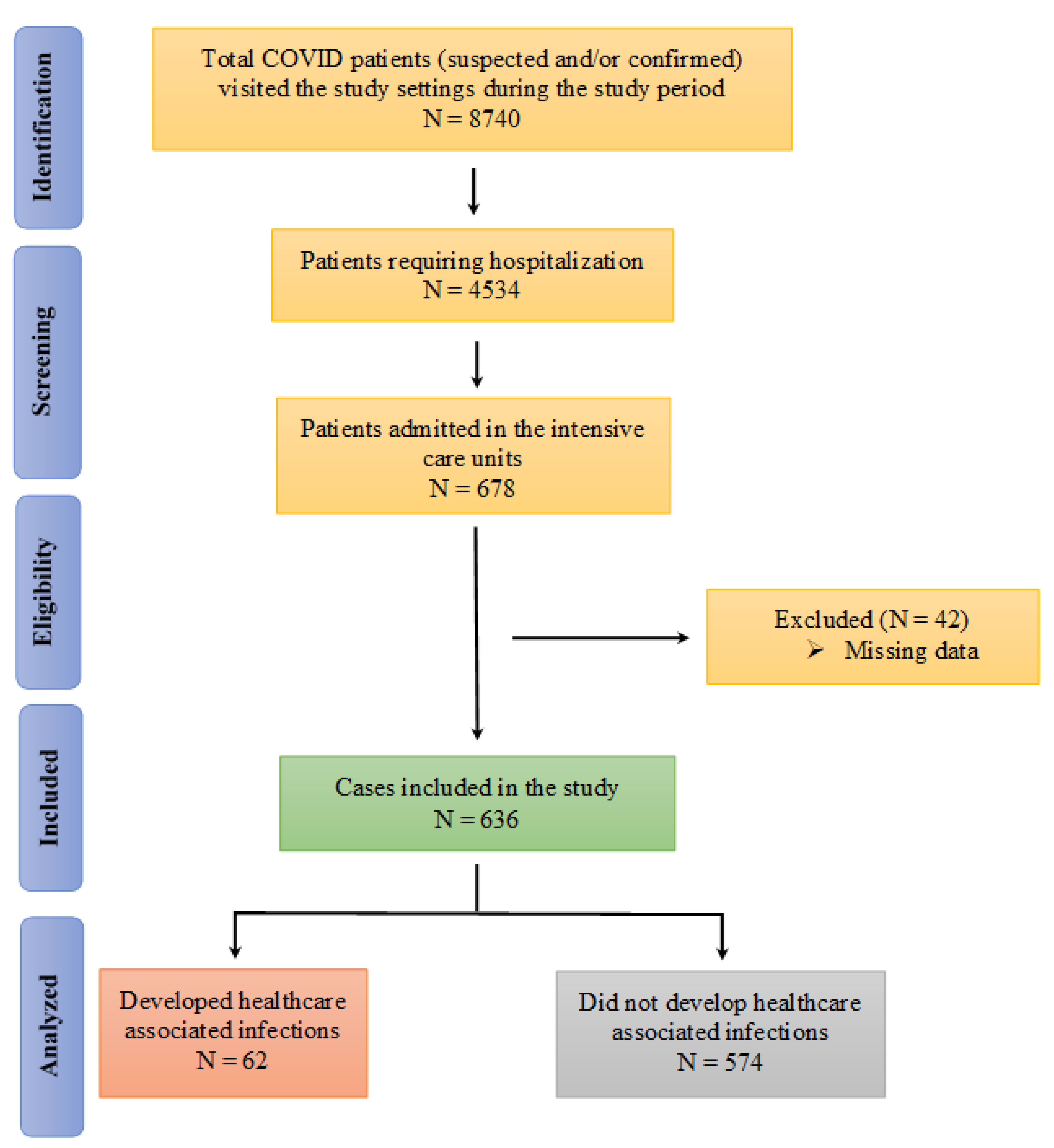
Predictors and Outcomes of Healthcare-Associated Infections among Patients with COVID-19 Admitted to Intensive Care Units in Punjab, Pakistan; Findings and Implications
Abstract
1. Introduction
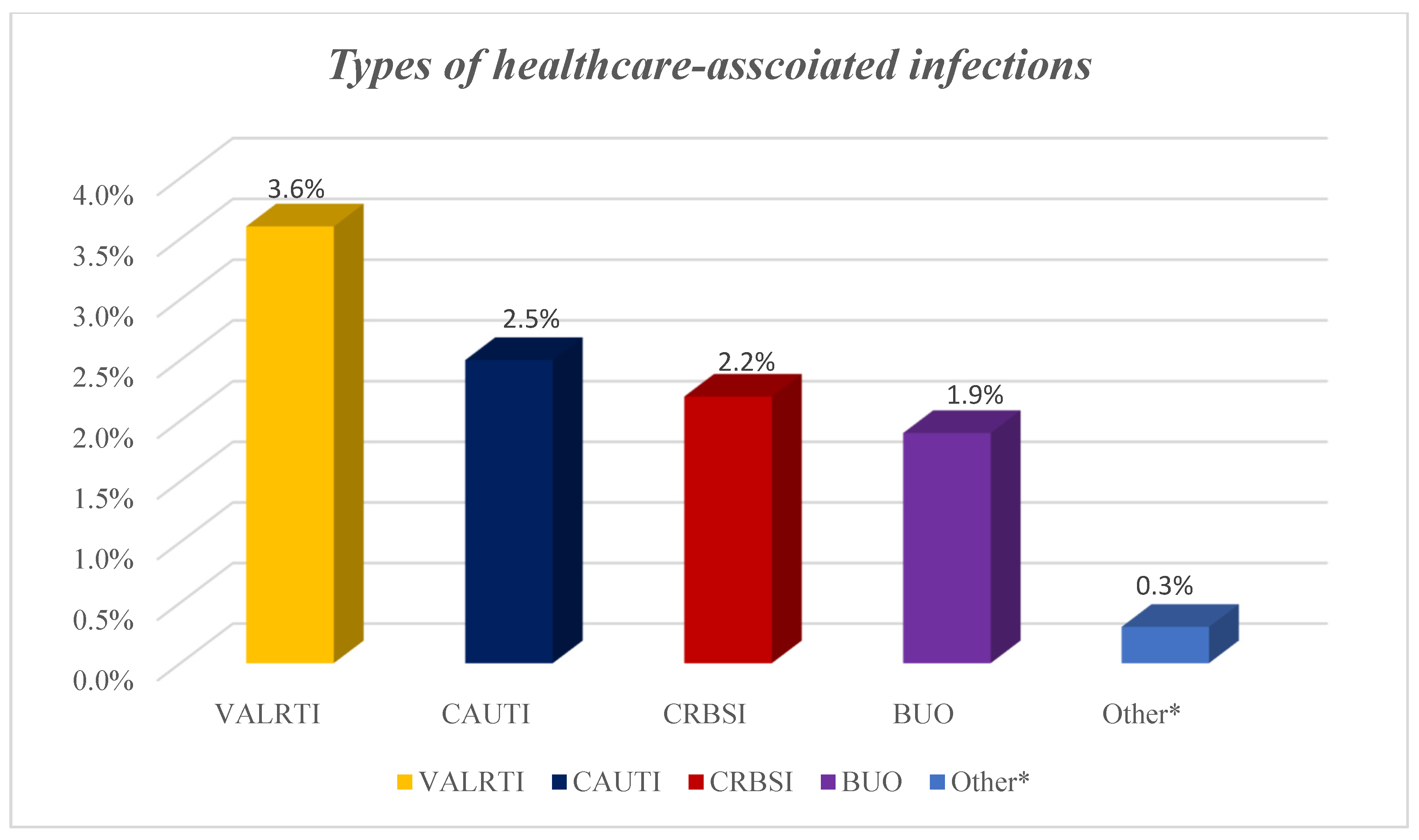
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. The Data Collection Form
4.3. The Data Collection Procedure
4.4. Inclusion and Exclusion Criteria
4.5. Data Analysis
4.6. Ethical Considerations
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pouwels, K.B.; Vansteelandt, S.; Batra, R.; Edgeworth, J.; Wordsworth, S.; Robotham, J.V. Estimating the Effect of Healthcare-Associated Infections on Excess Length of Hospital Stay Using Inverse Probability-Weighted Survival Curves. Clin. Infect. Dis. 2020, 71, e415–e420. [Google Scholar] [CrossRef]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Selby, L.M.; Rupp, M.E.; Cawcutt, K.A. Prevention of Central-Line Associated Bloodstream Infections: 2021 Update. Infect. Dis. Clin. N. Am. 2021, 35, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Godman, B.; Hassali, M.A.; Hashmi, F.K.; Azhar, F.; Rehman, I.U. Point prevalence surveys of health-care-associated infections: A systematic review. Pathog. Glob. Health 2019, 113, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Birhane, M.; Bekele, S.; Kibru, G.; Teshager, L.; Yilma, Y.; Ahmed, Y.; Fentahun, N.; Assefa, H.; Gashaw, M.; et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: Longitudinal study. Antimicrob. Resist. Infect. Control. 2018, 7, 2. [Google Scholar] [CrossRef]
- Nelson, R.E.; Schweizer, M.; Jones, M.; Stevens, V.W.; Khader, K.; Perencevich, E.; Rubin, M.; Samore, M. The Cost and Mortality Burden of Hospital-Onset Antimicrobial-Resistant Healthcare-Associated Infections in the USA. Open Forum Infect. Dis. 2017, 4 (Suppl. 1), S177–S178. [Google Scholar] [CrossRef][Green Version]
