Mechanisms of the Pioneering Peptide Antibiotics Tyrocidine and Gramicidin S. Am. Soc. Microbiol. 2018, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Stauss-Grabo, M.; Atiye, S.; Le, T.; Kretschmar, M. Decade-Long Use of the Antimicrobial Peptide Combination Tyrothricin Does Not Pose a Major Risk of Acquired Resistance with Gram-Positive Bacteria and Candida spp. Pharm. Int. J. Pharm. Sci. 2014, 69, 838–841. [Google Scholar]
- Essack, S.; Bell, J.; Burgoyne, D.S.; Duerden, M.; Shephard, A. Topical (Local) Antibiotics for Respiratory Infections with Sore Throat: An Antibiotic Stewardship Perspective. J. Clin. Pharm. Ther. 2019, 44, 829–837. [Google Scholar] [CrossRef]
- Palm, J.; Fuchs, K.; Stammer, H.; Schumacher-Stimpfl, A.; Milde, J. Efficacy and Safety of a Triple Active Sore Throat Lozenge in the Treatment of Patients with Acute Pharyngitis: Results of a Multi-Centre, Randomised, Placebo-Controlled, Double-Blind, Parallel-Group Trial (DoriPha). Int. J. Clin. Pract. 2018, 72, e13272. [Google Scholar] [CrossRef]
- Rammelkamp, C.H.; Weinstein, L. Toxic Effects of Tyrothricin, Gramicidin and Tyrocidine. J. Infect. Dis. 1942, 71, 166–173. [Google Scholar] [CrossRef]
- Van Epps, H.L. René Dubos: Unearthing Antibiotics. J. Exp. Med. 2006, 203, 259. [Google Scholar] [CrossRef]
- Wigger-Alberti, W.; Stauss-Grabo, M.; Grigo, K.; Atiye, S.; Williams, R.; Korting, H.C. Efficacy of a Tyrothricin-Containing Wound Gel in an Abrasive Wound Model for Superficial Wounds. Ski. Pharmacol. Physiol. 2013, 26, 52–56. [Google Scholar] [CrossRef]
- Juhl, D.W.; Rensburg, W.; Bossis, X.; Vosloo, J.A.; Rautenbach, M.; Bechinger, B. Tyrocidine A Interactions with Saccharides Investigated by CD and NMR Spectroscopies. J. Pept. Sci. 2019, 25, e3163. [Google Scholar] [CrossRef]
- Masoudi, Y.; van Rensburg, W.; Barnard-Jenkins, B.; Rautenbach, M. The Influence of Cellulose-Type Formulants on Anti-Candida Activity of the Tyrocidines. Antibiotics 2021, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, D. The Chemical Nature of Gramicidin and Tyrocidine. J. Biol. Chem. 1941, 141, 171–185. [Google Scholar] [CrossRef]
- Bagheri, M.; Keller, S.; Dathe, M. Interaction of W-Substituted Analogs of Cyclo-RRRWFW with Bacterial Lipopolysaccharides: The Role of the Aromatic Cluster in Antimicrobial Activity. Antimicrob. Agents Chemother. 2011, 55, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Yphantis, D.A.; Craig, L.C. Noncovalent Association of Tyrocidine B. Biochemistry 1972, 11, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Munyuki, G.; Jackson, G.E.; Venter, G.A.; Kövér, K.E.; Szilágyi, L.; Rautenbach, M.; Spathelf, B.M.; Bhattacharya, B.; van der Spoel, D. β-Sheet Structures and Dimer Models of the Two Major Tyrocidines, Antimicrobial Peptides from Bacillus aneurinolyticus. Biochemistry 2013, 52, 7798–7806. [Google Scholar] [CrossRef] [PubMed]
- Rautenbach, M.; Kumar, V.; Vosloo, J.A.; Masoudi, Y.; van Wyk, R.J.; Stander, M.A. Oligomerisation of Tryptocidine C, a Trp-Rich Cyclodecapeptide from the Antimicrobial Tyrothricin Complex. Biochimie 2020, 181, 123–133. [Google Scholar] [CrossRef]
- Rautenbach, M.; van Rensburg, W. Method for Preventing or Treating Microbial Growth on a Manufactured Product. U.S. Patent 10,512,271, 24 December 2017. [Google Scholar]
- van Rensburg, W. Characterization of Natural Antimicrobial Peptides Adsorbed to Different Matrices. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. Available online: https://scholar.sun.ac.za/handle/10019.1/97929 (accessed on 13 January 2020).
