Efficacy of Tigecycline as Salvage Therapy in Multidrug-Resistant Febrile Neutropenia in Patients with Acute Leukemia—A Single Center Analysis
Abstract
:1. Introduction
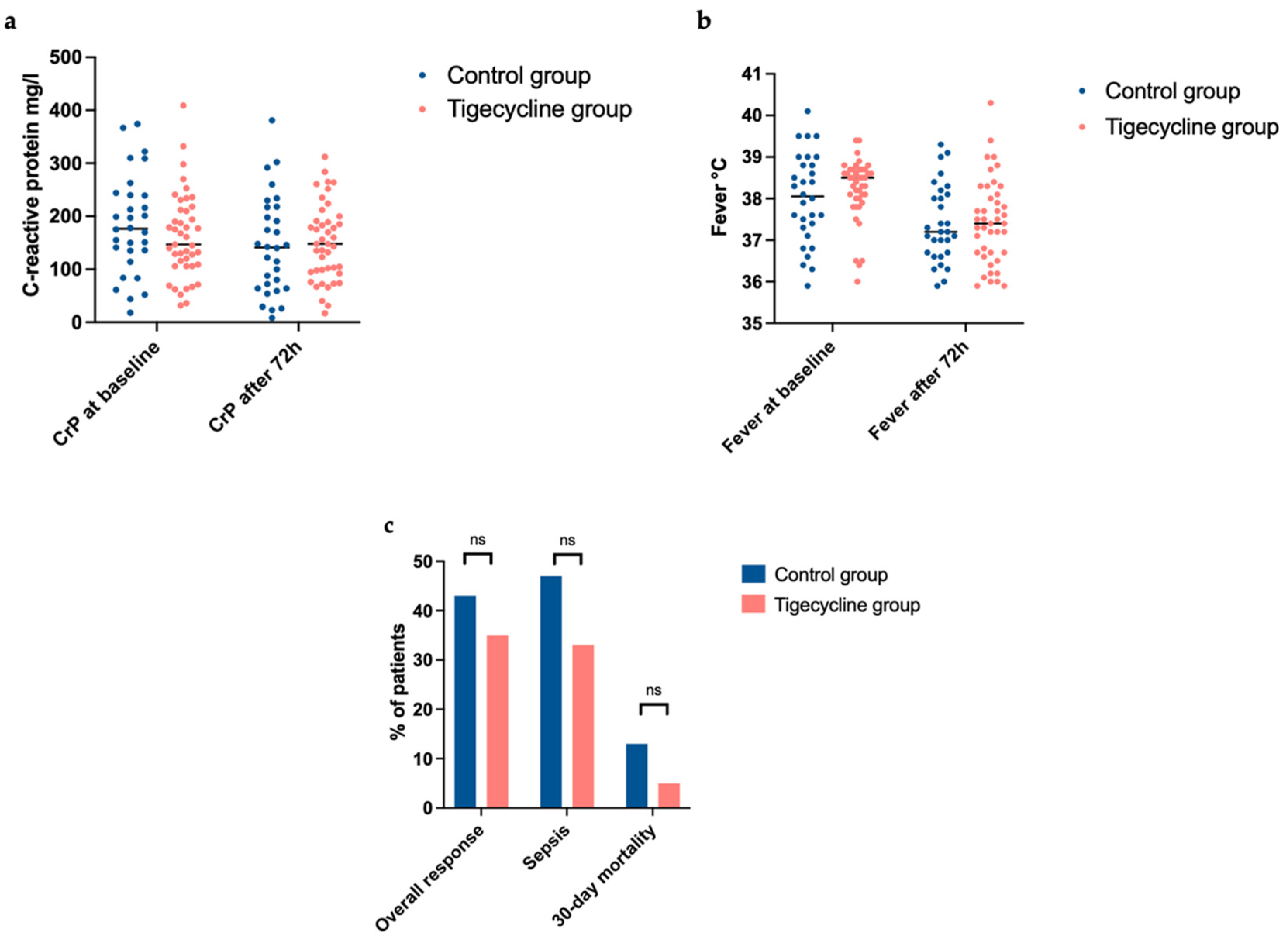
2. Results
3. Discussion
4. Materials and Methods
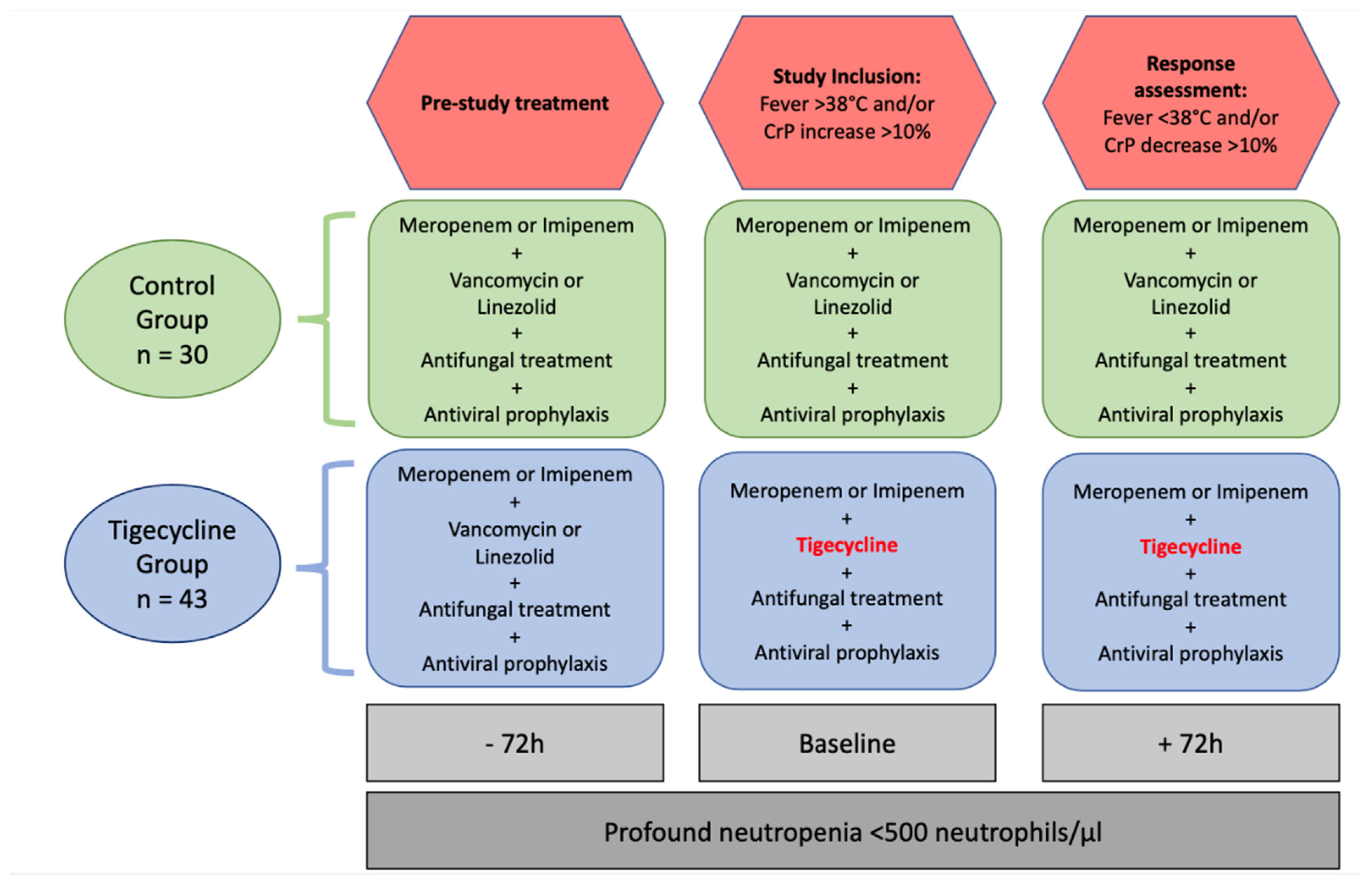
4.1. Study Population
4.2. Clinical Data Collection
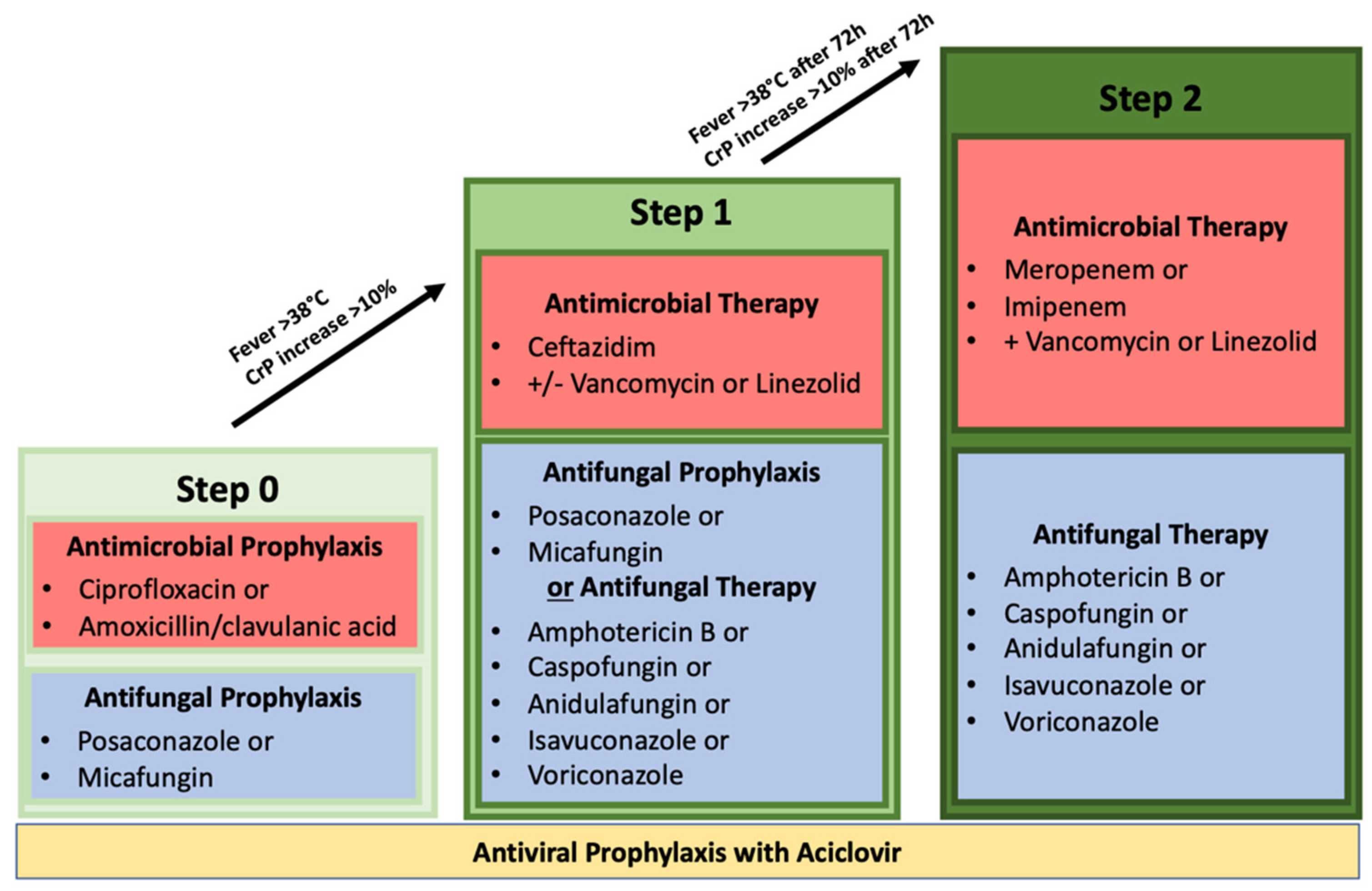
4.3. Definition of Time Points and Inclusion Criteria
- –
- Step 0: Prophylactic antibiotic administration with ciprofloxacin or amoxicillin/clavulanic acid. Prophylactic antiviral administration with acyclovir. Prophylactic antifungal administration with posaconazole or micafungin;
- –
- Step 1: Neutropenic fever or increase in inflammatory parameters; use of the antibiotic agent ceftazidime with or without vancomycin or linezolid. Switch of antifungal therapeutic therapy was not mandatory but possible. Therapeutic antifungal administrations include amphotericin B, caspofungin, anidulafungin, isavuconazole, and voriconazole. Prophylactic acyclovir administration had to be continued;
- –