- Nelson, R.E.; Hatfield, K.M.; Wolford, H.; Samore, M.H.; Scott, R.D.; Reddy, S.C.; Olubajo, B.; Paul, P.; Al Jernigan, J.; Baggs, J. National Estimates of Healthcare Costs Associated With Multidrug-Resistant Bacterial Infections among Hospitalized Patients in the United States. Clin. Infect. Dis. 2021, 72 (Suppl. 1), S17–S26. [Google Scholar] [CrossRef]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J. Changes in Prevalence of Health Care–Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Forrester, J.D.; Maggio, P.M.; Tennakoon, L. Cost of Health Care-Associated Infections in the United States. J. Patient Saf. 2022, 18, e477–e479. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, D.; Li, H.; Wang, Q.; Mao, Z.; Fang, L.; Ren, N.; Sun, J. Direct medical burden of antimicrobial-resistant healthcare-associated infections: Empirical evidence from China. J. Hosp. Infect. 2020, 105, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Robertson, C.; Pan, J.; Kennedy, S.; Dancer, S.; Haahr, L.; Manoukian, S.; Mason, H.; Kavanagh, K.; Cook, B.; et al. Epidemiology of healthcare-associated infection reported from a hospital-wide incidence study: Considerations for infection prevention and control planning. J. Hosp. Infect. 2021, 114, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.L.; Mwatondo, A.; Alimi, Y.H.; Varma, J.K.; Vilas, V. Healthcare-associated outbreaks of bacterial infections in Africa, 2009–2018: A review. Int. J. Infect. Dis. 2021, 103, 469–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Z.-F.; Chen, S.-X.; Zhou, D.-R.; Li, Z.-K.; Meng, Y.; Zhou, J.-F.; Hou, T.-Y. Prevalence of healthcare-associated infections and antimicrobial use in China: Results from the 2018 point prevalence survey in 189 hospitals in Guangdong Province. Int. J. Infect. Dis. 2019, 89, 179–184. [Google Scholar] [CrossRef]