- Cuq, J.C.; Vié, M.; Cheftel, J.C. Tryptophan Degradation during Heat Treatments: Part 2—Degradation of Protein-Bound Tryptophan. Food Chem. 1983, 12, 73–88. [Google Scholar] [CrossRef]
- Cuq, J.C.; Cheftel, J.C. Tryptophan Degradation during Heat Treatments: Part 1—The Degradation of Free Tryptophan. Food Chem. 1983, 12, 1–14. [Google Scholar] [CrossRef]
- Mello, G.D.S.; Cardoso, A.D.P.; Oliveira, E.W.; Siqueira, A.B. Tryptophan: A Proposal of the Mechanism of Thermal Decomposition. J. Therm. Anal. Calorim. 2015, 122, 1395–1401. [Google Scholar] [CrossRef]
- Patron, L.; Marinescu, G.; Culita, D.; Diamandescu, L.; Carp, O. Thermal Stability of Amino Acid-(Tyrosine and Tryptophan) Coated Magnetites. J. Therm. Anal. Calorim. 2008, 91, 627–632. [Google Scholar] [CrossRef]
- Azuma, T.; Demain, A.L. Interactions between Gramicidin S and Its Producer, Bacillus brevis. J. Ind. Microbiol. Biotechnol. 1996, 17, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Pschorn, W.; Paulus, H.; Hansen, J.; Ristow, H. Induction of Sporulation in Bacillus brevis. 2. Dependence on the Presence of the Peptide Antibiotics Tyrocidine and Linear Gramicidin. Eur. J. Biochem. FEBS 1982, 129, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Spathelf, B.M. Qualitative Structure-Activity Relationships of the Major Tyrocidines, Cyclic Decapeptides from Bacillus aneurinolyticus. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2010. Available online: https://scholar.sun.ac.za/handle/10019.1/4001 (accessed on 13 January 2020).
- Leussa, N.-N.A. Characterisation of Small Cyclic Peptides with Antilisterial and Antimalarial Activity. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2014. Available online: https://scholar.sun.ac.za/handle/10019.1/86161 (accessed on 13 January 2020).
- Troskie, A.M. Tyrocidines, Cyclic Decapeptides Produced by Soil Bacilli, as Potent Inhibitors of Fungal Pathogens. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2013. Available online: https://scholar.sun.ac.za/handle/10019.1/86162 (accessed on 13 January 2020).