- Step 2: Persistent neutropenic fever or increase in inflammatory parameters >72 h after step 1. Switch of antibiotic therapeutics to a carbapenem (either meropenem or imipenem/cilastatin) with concomitant administration of vancomycin or linezolid. Antifungal therapy had to be switched to a therapeutic approach with either amphotericin B, caspofungin, anidulafungin, isavuconazole, or voriconazole. Prophylactic acyclovir administration had to be continued.
4.4. Primary and Secondary Endpoints
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biswal, S.; Godnaik, C. Incidence and management of infections in patients with acute leukemia following chemotherapy in general wards. Ecancermedicalscience 2013, 7, 310. [Google Scholar]
- Nesher, L.; Rolston, K.V. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 2014, 42, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Dale, D.C.; Crawford, J.; Cosler, L.E.; Lyman, G.H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006, 106, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Bow, E. Risk Assessment of Adults with Chemotherapy-Induced Neutropenia. 2021. Available online: https://www.uptodate.com/contents/risk-assessment-of-adults-with-chemotherapy-induced-neutropenia (accessed on 11 November 2021).
- Van der Velden, W.J.; Herbers, A.H.; Netea, M.G.; Blijlevens, N.M. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: Introducing the paradigm febrile mucositis. Br. J. Haematol. 2014, 167, 441–452. [Google Scholar] [CrossRef]
- Malagola, M.; Peli, A.; Damiani, D.; Candoni, A.; Tiribelli, M.; Martinelli, G.; Piccaluga, P.P.; Paolini, S.; De Rosa, F.; Lauria, F.; et al. Incidence of bacterial and fungal infections in newly diagnosed acute myeloid leukaemia patients younger than 65 yr treated with induction regimens including fludarabine: Retrospective analysis of 224 cases. Eur. J. Haematol. 2008, 81, 354–363. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Cardozo-Espinola, C.; Puerta-Alcalde, P.; Marco, F.; Téllez, A.; Agüero, D.; Romero-Santana, F.; Díaz-Beyá, M.; Giné, E.; Morata, L.; et al. Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PLoS ONE 2018, 13, e0199531. [Google Scholar] [CrossRef]
- Mikulska, M.; Viscoli, C.; Orasch, C.; Livermore, D.M.; Averbuch, D.; Cordonnier, C.; Akova, M. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J. Infect. 2014, 68, 321–331. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.A.; Wendelbo, O.; Bruserud, O.; Hemsing, A.L.; Mosevoll, K.A.; Reikvam, H. Febrile Neutropenia in Acute Leukemia. Epidemiology, Etiology, Pathophysiology and Treatment. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020009. [Google Scholar] [CrossRef]