- Sun, J.; Qin, W.; Jia, L.; Sun, Z.; Xu, H.; Hui, Y.; Gu, A.; Li, W. Analysis of Continuous Prevalence Survey of Healthcare-Associated Infections Based on the Real-Time Monitoring System in 2018 in Shandong in China. BioMed Res. Int. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Tartari, E.; Huang, J.; Harbarth, S.; Pittet, D.; Zingg, W. The Prevalence of Healthcare-Associated Infections in Mainland China: A Systematic Review and Meta-analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 701–709. [Google Scholar] [CrossRef]
- Weinshel, K.; Dramowski, A.; Hajdu, Á.; Jacob, S.; Khanal, B.; Zoltán, M.; Mougkou, K.; Phukan, C.; Staneloni, M.I.; Singh, N. Gap analysis of infection control practices in low- and middle-income countries. Infect. Control Hosp. Epidemiol. 2015, 36, 1208–1214. [Google Scholar] [CrossRef]
- Sengupta, S.; Barman, P.; Lo, J. Opportunities to Overcome Implementation Challenges of Infection Prevention and Control in Low-Middle Income Countries. Curr. Treat. Options Infect. Dis. 2019, 11, 267–280. [Google Scholar] [CrossRef]
- Salman, M.; Raza, M.H.; Mustafa, Z.U.; Shrestha, S.; Ali, M.; Faham, H.; Asif, N.; Shehzadi, N.; Hussain, K. Knowledge, attitudes and practices of hand hygiene among Pakistani health professionals: A cross-sectional study. J. Infect. Dev. Ctries. 2018, 12, 063–066. [Google Scholar] [CrossRef]
- Shahida, S.I.A.; Dey, B.; Islam, F.; Venkatesh, K.; Goodman, A. Hospital Acquired Infections in Low and Middle Income Countries: Root Cause Analysis and the Development of Infection Control Practices in Bangladesh. Open J. Obstet. Gynecol. 2016, 6, 28–39. [Google Scholar] [CrossRef]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug. Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Ghali, H.; Ben Cheikh, A.; Bhiri, S.; Khefacha, S.; Latiri, H.S.; Ben Rejeb, M. Trends of Healthcare-associated Infections in a Tuinisian University Hospital and Impact of COVID-19 Pandemic. Inquiry 2021, 58, 469580211067930. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Mularoni, A.; Giacobbe, D.R.; Castaldo, N.; Vena, A. New Antibiotics for Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Semin Respir. Crit. Care Med. 2022, 43, 280–294. [Google Scholar] [CrossRef]