- Loll, P.J.; Upton, E.C.; Nahoum, V.; Economou, N.J.; Cocklin, S. The High Resolution Structure of Tyrocidine A Reveals an Amphipathic Dimer. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1199–1207. [Google Scholar] [CrossRef]
- Paradies, H.H. Aggregation of Tyrocidine in Aqueous Solutions. Biochem. Biophys. Res. Commun. 1979, 88, 810–817. [Google Scholar] [CrossRef]
- Thies, M.; Paradies, H.H. Self-Assembly of Tyrocidines in Nanotubular Structures. MRS Online Proc. Libr. Arch. 1997, 489, 145–151. [Google Scholar] [CrossRef]
- Ruttenberg, M.A.; King, T.; Craig, L.C. The Use of the Tyrocidines for the Study of Conformation and Aggregation Behavior. J. Am. Chem. Soc. 1965, 87, 4196–4198. [Google Scholar] [CrossRef]
- Paradies, H.H. Structure of Tyrocidine Micelles in Isotropic Aqueous Solution. J. Pharm. Sci. 1989, 78, 230–234. [Google Scholar] [CrossRef]
- Paradies, H.H.; Reichelt, H. Formation and Structures of Tyrocidine B Oligomers in Solution in the Presence of Water. AIP Adv. 2020, 10, 075007. [Google Scholar] [CrossRef]
- Stern, A.; Gibbons, W.A.; Craig, L.C. Effect of Assosiation on the Nuclear Magnetic Resonance Spectra of Tyrocidine B. J. Am. Chem. Soc. 1969, 91, 2794–2796. [Google Scholar] [CrossRef]
- Csonka, L.N. Physiological and Genetic Responses of Bacteria to Osmotic Stress. Microbiol. Mol. Biol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef]
- Kanda, M.; Hori, K.; Kurotsu, T.; Miura, S.; Nozoe, A.; Saito, Y. Studies on Gramicidin S Synthetase. J. Biochem. 1978, 84, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Gevers, W.; Kleinkauf, H.; Lipmann, F. The Activation of Amino Acids for Biosynthesis of Gramicidin S. Proc. Natl. Acad. Sci. USA 1968, 60, 269. [Google Scholar] [CrossRef] [PubMed]
- Kleinkauf, H.; Gevers, W.; Lipmann, F. Interrelation between Activation and Polymerization in Gramicidin S Biosynthesis. Proc. Natl. Acad. Sci. USA 1968, 62, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zuo, R.; Wood, T.K. Inhibiting Mild Steel Corrosion from Sulfate-Reducing and Iron-Oxidizing Bacteria Using Gramicidin-S-Producing Biofilms. Appl. Microbiol. Biotechnol. 2004, 65, 747–753. [Google Scholar] [CrossRef]
- De Melo Carrasco, L.D.; Bertolucci, R.; Ribeiro, R.T.; Sampaio, J.L.M.; Carmona-Ribeiro, A.M. Cationic Nanostructures against Foodborne Pathogens. Front. Microbiol. 2016, 7, 1804. [Google Scholar] [CrossRef]
- Kondejewski, L.H.; Farmer, S.W.; Wishart, D.S.; Hancock, R.E.; Hodges, R.S. Gramicidin S Is Active against Both Gram-Positive and Gram-Negative Bacteria. Int. J. Pept. Protein Res. 1996, 47, 460–466. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a Class of Antimiorobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial CDNA Sequence of a Precursor. J. Occup. Environ. Med. 1988, 30, 470. [Google Scholar] [CrossRef]
- Soravia, E.; Martini, G.; Zasloff, M. Antimicrobial Properties of Peptides from Xenopus Granular Gland Secretions. FEBS Lett. 1988, 228, 337–340. [Google Scholar] [CrossRef]
- Gravesen, A.; Ramnath, M.; Rechinger, K.B.; Andersen, N.; Jänsch, L.; Héchard, Y.; Hastings, J.W.; Knøchel, S. High-Level Resistance to Class IIa Bacteriocins Is Associated with One General Mechanism in Listeria monocytogenes. Microbiology 2002, 148, 2361–2369. [Google Scholar] [CrossRef]