- Garnica, M.; Nouér, S.A.; Pellegrino, F.L.; Moreira, B.M.; Maiolino, A.; Nucci, M. Ciprofloxacin prophylaxis in high risk neutropenic patients: Effects on outcomes, antimicrobial therapy and resistance. BMC Infect. Dis. 2013, 13, 356. [Google Scholar] [CrossRef] [Green Version]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826–1835. [Google Scholar] [CrossRef] [Green Version]
- Gustinetti, G.; Mikulska, M. Bloodstream infections in neutropenic cancer patients: A practical update. Virulence 2016, 7, 280–297. [Google Scholar] [CrossRef]
- Greer, N.D. Tigecycline (Tygacil): The First in the Glycylcycline Class of Antibiotics. Bayl. Univ. Med. Cent. Proc. 2006, 19, 155–161. [Google Scholar] [CrossRef]
- Scheinfeld, N. Tigecycline: A review of a new glycylcycline antibiotic. J. Dermatol. Treat. 2005, 16, 207–212. [Google Scholar] [CrossRef]
- Bucaneve, G.; Micozzi, A.; Picardi, M.; Ballanti, S.; Cascavilla, N.; Salutari, P.; Specchia, G.; Fanci, R.; Luppi, M.; Cudillo, L.; et al. Results of a Multicenter, Controlled, Randomized Clinical Trial Evaluating the Combination of Piperacillin/Tazobactam and Tigecycline in High-Risk Hematologic Patients With Cancer With Febrile Neutropenia. J. Clin. Oncol. 2014, 32, 1463–1471. [Google Scholar] [CrossRef]
- Babinchak, T.; Ellis-Grosse, E.; Dartois, N.; Rose, G.M.; Loh, E.; Tigecycline 301 Study Group; Tigecycline 306 Study Group. The Efficacy and Safety of Tigecycline for the Treatment of Complicated Intra-Abdominal Infections: Analysis of Pooled Clinical Trial Data. Clin. Infect. Dis. 2005, 41 (Suppl. S5), S354–S367. [Google Scholar] [CrossRef]
- Sacchidanand, S.; Penn, R.L.; Embil, J.M.; Campos, M.E.; Curcio, D.; Ellis-Grosse, E.; Loh, E.; Rose, G. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: Results from a phase 3, randomized, double-blind trial. Int. J. Infect. Dis. 2005, 9, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Bergallo, C.; Jasovich, A.; Teglia, O.; Oliva, M.E.; Lentnek, A.; de Wouters, L.; Zlocowski, J.C.; Dukart, G.; Cooper, A.; Mallick, R. Safety and efficacy of intravenous tigecycline in treatment of community-acquired pneumonia: Results from a double-blind randomized phase 3 comparison study with levofloxacin. Diagn. Microbiol. Infect. Dis. 2009, 63, 52–61. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. 2018. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=208744 (accessed on 21 October 2020).