- Bennett, E.E.; VanBuren, J.; Holubkov, R.; Bratton, S.L. Presence of Invasive Devices and Risks of Healthcare-Associated Infections and Sepsis. J. Pediatr. Intensive Care 2018, 7, 188–195. [Google Scholar]
- de Hesselle, M.L.; Borgmann, S.; Rieg, S.; Vehreshild, J.J.; Spinner, C.D.; Koll, C.E.M.; Hower, M.; Stecher, M.; Ebert, D.; Hanses, F.; et al. Invasiveness of Ventilation Therapy Is Associated to Prevalence of Secondary Bacterial and Fungal Infections in Critically Ill COVID-19 Patients. J. Clin. Med. 2022, 11, 5239. [Google Scholar] [CrossRef]
- Onyedibe, K.; Shehu, N.; Igbanugo, J.; Okolo, M.; Gomerep, S.; Isa, S.; Egah, D. Hand hygiene knowledge, training and practice: A cross-sectional study in a tertiary health institution, North-central Nigeria. Niger. J. Clin. Pr. 2019, 22, 1008–1013. [Google Scholar] [CrossRef]
- Sarani, H.; Balouchi, A.; Masinaeinezhad, N.; Ebrahimitabas, E. Knowledge, Attitude and Practice of Nurses about Standard Precautions for Hospital-Acquired Infection in Teaching Hospitals Affiliated to Zabol University of Medical Sciences (2014). Glob. J. Health Sci. 2015, 8, 193–198. [Google Scholar] [CrossRef]
- Sharif, I.; Rashid, Z.; Tariq, N.; Mashhadi, F.; Din, M.; Wazir, L.; Dogar, M.A.; Asif, Y.; Jadoon, A. Gaps in knowledge and practices regarding nosocomial infections among nursing staff of a tertiary care hospital of Rawalpindi. PAFMJ 2019, 69, 1210–1225. [Google Scholar]
- Asfaw, N. Knowledge and practice of nurses towards prevention of hospital acquired infections and its associated factors. Int. J. Afr. Nurs. Sci. 2021, 15, 100333. [Google Scholar] [CrossRef]
- Zaidi, N.J.N.; Naz, S.; Mumtaz, A. Gaps in Knowledge and Practices About Health Care Associated Infections Among Health Care Workers at a Tertiary Care Hospital. J. Islamabad Med. Dent. Coll. (JIMDC). 2016, 5, 84–87. [Google Scholar]
- CDC. Types of Healthcare-Associated Infections. 2014. Available online: https://www.cdc.gov/hai/infectiontypes.html (accessed on 2 September 2022).
