Association of blaVIM-2, blaPDC-35, blaOXA-10, blaOXA-488 and blaVEB-9 β-Lactamase Genes with Resistance to Ceftazidime–Avibactam and Ceftolozane–Tazobactam in Multidrug-Resistant Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Methods
2.1. Bacterial Identification and Antimicrobial Susceptibility Testing
2.2. Genomic and Phylogenetic Analyses
2.3. Statistical Analysis
3. Results
3.1. Antimicrobial Susceptibility, Clinical Source, and Distribution of MDR-P. aeruginosa Isolates
3.2. The Frequency of β-Lactamases and Sequence Types among MDR-P. aeruginosa
3.3. The Association between the β-Lactamase Genes and the Minimum Inhibitory Concentration of Ceftazidime–Avibactam and Ceftolozane–Tazobactam
3.4. Correlation of Specific β-Lactamase Genes to Ceftazidime–Avibactam and Ceftolozane–Tazobactam Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [Green Version]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallan, A.; Limbago, B.; Fridkin, S. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Hawkey, P.M.; Warren, R.E.; Livermore, D.M.; McNulty, C.A.; Enoch, D.A.; Otter, J.A.; Wilson, A.P.R. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: Report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J. Antimicrob. Chemother. 2018, 73, iii2–iii78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, E.B.; Tam, V.H. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert. Rev. Pharmacoecon. Outcomes Res. 2010, 10, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, Cd003543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liscio, J.L.; Mahoney, M.V.; Hirsch, E.B. Ceftolozane/tazobactam and ceftazidime/avibactam: Two novel β-lactam/β-lactamase inhibitor combination agents for the treatment of resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents 2015, 46, 266–271. [Google Scholar] [CrossRef]
- Ehmann, D.E.; Jahic, H.; Ross, P.L.; Gu, R.-F.; Hu, J.; Durand-Réville, T.F.; Lahiri, S.; Thresher, J.; Livchak, S.; Gao, N.; et al. Kinetics of avibactam inhibition against Class A, C, and D β-lactamases. J. Biol. Chem. 2013, 288, 27960–27971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, M.C.; Hsu, D.I.; Bounthavong, M. Ceftolozane/tazobactam: A novel antipseudomonal cephalosporin and β-lactamase-inhibitor combination. Infect. Drug Resist. 2013, 6, 215–223. [Google Scholar]