- Wu, X.; Singh, A.K.; Wu, X.; Lyu, Y.; Bhunia, A.K.; Narsimhan, G. Characterization of Antimicrobial Activity against Listeria and Cytotoxicity of Native Melittin and Its Mutant Variants. Colloids Surf. B Biointerfaces 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, H.; Weisshaar, J.C. Melittin-Induced Permeabilization, Re-Sealing, and Re-Permeabilization of E. Coli Membranes. Biophys. J. 2018, 114, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Picoli, T.; Peter, C.M.; Zani, J.L.; Waller, S.B.; Lopes, M.G.; Boesche, K.N.; Vargas, G.D.; de Oliveira Hübner, S.; Fischer, G. Melittin and Its Potential in the Destruction and Inhibition of the Biofilm Formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa Isolated from Bovine Milk. Microb. Pathog. 2017, 112, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Rakic-Martinez, M.; Drevets, D.A.; Dutta, V.; Katic, V.; Kathariou, S. Listeria monocytogenes Strains Selected on Ciprofloxacin or the Disinfectant Benzalkonium Chloride Exhibit Reduced Susceptibility to Ciprofloxacin, Gentamicin, Benzalkonium Chloride, and Other Toxic Compounds. Appl. Environ. Microbiol. 2011, 77, 8714–8721. [Google Scholar] [CrossRef] [PubMed]
- Adwan, K.; Abu-Hasan, N. Gentamicin Resistance in Clinical Strains of Enterobacteriaceae Associated with Reduced Gentamicin Uptake. Folia Microbiol. 1998, 43, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.C.; Medoff, G.; Leech, I.; Wennersten, C.; Kunz, L.J. Antibiotic Synergism against Listeria monocytogenes. Antimicrob. Agents Chemother. 1972, 1, 30–34. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, W.; Laubscher, W.E.; Rautenbach, M. High Throughput Method to Determine the Surface Activity of Antimicrobial Polymeric Materials. MethodsX 2021, 8, 101593. [Google Scholar] [CrossRef]
- van de Lagemaat, M.; Grotenhuis, A.; van de Belt-Gritter, B.; Roest, S.; Loontjens, T.J.A.; Busscher, H.J.; van der Mei, H.C.; Ren, Y. Comparison of Methods to Evaluate Bacterial Contact-Killing Materials. Acta Biomater. 2017, 59, 139–147. [Google Scholar] [CrossRef]
- Vosloo, J.A.; Stander, M.A.; Leussa, A.N.N.; Spathelf, B.M.; Rautenbach, M. Manipulation of the Tyrothricin Production Profile of Bacillus aneurinolyticus. Microbiology 2013, 159, 2200–2211. [Google Scholar] [CrossRef]
- Tang, X.J.; Thibault, P.; Boyd, R.K. Characterisation of the Tyrocidine and Gramicidin Fractions of the Tyrothricin Complex from Bacillus brevis Using Liquid Chromatography and Mass Spectrometry. Int. J. Mass Spectrom. Ion Process. 1992, 122, 153–179. [Google Scholar] [CrossRef]
- Ruttenberg, M.A.; Mach, B. Studies on Amino Acid Substitution in the Biosynthesis of the Antibiotic Polypeptide Tyrocidine. Biochemistry 1966, 5, 2864–2869. [Google Scholar] [CrossRef]
- Fujikawa, K.; Sakamoto, Y.; Suzuki, T.; Kurahashi, K. Biosynthesis of Tyrocidine by a Cell-Free Enzyme System of Bacillus brevis ATCC 8185. II. Amino Acid Substitution in Tyrocidine. Biochim. Biophys. Acta 1968, 169, 520–533. [Google Scholar] [CrossRef]
- Mach, B.; Tatum, E.L. Environmental Control of Amino Acid Substitutions in the Biosynthesis of the Antibiotic Polypeptide Tyrocidine. Proc. Natl. Acad. Sci. USA 1964, 52, 876–884. [Google Scholar] [CrossRef] [PubMed]

| Material Name | Application | Chemical Composition | General Chemical Character |
|---|---|---|---|
| GSWP | Solvent filtration | Mixed cellulose esters | Hydrophilic, porous |
| HAWP | Hydrophilic, porous | ||
| PC | General sterile filtration | Polycarbonate | Hydrophobic, porous |