- Simpson, C.R.; Robertson, C.; Vasileiou, E.; Moore, E.; McCowan, C.; Agrawal, U.; Stagg, H.R.; Docherty, A.; Mulholland, R.; Murray, J.L.; et al. Temporal trends and forecasting of COVID-19 hospitalisations and deaths in Scotland using a national real-time patient-level data platform: A statistical modelling study. Lancet Digit. Health 2021, 3, e517–e525. [Google Scholar] [CrossRef]
- Sapiano, M.R.P.; Dudeck, M.A.; Soe, M.; Edwards, J.R.; O’Leary, E.N.; Wu, H.; Allen-Bridson, K.; Amor, A.; Arcement, R.; Tejedor, S.C.; et al. Impact of coronavirus disease 2019 (COVID-19) on US Hospitals and Patients, April-July 2020. Infect. Control Hosp. Epidemiol. 2022, 43, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Odone, A.; Delmonte, D.; Scognamiglio, T.; Signorelli, C. COVID-19 deaths in Lombardy, Italy: Data in context. Lancet Public Health 2020, 5, e310. [Google Scholar] [CrossRef]
- Peters, A.W.; Chawla, K.S.; Turnbull, Z.A. Transforming ORs into ICUs. N. Engl. J. Med. 2020, 382, e52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Mazzucchelli, R.; Agudo Dieguez, A.; Dieguez Costa, E.M.; Crespí Villarías, N. Democracy and COVID-19 mortality in Europe. Rev. Esp. Salud. Publica. 2020, 94, e202006073. [Google Scholar]
- Ng, Y.; Li, Z.; Chua, Y.X.; Chaw, W.L.; Zhao, Z.; Er, B.; Pung, R.; Chiew, C.J.; Lye, D.; Heng, D.; et al. Evaluation of the Effectiveness of Surveillance and Containment Measures for the First 100 Patients with COVID-19 in Singapore—January 2–February 29, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 307–311. [Google Scholar] [CrossRef]
- Thai, P.Q.; Rabaa, M.A.; Luong, D.H.; Tan, D.Q.; Quang, T.D.; Quach, H.-L.; Thi, H.N.-A.; Dinh, P.C.; Nghia, N.D.; Tu, T.A.; et al. The First 100 Days of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Control in Vietnam. Clin. Infect. Dis. 2021, 72, e334–e342. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Basu, D.; Mueller, D.; Sneddon, J.; Seaton, R.A.; Yinka-Ogunleye, A.F.; Wamboga, J.; Miljković, N.; Mwita, J.C.; Rwegerera, G.M.; et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharmacol. 2020, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, O.O.; Godman, B.; Fadare, J.O.; Mudenda, S.; Adeoti, A.O.; Yinka-Ogunleye, A.F.; Ogundele, S.O.; Oyawole, M.R.; Schönfeldt, M.; Rashed, W.M.; et al. Coronavirus Disease 2019 (COVID-19) Pandemic across Africa: Current Status of Vaccinations and Implications for the Future. Vaccines 2022, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Myatra, S.N.; Divatia, J.V.; Biswas, S.; Shrivastava, A.; Al-Ruzzieh, M.A.; Ayaad, O.; Bat-Erdene, A.; Bat-Erdene, I.; Narankhuu, B.; et al. The impact of COVID-19 on health care-associated infections in intensive care units in low- and middle-income countries: International Nosocomial In-fection Control Consortium (INICC) findings. Int. J. Infect. Dis. 2022, 118, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Porto, A.P.M.; Borges, I.C.; Buss, L.; Machado, A.; Bassetti, B.R.; Cocentino, B.; Bicalho, C.S.; Carrilho, C.M.; Rodrigues, C.; Neto, E.A.S.; et al. Healthcare-associated infections on the intensive care unit in 21 Brazilian hospitals during the early months of the coronavirus disease 2019 (COVID-19) pandemic: An ecological study. Infect. Control. Hosp. Epidemiology 2022, 1–7. [Google Scholar] [CrossRef]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.C.; Giannini, D.; Marotta, D.; Marte, M.; Alessandri, F.; Mazzalai, E.; et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control. 2021, 10, 87. [Google Scholar] [CrossRef]
- Ramzan, K.; Shafiq, S.; Raees, I.; Mustafa, Z.U.; Salman, M.; Khan, A.H.; Meyer, J.C.; Godman, B. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalized with COVID-19 during the First Five Waves of the Pandemic in Pakistan; Findings and Implications. Antibiotics 2022, 11, 789. [Google Scholar] [CrossRef]
- Salman, M.; Mustafa, Z.U.; Khan, T.M.; Shehzadi, N.; Hussain, K. How Prepared Was Pakistan for the COVID-19 Outbreak? Disaster Med. Public Health Prep. 2020, 14, e44–e45. [Google Scholar] [CrossRef]
- Kamran, S.H.; Ul Mustafa, Z.; Rao, A.Z.; Hasan, S.S.; Zahoor, F.; Sarwar, M.U.; Khan, S.; Butt, S.; Rameez, M.; Abbas, M.A. SARS-CoV-2 infection pattern, transmission and treatment: Multi-center study in low to middle-income districts hospitals in Punjab, Pakistan. Pak. J. Pharm. Sci. 2021, 34, 1135–1142. [Google Scholar]
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 2 September 2022).