- Farrell, D.J.; Flamm, R.K.; Sader, H.S.; Jones, R.N. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. Hospitals (2011–2012). Antimicrob. Agents Chemother. 2013, 57, 6305–6310. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.L.; Papp-Wallace, K.M.; Hujer, A.M.; Domitrovic, T.N.; Hujer, K.M.; Hurless, K.N.; Tuohy, M.; Hall, G.; Bonomo, R.A. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: Resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 1020–1029. [Google Scholar] [CrossRef] [Green Version]
- Fraile-Ribot, P.A.; Cabot, G.; Mulet, X.; Periañez, L.; Martín-Pena, M.L.; Juan, C.; Pérez, J.L.; Oliver, A. Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2018, 73, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Sid Ahmed, M.A.; Abdel Hadi, H.; Hassan, A.A.I.; Jarir, S.A.; Al-Maslamani, M.A.; Eltai, N.O.; Dousa, K.M.; Hujer, A.M.; Sultan, A.A.; Soderquist, B.; et al. Evaluation of in vitro activity of ceftazidime/avibactam and ceftolozane/tazobactam against MDR Pseudomonas aeruginosa isolates from Qatar. J. Antimicrob. Chemother. 2019, 74, 3497–3504. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falgas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI). Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar]
- Wayne, P.A. Performance Standards for Antimicrobial Susceptibility Testing; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prijbelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Ponten, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Abdouchakour, F.; Aujoulat, F.; Licznar-Fajardo, P.; Marchandin, H.; Toubiana, M.; Parer, S.; Lotthé, A.; Jumas-Bilak, E. Intraclonal variations of resistance and phenotype in Pseudomonas aeruginosa epidemic high-risk clone ST308: A key to success within a hospital? Int. J. Med. Microbiol. 2018, 308, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi-Akiyama, T.; Tada, T.; Ohmagari, N.; Hung, N.V.; Tharavichitkul, P.; Pokhrel, B.M.; Gniadkowski, M.; Shimojima, M.; Kirikae, T. Emergence and Spread of Epidemic Multidrug-Resistant Pseudomonas aeruginosa. Genome Biol. Evol. 2017, 9, 3238–3245. [Google Scholar] [CrossRef]