| CN | Cellulose nitrate | Hydrophilic, porous | |
| CL | Cellulose | Hydrophilic, porous | |
| BP | Fluorescence assays | Polystyrene | Hard plastic |
| AP | Micro-dilution assays | Polystyrene | Hard plastic |
| PPR | Micro-dilution preparation plate | Polypropylene | Hard plastic |
| PSR | Polystyrene | Hard plastic | |
| Plastic tray | Fruit packaging | Unknown | Hydrophobic, Hard plastic |
| Tissue paper | Fruit wrapping | Cellulose | Hydrophilic, layered cellulose |
| Ripple carton | Fruit packaging | Cellulose | Hydrophilic, layered cellulose |
| Carton box | Fruit shipping/transport | Cellulose | Hydrophilic, multiple layered cellulose |
| Meat paper- PL | Meat wrapping | Cellulose & polylactate | Hydrophobic |
| Meat paper | Meat wrapping | Cellulose | Hydrophilic |
| White bag | Waste bag—home use | Polylactate | Hydrophobic |
| Green bag | Waste bag—industrial use | Polylactate | Hydrophobic |
| Cling film | Food wrapping | Polylactate | Hydrophobic |
| Compound | Origin | Character | Minimum Inhibitory Concentration (μg/mL) | |
|---|---|---|---|---|
| L. monocytogenes | E. coli | |||
| Tcn | Peptide complex produced by Brevibacillus parabrevis | Mixture of cationic cyclo-decapeptides and neutral pentadecapeptides, soluble in ≥50% v/v acetonitrile/organic solvents | 21 ± 0.10 [34] | Not available |
| Trc mix | Purified from Tcn | Cationic, cyclic decapeptide complex, soluble in ≥50% v/v acetonitrile/organic solvents | 23 ± 0.63 [34] | >100 [34] |
| GD | Purified from Tcn | Neutral linear pentadecapeptide complex, haemolytic, soluble in organic solvent | 19 [83] | 9 [83] |
| GS | Aneurinibacillus migulanus | Cationic, amphipathic cyclo- decapeptide, 50% identity to tyrocidine A, haemolytic, water-soluble | 11 ± 0.20 [34] | 3–12.5 [84] |
| Magainin 2 | Skin of Xenopus laevis | Linear 23-mer cationic, amphipathic α-helical peptide, water soluble, non-haemolytic, water-soluble | Not available | 10–50 [85] |
| PGLa | Skin of Xenopus laevis | Linear 21-mer cationic, amphipathic α-helical peptide amide, haemolytic, water-soluble | Not available | 10–50 [86] |
| Leucocin A | Leuconostoc gelidum UAL187 | 37-mer cationic, amphipathic disulphide-bonded bacteriocin, non-haemolytic, water-soluble | 0.98–1.98 [87] | Not available |
| Melittin | Venom of European honey-bee (Apis mellifera) | Linear 26-mer cationic, amphipathic α -helical peptide, haemolytic, water-soluble | 0.315 ± 0.008 [88] | 15–42.5 [89,90] |
| Gentamicin | Micromonospora purpurea | Water-soluble aminoglycoside antibiotic | 0.5–4.0 [91,92] | 0.156–1.25 [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Rensburg, W.; Rautenbach, M. Creating Robust Antimicrobial Materials with Sticky Tyrocidines. Antibiotics 2022, 11, 174. https://doi.org/10.3390/antibiotics11020174
van Rensburg W, Rautenbach M. Creating Robust Antimicrobial Materials with Sticky Tyrocidines. Antibiotics. 2022; 11(2):174. https://doi.org/10.3390/antibiotics11020174
Chicago/Turabian Stylevan Rensburg, Wilma, and Marina Rautenbach. 2022. "Creating Robust Antimicrobial Materials with Sticky Tyrocidines" Antibiotics 11, no. 2: 174. https://doi.org/10.3390/antibiotics11020174
APA Stylevan Rensburg, W., & Rautenbach, M. (2022). Creating Robust Antimicrobial Materials with Sticky Tyrocidines. Antibiotics, 11(2), 174. https://doi.org/10.3390/antibiotics11020174