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Saleem, M.S.; Ikram, M.N.; Salman, M.; Butt, S.A.; Khan, S.; Godman, B.; Seaton, R.A. Co-infections and antimicrobial use among hospitalized COVID-19 patients in Punjab, Pakistan: Findings from a multicenter, point prevalence survey. Pathog. Glob. Health 2021, 116, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, m1983. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Naghavi, M. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Bank Group. Pulling Together to Beat Superbugs Knowledge and Implementation Gaps in Addressing Antimicrobial Resistance. 2019. Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/32552/Pulling-Together-to-Beat-Superbugs-Knowledge-and-Implementation-Gaps-in-Addressing-Antimicrobial-Resistance.pdf?sequence=1&isAllowed=y (accessed on 2 September 2022).
- Zeshan, B.; Karobari, M.I.; Afzal, N.; Siddiq, A.; Basha, S.; Basheer, S.N.; Peeran, S.W.; Mustafa, M.; Daud, N.H.A.; Ahmed, N.; et al. The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance. Antibiotics 2021, 11, 35. [Google Scholar] [CrossRef]
- Ministry of National Health Services Regulations; Coordination Government of Pakistan. National AMR Action Plan for Pakistan. 2017. Available online: https://www.nih.org.pk/wp-content/uploads/2018/08/AMR-National-Action-Plan-Pakistan.pdf (accessed on 2 September 2022).
- Saleem, Z.; Hassali, M.A.; Hashmi, F.K. Pakistan’s national action plan for antimicrobial resistance: Translating ideas into reality. Lancet Infect Dis. 2018, 18, 1066–1067. [Google Scholar] [CrossRef]
- Saleem, Z.; Godman, B.; Azhar, F.; Kalungia, A.C.; Fadare, J.; Opanga, S.; Markovic-Pekovic, V.; Hoxha, I.; Saeed, A.; Al-Gethamy, M. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti-Infect. Ther. 2022, 20, 71–93. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Wang, Z.; Chen, H.; Tian, L.; Liu, D. Nosocomial infection among patients with COVID-19: A retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect. Control Hosp. Epidemiol. 2020, 41, 982–983. [Google Scholar] [CrossRef]
- Ong, C.C.H.; Farhanah, S.; Linn, K.Z.; Tang, Y.W.; Poon, C.Y.; Lim, A.Y.; Tan, H.R.; Hamed, N.H.B.; Huan, X.; Puah, S.H.; et al. Nosocomial infections among COVID-19 patients: An analysis of intensive care unit surveillance data. Antimicrob. Resist. Infect. Control. 2021, 10, 119. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2020, 76, 1078–1084. [Google Scholar] [CrossRef]
- De Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; De Pauw, I.; Stessel, B.; Dubois, J. Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation. BMC Infect. Dis. 2022, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Ihsan, B.; Malik, I.; Ahmad, N.; Saleem, Z.; Sehar, A.; Baber, Z.U. Antibiotic stewardship program in Pakistan: A multicenter qualitative study exploring medical doctors’ knowledge, perception and practices. BMC Infect. Dis. 2021, 21, 374. [Google Scholar] [CrossRef]
- Niaz, Q.; Godman, B.; Campbell, S.; Kibuule, D. Compliance to prescribing guidelines among public health care facilities in Namibia; findings and implications. Int. J. Clin. Pharm. 2020, 42, 1227–1236. [Google Scholar] [CrossRef]
- Campbell, S.M.; Meyer, J.; Godman, B. Why Compliance to National Prescribing Guidelines is Important Especially across Sub-Saharan Africa and Suggestions for the Future. Biomed. Pharm. Sci. 2021, 4, 1–7. [Google Scholar]
- Meawed, T.E.; Ahmed, S.M.; Mowafy, S.M.S.; Samir, G.M.; Anis, R.H. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J. Infect. Public Health 2021, 14, 1375–1380. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care. 2021, 25, 25. [Google Scholar] [CrossRef]