- Perez, F.; Hujer, A.M.; Marshall, S.H.; Ray, A.J.; Rather, P.N.; Suwantarat, N.; Dumford, D., III; O’Shea, P.; Domitrovic, T.N.J.; Salata, R.A.; et al. Extensively drug-resistant Pseudomonas aeruginosa isolates containing blaVIM-2 and elements of Salmonella genomic island 2: A new genetic resistance determinant in Northeast Ohio. Antimicrob. Agents Chemother. 2014, 58, 5929–5935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialvaei, Z.A.; Kafil, S.H.; Leylabadlo, E.H.; Asgharzadeh, M.; Aghazadeh, M. Dissemination of carbapenemases producing Gram negative bacteria in the Middle East. Iran. J. Microbiol. 2015, 7, 226–246. [Google Scholar]
- Zowawi, H.M.; Balkhy, H.H.; Walsh, T.R.; Paterson, D.L. β-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin. Microbiol. Rev. 2013, 26, 361–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotondo, C.M.; Wright, G.D. Inhibitors of metallo-beta-lactamases. Curr. Opin. Microbiol. 2017, 39, 96–105. [Google Scholar] [CrossRef]
- Ortiz de la Rosa, J.-M.; Nordmann, P.; Poirel, L. ESBLs and resistance to ceftazidime/avibactam and ceftolozane/tazobactam combinations in Escherichia coli and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2019, 74, 1934–1939. [Google Scholar] [CrossRef]
- Aubert, D.; Girlich, D.; Naas, T.; Nagarajan, S.; Nordmann, P. Functional and structural characterization of the genetic environment of an extended-spectrum beta-lactamase blaVEB gene from a Pseudomonas aeruginosa isolate obtained in India. Antimicrob. Agents Chemother. 2004, 48, 3284–3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laudy, A.E.; Róg, P.; Smolińska-Król, K.; Ćmiel, M.; Słoczyńska, A.; Patzer, J.; Dzierżanowska, D.; Wolinowska, R.; Starościak, B.; Tyski, S. Prevalence of ESBL-producing Pseudomonas aeruginosa isolates in Warsaw, Poland, detected by various phenotypic and genotypic methods. PLoS ONE 2017, 12, e0180121. [Google Scholar] [CrossRef] [Green Version]
- Al-Agamy, M.H.; Jeannot, K.; El-Mahdy, T.S.; Samaha, H.A.; Shibl, A.M.; Plésiat, P.; Courvalin, P. Diversity of Molecular Mechanisms Conferring Carbapenem Resistance to Pseudomonas aeruginosa Isolates from Saudi Arabia. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 4379686. [Google Scholar] [CrossRef] [Green Version]