- Stein, G.E.; Babinchak, T. Tigecycline: An update. Diagn. Microbiol. Infect. Dis. 2013, 75, 331–336. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Wang, P.S.; Avorn, J.; Glynn, R.J. Improved Comorbidity Adjustment for Predicting Mortality in Medicare Populations. Heal. Serv. Res. 2003, 38, 1103–1120. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Schwab, K.S.; Hahn-Ast, C.; Heinz, W.J.; Germing, U.; Egerer, G.; Glasmacher, A.; Leyendecker, C.; Marklein, G.; Nellessen, C.M.; Brossart, P.; et al. Tigecycline in febrile neutropenic patients with haematological malignancies: A retrospective case documentation in four university hospitals. Infection 2014, 42, 97–104. [Google Scholar] [CrossRef]
- Zhou, X.-P.; Ye, X.-J.; Shen, J.-P.; Lan, J.-P.; Jiang, H.-F.; Zhang, J.; Zhang, X.-J.; Li, L.; Qian, S.-X.; Tong, H.-Y. Salvage tigecycline in high risk febrile neutropenic patients with hematological malignancies: A prospective multicenter study. Leuk. Lymphoma 2018, 59, 2679–2685. [Google Scholar] [CrossRef]
- Ballo, O.; Tarazzit, I.; Stratmann, J.; Reinheimer, C.; Hogardt, M.; Wichelhaus, T.A.; Kempf, V.; Serve, H.; Finkelmeier, F.; Brandts, C. Colonization with multidrug resistant organisms determines the clinical course of patients with acute myeloid leukemia undergoing intensive induction chemotherapy. PLoS ONE 2019, 14, e0210991. [Google Scholar] [CrossRef]
- Muralidharan, G.; Micalizzi, M.; Speth, J.; Raible, D.; Troy, S. Pharmacokinetics of Tigecycline after Single and Multiple Doses in Healthy Subjects. Antimicrob. Agents Chemother. 2005, 49, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Pan, Y.; Shen, J.; Xu, Y. The efficacy and safety of tigecycline for the treatment of bloodstream infections: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yaghoubi, S.; Zekiy, A.O.; Krutova, M.; Gholami, M.; Kouhsari, E.; Sholeh, M.; Ghafouri, Z.; Maleki, F. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: Narrative review. Eur. J. Clin. Microbiol. 2021, 1–20. [Google Scholar] [CrossRef]
- Oncology NCPGi. Prevention and Treatment of Cancer-Related Infections. 2021. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1457 (accessed on 2 July 2021).
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 27 November 2017).
| Control Group (n = 30) | Tigecycline Group (n = 43) | |
|---|---|---|
| Male, no. (%) | 16 (53) | 25 (58) |
| Age, median (IQR) | 60.5 (44–68) | 59 (43–68) |
| Relevant comorbidities, no. (%) | 8 (27) | 12 (28) |
| Cerebrovascular disease | / | 1 (2) |
| Breast cancer | 3 (10) | 4 (9) |
| Bladder cancer | / | 1 (2) |
| Prostate cancer | / | 2 (5) |
| Lung emphysema | / | 1 (2) |
| Multiple Myeloma | / | 1 (2) |
| Acute myeloid leukemia | / | 1 (2) |
| Diabetes mellitus II | 2 (7) | 1 (2) |
| Peripheral artery disease | 1 (3) | / |
| Coronary heart disease | 2 (7) | / |
| Charlson-Comorbidity-Index (CCI), median (IQR) 1 | 4 (2–5) | 4 (2–5) |
| Underlying hematological malignancy, no. (%) | ||
| Acute myeloid leukemia | 25 (83) | 37 (86) |
| Acute lymphoblastic leukemia | 5 (17) | 6 (14) |
| Remission status, no. (%) | ||
| First diagnosis | 25 (83) | 33 (77) |
| Relapse/refractory | 5 (17) | 10 (23) |
| Under intensive treatment, no. (%) | 28 (93) | 38 (88) |
| Duration (days) of profound neutropenia (<500 neutrophils/µL), median (IQR) | 25 (20–28) | 21 (17–30) |