- Russo, A.; Olivadese, V.; Trecarichi, E.M.; Torti, C. Bacterial Ventilator-Associated Pneumonia in COVID-19 Patients: Data from the Second and Third Waves of the Pandemic. J. Clin. Med. 2022, 11, 2279. [Google Scholar] [CrossRef]
- Kumar, G.; Adams, A.; Hererra, M.; Rojas, E.R.; Singh, V.; Sakhuja, A.; Meersman, M.; Dalton, D.; Kethireddy, S.; Nanchal, R.; et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int. J. Infect. Dis. 2020, 104, 287–292. [Google Scholar] [CrossRef]
- Smith, L.; Karaba, S.M.; Amoah, J.; Jones, G.; Avery, R.K.; Dzintars, K.; Helsel, T.; Cosgrove, S.E.; Fabre, V.; Hesel, T.; et al. Hospital-acquired infections among adult patients admitted for coronavirus disease 2019 (COVID-19). Infect. Control Hosp. Epidemiol. 2022, 43, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE. 2022, 17, e0272375. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; I de Silva, T.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Haque, M.; Shetty, A.; Acharya, J.; Kumar, M.; Sinha, V.; Manohar, B.; Gowere, M.; Godman, B. Current management of children with COVID-19 in hospitals in India; Pilot study and findings. Adv. Hum. Biol. 2022, 12, 16–21. [Google Scholar]
- Chowdhury, K.; Haque, M.; Nusrat, N.; Adnan, N.; Islam, S.; Lutfor, A.B.; Begum, D.; Rabbany, A.; Karim, E.; Malek, A.; et al. Management of Children Admitted to Hospitals across Bangladesh with Suspected or Confirmed COVID-19 and the Implications for the Future: A Nationwide Cross-Sectional Study. Antibiotics 2022, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, N.; Haque, M.; Chowdhury, K.; Adnan, N.; Lutfor, A.B.; Karim, E.; Hassan, M.; Rabbany, A.; Begum, D.; Hasan, M.N.; et al. Pilot Study on the Current Management of Children with COVID-19 In Hospitals in Bangladesh; Findings and Implications. Bangladesh J. Med Sci. 2021, 188–198. [Google Scholar] [CrossRef]
- Kumar, S.; Haque, M.; Shetty, A.; Choudhary, S.; Bhatt, R.; Sinha, V.; Manohar, B.; Chowdhury, K.; Nusrat, N.; Jahan, N.; et al. Characteristics and Management of Children With Suspected COVID-19 Admitted to Hospitals in India: Implications for Future Care. Cureus 2022, 14, e27230. [Google Scholar] [CrossRef]
- Paramadhas, B.D.A.; Tiroyakgosi, C.; Mpinda-Joseph, P.; Morokotso, M.; Matome, M.; Sinkala, F.; Gaolebe, M.; Malone, B.; Molosiwa, E.; Shanmugam, M.G.; et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert. Rev. Anti. Infect Ther. 2019, 17, 535–546. [Google Scholar] [CrossRef]
- Government of Pakistan; Ministry of National Health Services, Regulations and Coordination. Clinical Management Guidelines for COVID-19 Infections. 2020. Available online: https://nhsrc.gov.pk/SiteImage/Misc/files/20200704%20Clinical%20Management%20Guidelines%20for%20COVID-19%20infections_1203.pdf (accessed on 3 September 2022).
- ECDC. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial use in European Acute Care Hospitals—ECDC PPS Validation Protocol Version 3.1.2. 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/PPS-HAI-AMR-protocol.pdf (accessed on 3 September 2022).
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital Acquired Infections; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Boev, C.; Kiss, E. Hospital-Acquired Infections: Current Trends and Prevention. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65. [Google Scholar] [CrossRef]
- ECDC. Healthcare-Associated Infections. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections (accessed on 3 September 2022).