- Barnes, M.D.; Taracila, M.A.; Rutter, J.D.; Bethel, C.R.; Galdadas, I.; Hujer, A.M.; Caselli, E.; Prati, F.; Dekker, J.P.; Papp-Wallace, K.M.; et al. Deciphering the Evolution of Cephalosporin Resistance to Ceftolozane-Tazobactam in Pseudomonas aeruginosa. mBio 2018, 9, e02085-18. [Google Scholar] [CrossRef] [Green Version]
- Papp-Wallace, K.M.; Zeiser, E.T.; Becka, S.A.; Park, S.; Wilson, B.M.; Winkler, M.L.; D’Souza, R.; Singh, I.; Sutton, G.; Fouts, E.D.; et al. Ceftazidime-Avibactam in Combination With Fosfomycin: A Novel Therapeutic Strategy Against Multidrug-Resistant Pseudomonas aeruginosa. J. Infect. Dis. 2019, 220, 666–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, J.Q.-M.; Lim, J.C.; Tang, C.Y.; Tan, S.H.; Sim, J.H.-C.; Ong, R.T.-H.; Kwa, A.L.-H. Ceftolozane/Tazobactam Resistance and Mechanisms in Carbapenem-Nonsusceptible Pseudomonas aeruginosa. mSphere 2021, 6, e01026-20. [Google Scholar] [CrossRef]
- So, W.; Shurko, J.; Galega, R.; Quilitz, R.; Greene, J.N.; Lee, G.C. Mechanisms of high-level ceftolozane/tazobactam resistance in Pseudomonas aeruginosa from a severely neutropenic patient and treatment success from synergy with tobramycin. J. Antimicrob. Chemother. 2019, 74, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Danel, F.; Hall, L.M.; Duke, B.; Gur, D.; Livermore, D.M. OXA-17, a further extended-spectrum variant of OXA-10 beta-lactamase, isolated from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1999, 43, 1362–1366. [Google Scholar] [CrossRef] [Green Version]
- Arca-Suárez, J.; Lasarte-Monterrubio, C.; Rodiño-Janeiro, B.-K.; Cabot, G.; Vázquez-Ucha, J.C.; Rodríguez-Iglesias, M.; Galán-Sánchez, F.; Beceiro, A.; González-Bello, C.; Oliver, A.; et al. Molecular mechanisms driving the in vivo development of OXA-10-mediated resistance to ceftolozane/tazobactam and ceftazidime/avibactam during treatment of XDR Pseudomonas aeruginosa infections. J. Antimicrob. Chemother. 2020, 76, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, F.; Bletz, S.; Mellmann, A.; Becker, K.; Idelevich, E.A. Comparison of methods to analyse susceptibility of German MDR/XDR Pseudomonas aeruginosa to ceftazidime/avibactam. Int. J. Antimicrob. Agents 2019, 54, 255–260. [Google Scholar] [CrossRef]