| Control Group (n = 30) | Tigecycline Group (n = 43) | |
|---|---|---|
| Carbapenem, no. (%) | 30 (100) | 43 (100) |
| Meropenem | 27 (90) | 43 (100) |
| Imipenem/Cilastatin | 3 (10) | 0 (0) |
| Vancomycin, no. (%) | 26 (87) | 41 (95) |
| Linezolid, no. (%) | 13 (43) | 13 (30) |
| Vancomycin and linezolid sequentially, no. (%) | 10 (33) | 11 (26) |
| Control Group (n = 30) | Tigecycline Group (n = 43) | p-Value | |
|---|---|---|---|
| Overall response rate, no. (%) | 13 (43) | 15 (35) | 0.476 |
| Infection-associated 30-day mortality, no. (%) | 4 (13) | 2 (5) | not done |
| Sepsis, no. (%) | 14 (47) | 14 (33) | not done |
| Pneumonia in X-ray or computed chest tomography, no. (%) | 16 (53) | 26 (60) | not done |
| Catheter-associated infections, no. (%) | 4 (13) | 16 (37) | not done |
| Infections of urinary tract, no. (%) | 3 (10) | 6 (14) | not done |
| Positive blood culture samples, no. (%) | 9 (30) | 22 (51) | not done |
| Staphylococcus haemolyticus | 4 (13) * | 5 (12) | |
| Staphylococcus hominis | 1 (3) | 4 (9) | |
| Staphylococcus epidermidis | 4 (13) | 9 (21) | |
| Staphylococcus lugdunensis | / | 1 (2) | |
| Stenotrophomonas maltophilia | / | 2 (5) | |
| Citrobacter freundii | / | 1 (2) | |
| Enterococcus faecium (non-VRE) | / | 3 (7) | |
| Enterococcus faecalis (non-VRE) | 1 (3) | 1 (2) | |
| Enterococcus faecium (VRE) | / | 1 (2) | |
| Escherichia coli | / | 1 (2) | |
| Pseudomonas aeruginosa | 1 (3) | / | |
| Positive BAL, no. (%) | 15 of 18 (83) | 8 of 13 (66) | |
| Yeasts | 3 (10) | 7 (16) | |
| Coagulase-neg. staphylococci | 5 (17) | 10 (23) | |
| Enterococcus spp. | 4 (14) | 3 (7) | |
| Herpes simplex virus 1 | 2 (7) | 2 (5) | |
| Enterovirus | 1 (3) | / | |
| Rhinovirus | 1 (3) | / | |
| Streptococcus viridans | / | 4 (9) | |
| Metapneumonia virus | / | 1 (2) | |
| Aspergillus fumigatus | / | 1 (2) | |
| Escherichia coli | / | 1 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modemann, F.; Härterich, S.; Schulze zur Wiesch, J.; Rohde, H.; Lindeman, N.B.; Bokemeyer, C.; Fiedler, W.; Ghandili, S. Efficacy of Tigecycline as Salvage Therapy in Multidrug-Resistant Febrile Neutropenia in Patients with Acute Leukemia—A Single Center Analysis. Antibiotics 2022, 11, 128. https://doi.org/10.3390/antibiotics11020128
Modemann F, Härterich S, Schulze zur Wiesch J, Rohde H, Lindeman NB, Bokemeyer C, Fiedler W, Ghandili S. Efficacy of Tigecycline as Salvage Therapy in Multidrug-Resistant Febrile Neutropenia in Patients with Acute Leukemia—A Single Center Analysis. Antibiotics. 2022; 11(2):128. https://doi.org/10.3390/antibiotics11020128
Chicago/Turabian StyleModemann, Franziska, Steffen Härterich, Julian Schulze zur Wiesch, Holger Rohde, Nick Benjamin Lindeman, Carsten Bokemeyer, Walter Fiedler, and Susanne Ghandili. 2022. "Efficacy of Tigecycline as Salvage Therapy in Multidrug-Resistant Febrile Neutropenia in Patients with Acute Leukemia—A Single Center Analysis" Antibiotics 11, no. 2: 128. https://doi.org/10.3390/antibiotics11020128
APA StyleModemann, F., Härterich, S., Schulze zur Wiesch, J., Rohde, H., Lindeman, N. B., Bokemeyer, C., Fiedler, W., & Ghandili, S. (2022). Efficacy of Tigecycline as Salvage Therapy in Multidrug-Resistant Febrile Neutropenia in Patients with Acute Leukemia—A Single Center Analysis. Antibiotics, 11(2), 128. https://doi.org/10.3390/antibiotics11020128