- Fraile-Ribot, P.A.; Mulet, X.; Cabot, G.; del Barrio-Tofiño, E.; Juan, C.; Pérez, J.L.; Oliver, A. In Vivo Emergence of Resistance to Novel Cephalosporin-β-Lactamase Inhibitor Combinations through the Duplication of Amino Acid D149 from OXA-2 β-Lactamase (OXA-539) in Sequence Type 235 Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01117-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagès, J.M.; Peslier, S.; Keating, T.A.; Lavigne, J.P.; Nichols, W.W. Role of the Outer Membrane and Porins in Susceptibility of β-Lactamase-Producing Enterobacteriaceae to Ceftazidime-Avibactam. Antimicrob. Agents Chemother. 2015, 60, 1349–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, R.M.; Hindler, J.A.; Magnano, P.; Wong-Beringer, A.; Tibbetts, R.; Miller, S.A. Performance of Ceftolozane-Tazobactam Etest, MIC Test Strips, and Disk Diffusion Compared to Reference Broth Microdilution for β-Lactam-Resistant Pseudomonas aeruginosa Isolates. J. Clin. Microbiol. 2018, 56, e01633-17. [Google Scholar] [CrossRef] [Green Version]
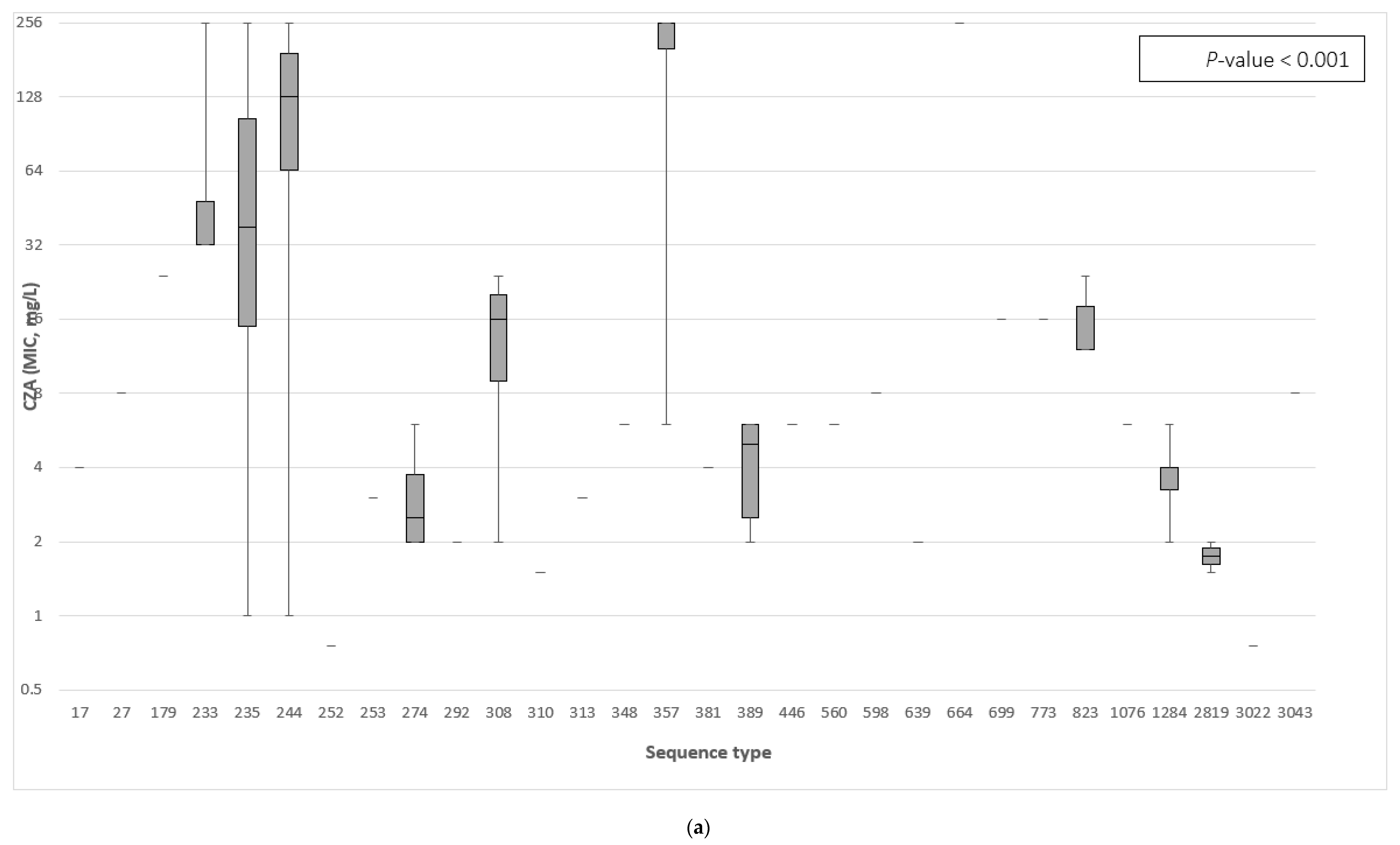
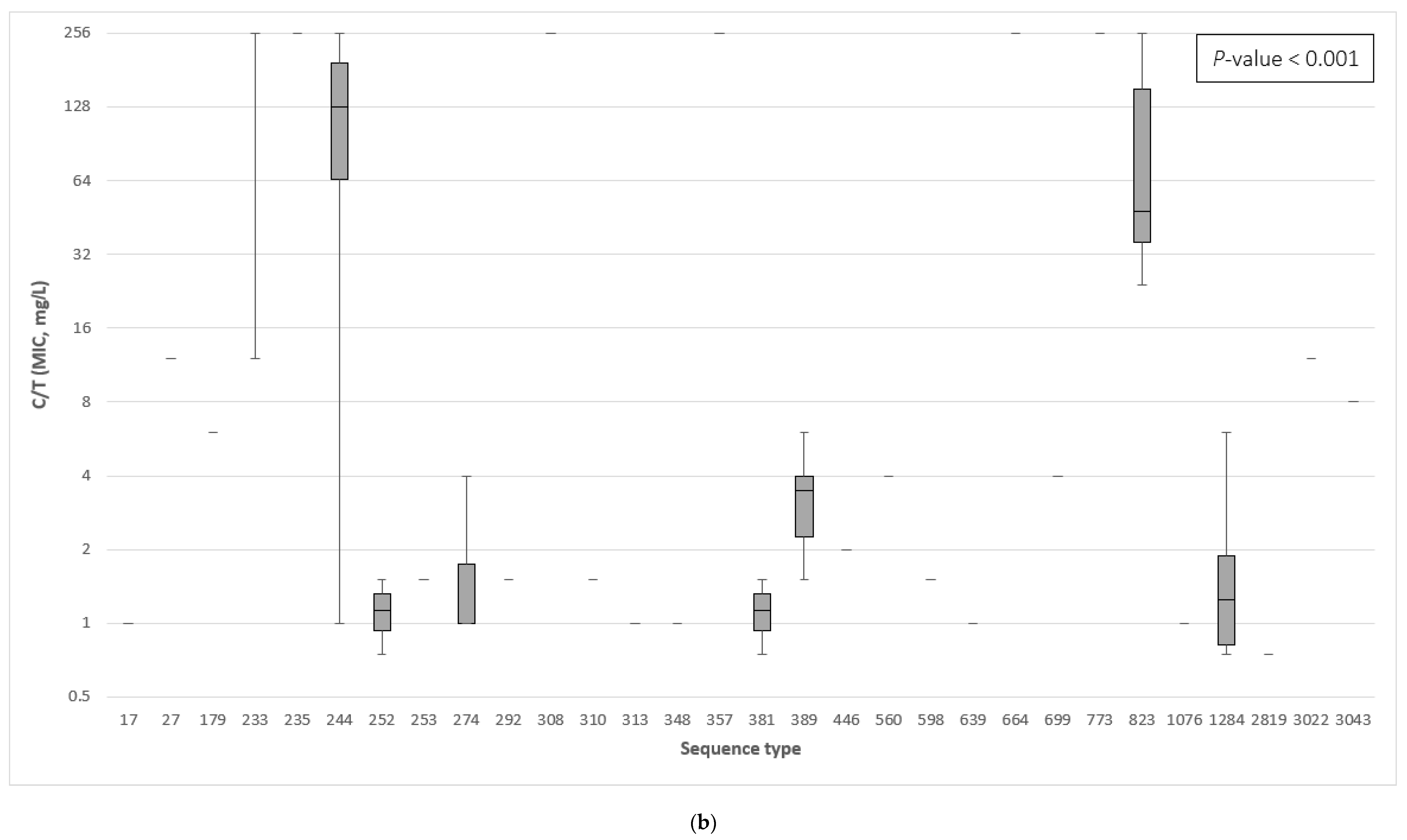
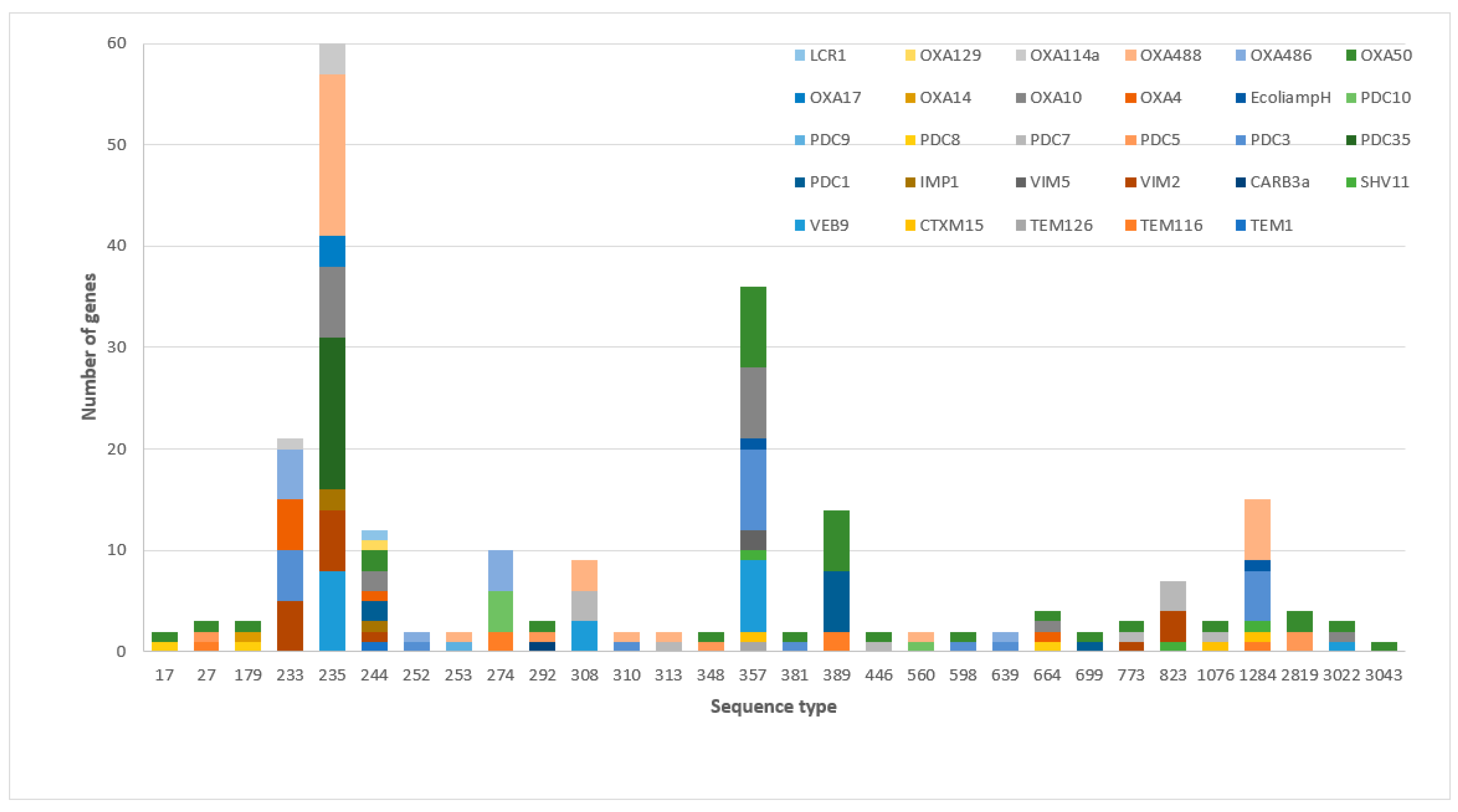
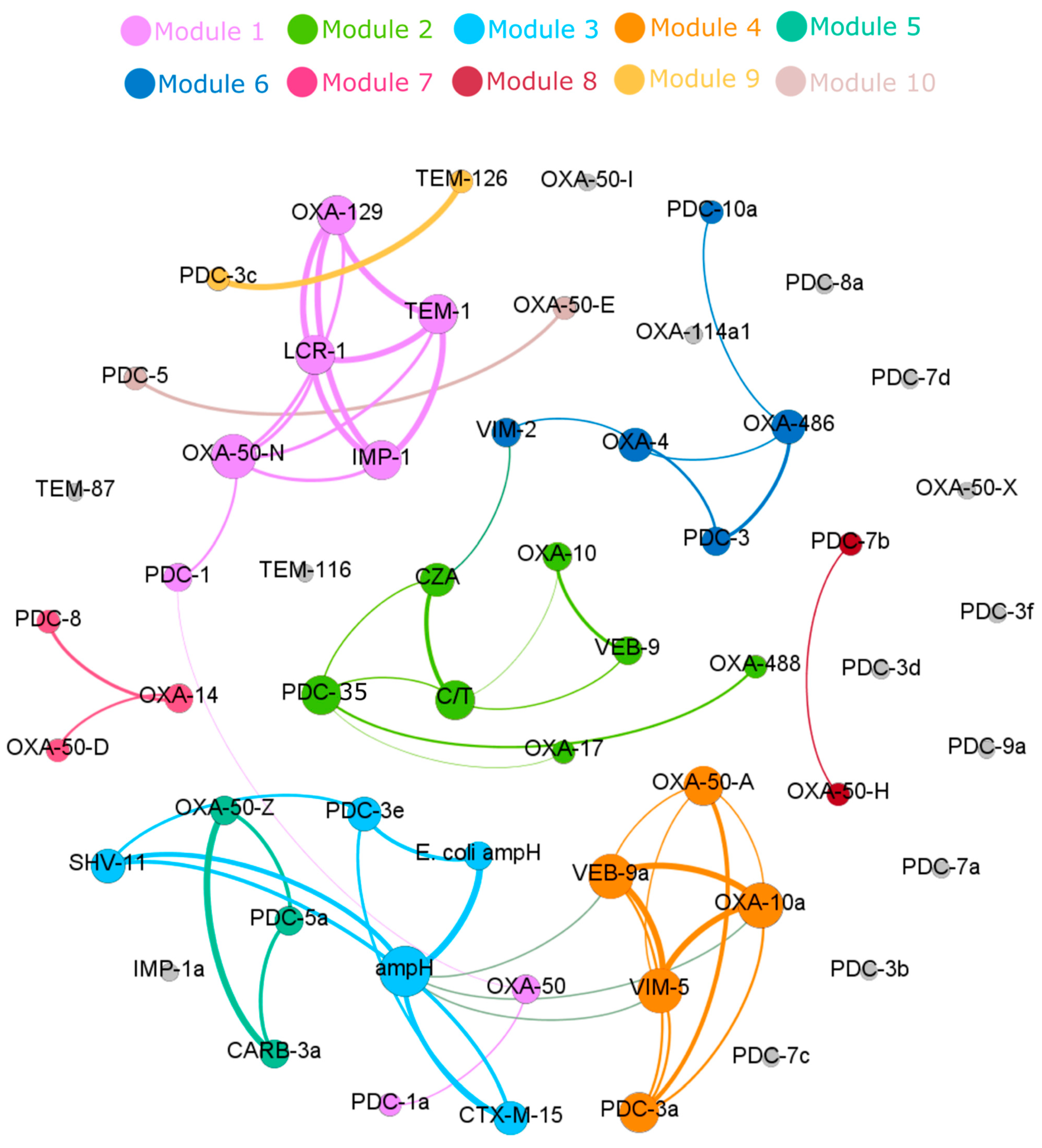
| Sequence Type | No. Strains (Frequency) | β-Lactamase Class | Antimicrobial Susceptibility | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class A | Class B | Class C | Class D | CZA | C/T | ||||||||
| No | Yes | No | Yes | No | Yes | No | Yes | R | S | R | S | ||
| 235 | 16 (21.3) | 8 (17.8) | 8 (26.7) | 8 (14.5) | 8 (40) | 1 (33.3) | 15 (20.8) | 0 | 16 (22.2) | 15 (40.5) | 1 (2.6) | 16 (40) | 0 |
| 357 | 8 (10.7) | 1 (2.2) | 7 (23.3) | 6 (10.9) | 2 (10) | 0 | 8 (11.1) | 0 | 8 (11.1) | 7 (18.9) | 1 (2.6) | 8 (20) | 0 |
| 389 | 6 (8) | 4 (8.9) | 2 (6.7) | 6 (10,9) | 0 | 0 | 6 (8.3) | 0 | 6 (8.3) | 0 | 6 (15.8) | 0 | 6 (17.1) |
| 1284 | 6 (8) | 4 (8.9) | 2 (6.7) | 6 (10,9) | 0 | 0 | 6 (8.3) | 0 | 6 (8.3) | 0 | 6 (15.8) | 0 | 6 (17.1) |
| 233 | 5 (6.7) | 5 (11.1) | 0 | 0 | 5 (25) | 0 | 5 (6.9) | 0 | 5 (6.9) | 5 (13.5) | 0 | 5 (12.5) | 0 |
| 274 | 4 (5.3) | 2 (4.4) | 2 (6.7) | 4 (7.3) | 0 | 0 | 4 (5.6) | 0 | 4 (5.6) | 0 | 4 (10.5) | 0 | 4 (11.4) |
| 308 | 3 (3) | 0 | 3 (10) | 3 (5.5) | 0 | 0 | 3 (4.2) | 0 | 3 (4.2) | 2 (5.4) | 1 (2.6) | 3 (7.5) | 0 |
| 823 | 3 (4) | 2 (4.4) | 1 (3.3) | 0 | 3 (15) | 0 | 3 (4.2) | 3 (100) | 0 | 3 (8.1) | 0 | 3 (7.5) | 0 |
| 244 | 2 (2.7) | 1 (2.2) | 1 (3.3) | 1 (1.8) | 1 (5) | 0 | 2 (2.8) | 0 | 2 (2.8) | 1 (2.7) | 1 (2.6) | 1 (2.5) | 1 (2.9) |
| 2819 | 2 (2.7) | 2 (4.4) | 0 | 2 (3.6) | 0 | 0 | 2 (2.8) | 0 | 2 (2.8) | 0 | 2 (5.3) | 0 | 2 (5.7) |
| 17 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 27 | 1 (1.3) | 0 | 1 (3.3) | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 1 (2.5) | 0 |
| 179 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 2 (1.4) | 1 (2.7) | 0 | 0 | 1 (2.9) |
| 252 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 253 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 292 | 1 (1.3) | 0 | 1 (3.3) | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 310 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 313 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 348 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 381 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 446 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 560 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 598 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 639 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 664 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 1 (2.7) | 0 | 1 (2.5) | 0 |
| 699 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 1 (2.7) | 0 | 0 | 1 (2.9) |
| 773 | 1 (1.3) | 1 (2.2) | 0 | 0 | 1 (5) | 0 | 1 (1.4) | 0 | 1 (1.4) | 1 (2.7) | 0 | 1 (2.5) | 0 |
| 1076 | 1 (1.3) | 0 | 1 (3.3) | 1 (1.8) | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| 3022 | 1 (1.3) | 0 | 1 (3.3) | 1 (1.8) | 0 | 1 (33.3) | 0 | 0 | 1 (1.4) | 0 | 1 (2.6) | 1 (2.5) | 0 |
| 3043 | 1 (1.3) | 1 (2.2) | 0 | 1 (1.8) | 0 | 2 (33.3) | 0 | 0 | 1 (1.4) | 0 | 1 (2.6) | 0 | 1 (2.9) |
| Total | 75 (100) | 45 (60) | 30 (40) | 55 (73.3) | 20 (26.7) | 3 (4) | 72 (96) | 3 (4) | 72 (96) | 37 (49,3) | 38 (50.7) | 40 (53.3) | 35 (46.7) |
| Antibiotic | β-Lactamase Class | Resistant | Susceptible | p-Value * |
|---|---|---|---|---|
| N = 37 | N = 38 | |||
| Ceftazidime–avibactam | Class A | 48.6% | 31.6% | 0.131 |
| Class B | 54.1% | 0% | <0.001 | |
| Class C | 100% | 92.1% | 0.240 † | |
| Class D | 91.9% | 100% | 0.115 † | |
| Ceftolozane–tazobactam | N = 40 | N = 35 | ||
| Class A | 55% | 22.9% | 0.005 | |
| Class B | 50% | 0% | <0.001 | |
| Class C | 95% | 97.1% | 0.99 † | |
| Class D | 92.5% | 100% | 0.243 † |
| Antimicrobial Agent | Ceftazidime–Avibactam | Ceftolozane–Tazobactam | Total Genes | ||||
|---|---|---|---|---|---|---|---|
| Susceptibility Result | Susceptible | Resistant | Extremely Resistant | Susceptible | Resistant | Extremely Resistant | |
| MIC 0.75–8 | MIC 12–192 | MIC ≥ 256 | MIC 0.75–8 | MIC 12–48 | MIC ≥ 256 | ||
| Gene | Frequency (%) | Frequency (%) | |||||
| Class A β-lactamase | |||||||
| VEB-9 | 3 (15.8) | 10 (52.6) | 6 (31.6) | 0 | 1 (5.6) | 18 (94.7) | 19 |
| TEM-116 | 6 (100) | 0 | 0 | 5 (83.3) | 1 (16.7) | 0 | 6 |
| CTX-M-15 | 2 (66.7) | 0 | 1 (33.3) | 2 (66.7) | 0 | 1 (33.3) | 3 |
| SHV-11 | 1 (33.33) | 1 (33.33) | 1 (33.33) | 1 (33.33) | 1 (33.33) | 1 (33.33) | 3 |
| TEM-1 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 |
| TEM-126 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 |
| CARB-3a | 1 (100) | 0 | 0 | 1 (100) | 0 | 0 | 1 |
| p-value † | 0.002 | <0.001 | |||||
| Class B β-lactamase | |||||||
| VIM-2 | 0 | 14 (87.5) | 2 (12.5) | 0 | 3 (18.7) | 13 (81.3) | 16 |
| IMP-1 | 0 | 0 | 3 (100) | 0 | 0 | 3 (100) | 3 |
| VIM-5 | 0 | 0 | 2 (100) | 0 | 0 | 2 (100) | 2 |
| p-value † | 0.001 | 0.99 | |||||
| Class C β-lactamase | |||||||
| PDC-3 | 11 (47.8) | 5 (21.7) | 7 (30.4) | 10 (43.5) | 1 (4.3) | 12 (52.2) | 23 |
| PDC-35 | 0 | 13 (86.7) | 2 (13.3) | 0 | 0 | 15 (100) | 15 |
| PDC-7 | 4 (40) | 6 (60) | 0 | 3 (30) | 2 (20) | 5 (50) | 10 |
| PDC-1 | 7 (77.8) | 1 (11.1) | 1 (11.1) | 8 (88.9) | 0 | 1 (11.1) | 9 |
| PDC-5 | 5 (100) | 0 | 0 | 4 (80) | 1 (20) | 0 | 5 |
| PDC-10 | 5 (100) | 0 | 0 | 5 (100) | 0 | 0 | 5 |
| PDC-8 | 1 (33.33) | 1 (33.33) | 1 (33.33) | 2 (66.7) | 0 | 1 (33.33) | 3 |
| PDC-9 | 1 (100) | 0 | 0 | 1 (100) | 0 | 0 | 1 |
| p-value † | <0.001 | <0.001 | |||||
| Class D β-lactamase | |||||||
| OXA-50 | 20 (64.5) | 4 (12.9) | 8 (25.8) | 19 (59.4) | 2 (6.2) | 11 (34.4) | 32 |
| OXA-488 | 12 (42.8) | 15 (53.6) | 2 (7.14) | 10 (34.5) | 0 | 19 (56.5) | 29 |
| OXA-10 | 3 (16.7) | 7 (38.9) | 8 (44.4) | 1 (5.6) | 1 (5.6) | 16 (88.9) | 18 |
| OXA-486 | 6 (54.5) | 4 (36.4) | 1 (9.1) | 6 (54.5) | 1 (9.1) | 4 (36.4) | 11 |
| OXA-4 | 0 | 4 (57.1) | 3 (42.9) | 0 | 1 (14.3) | 6 (85.7) | 7 |
| OXA-114a | 1 (25) | 2 (50) | 1 (25) | 0 | 0 | 4 (100) | 4 |
| OXA-17 | 0 | 3 (100) | 0 | 0 | 0 | 3 (100) | 3 |
| E. coli ampH | 1 (50) | 0 | 1 (50) | 1 (50) | 0 | 1 (50) | 2 |
| OXA-14 | 0 | 1 (100) | 0 | 1 (100) | 0 | 0 | 1 |
| OXA-129 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 |
| LCR-1 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 |
| p-value † | <0.001 | 0.001 | |||||
| Total of genes | 90 (38.3) | 91 (38.7) | 54 (23) | 80 (34) | 15 (6.4) | 140 (59.6) | 235 |
| Total No. of isolates | 38 (50.6) | 26 (34.7) | 11 (14.7) | 35 (46.7) | 5 (6.6) | 35 (46.7) | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sid Ahmed, M.A.; Khan, F.A.; Hadi, H.A.; Skariah, S.; Sultan, A.A.; Salam, A.; Al Khal, A.L.; Söderquist, B.; Ibrahim, E.B.; Omrani, A.S.; et al. Association of blaVIM-2, blaPDC-35, blaOXA-10, blaOXA-488 and blaVEB-9 β-Lactamase Genes with Resistance to Ceftazidime–Avibactam and Ceftolozane–Tazobactam in Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2022, 11, 130. https://doi.org/10.3390/antibiotics11020130
Sid Ahmed MA, Khan FA, Hadi HA, Skariah S, Sultan AA, Salam A, Al Khal AL, Söderquist B, Ibrahim EB, Omrani AS, et al. Association of blaVIM-2, blaPDC-35, blaOXA-10, blaOXA-488 and blaVEB-9 β-Lactamase Genes with Resistance to Ceftazidime–Avibactam and Ceftolozane–Tazobactam in Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics. 2022; 11(2):130. https://doi.org/10.3390/antibiotics11020130
Chicago/Turabian StyleSid Ahmed, Mazen A., Faisal Ahmad Khan, Hamad Abdel Hadi, Sini Skariah, Ali A. Sultan, Abdul Salam, Abdul Latif Al Khal, Bo Söderquist, Emad Bashir Ibrahim, Ali S. Omrani, and et al. 2022. "Association of blaVIM-2, blaPDC-35, blaOXA-10, blaOXA-488 and blaVEB-9 β-Lactamase Genes with Resistance to Ceftazidime–Avibactam and Ceftolozane–Tazobactam in Multidrug-Resistant Pseudomonas aeruginosa" Antibiotics 11, no. 2: 130. https://doi.org/10.3390/antibiotics11020130
APA StyleSid Ahmed, M. A., Khan, F. A., Hadi, H. A., Skariah, S., Sultan, A. A., Salam, A., Al Khal, A. L., Söderquist, B., Ibrahim, E. B., Omrani, A. S., & Jass, J. (2022). Association of blaVIM-2, blaPDC-35, blaOXA-10, blaOXA-488 and blaVEB-9 β-Lactamase Genes with Resistance to Ceftazidime–Avibactam and Ceftolozane–Tazobactam in Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics, 11(2), 130. https://doi.org/10.3390/antibiotics11020130