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 1988, 16, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect Control. 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Momanyi, L.; Opanga, S.; Nyamu, D.; Oluka, M.; Kurdi, A.; Godman, B. Antibiotic Prescribing Patterns at a Leading Referral Hospital in Kenya: A Point Prevalence Survey. J. Res. Pharm. Pract. 2019, 8, 149–154. [Google Scholar] [PubMed]
- Mustafa, Z.U.; Salman, M.; Yasir, M.; Godman, B.; Majeed, H.A.; Kanwal, M.; Iqbal, M.; Riaz, M.B.; Hayat, K.; Hasan, S.S. Antibiotic consumption among hospitalized neonates and children in Punjab province, Pakistan. Expert. Rev. Anti. Infect Ther. 2022, 20, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Z.U.; Salman, M.; Aslam, N.; Asif, N.; Hussain, K.; Shehzadi, N.; Hayat, K. Antibiotic use among hospitalized children with lower respiratory tract infections: A multicenter, retrospective study from Punjab, Pakistan. Expert. Rev. Anti. Infect Ther. 2022, 20, 131–136. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Hashmi, F.K.; Saleem, F. A multicenter point prevalence survey of healthcare-associated infections in Pakistan: Findings and implications. Am. J. Infect. Control. 2019, 47, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Skosana, P.P.; Schellack, N.; Godman, B.; Kurdi, A.; Bennie, M.; Kruger, D.; Mayer, J.C. A national, multicentre web-based point prevalence survey of antimicrobial use in community healthcare centres across South Africa and the implications. Hosp. Pract. 2022, 50, 306–317. [Google Scholar] [CrossRef]

| Variable | N (%) | p-Value | ||
|---|---|---|---|---|
| Overall | HAI (N = 62) | No HAI (N = 574) | ||
| Age (years) | 0.039 | |||
| <30 | 21 (31.3) | 0 (0.0) | 21 (3.7) | |
| 31–50 | 130 (20.4) | 7 (11.3) | 123 (21.5) | |
| >50 | 485 (76.3) | 55 (88.7) | 430 (74.9) | |
| Sex | 0.784 * | |||
| Male | 398 (62.6) | 40 (64.5) | 358 (62.4) | |
| Female | 238 (37.4) | 22 (35.5) | 216 (37.6) | |
| Residence | 0.673 * | |||
| Rural | 423 (66.5) | 43 (69.4) | 380 (66.2) | |
| Urban | 213 (33.5) | 19 (30.6) | 164 (33.8) | |
| Comorbidities | ||||
| Diabetes | 142 (22.3) | 16 (25.8) | 126 (22.0) | 0.521 * |
| Hypertension | 127 (20.0) | 13 (21.0) | 114 (19.9) | 0.867 * |
| Heart disease | 64 (10.1) | 11 (17.7) | 53 (9.2) | 0.044 * |
| Respiratory disorder | 38 (6.0) | 4 (6.5) | 34 (5.9) | 0.780 * |
| Other † | 10 (1.6) | 1 (1.6) | 9 (1.6) | 1.000 * |
| Symptoms at presentation | 0.144 | |||
| Fever + cough | 24 (3.8) | 4 (6.5) | 20 (3.5) | |
| Fever + myalgia | 65 (10.2) | 5 (8.1) | 60 (10.5) | |
| Fever + sore throat | 100 (15.7) | 5 (8.1) | 95 (16.6) | |
| Fever + dyspnea | 122 (19.2) | 7 (11.3) | 115 (20.0) | |
| Fever + cough + dyspnea | 97 (15.3) | 13 (21.0) | 84 (14.6) | |
| Fever + sore throat + body ache | 59 (9.3) | 7 (11.3) | 52 (9.1) | |
| More than three symptoms | 169 (26.6) | 21 (33.9) | 148 (25.8) | |
| COVID severity †† | <0.001 | |||
| Moderate | 43 (6.8) | 1 (1.6) | 42 (7.3) | |
| Severe | 441 (69.3) | 12 (19.4) | 429 (74.7) | |
| Critical | 152 (23.9) | 49 (79.0) | 103 (17.9) | |
| Laboratory findings †† | ||||
| Abnormal X-ray | 591 (92.9) | 61 (98.4) | 530 (92.3) | 0.112 * |
| Abnormal WBC | 497 (78.1) | 56 (90.3) | 441 (76.8) | 0.014 * |
| Abnormal CRP | 462 (72.6) | 56 (90.3) | 406 (70.7) | 0.001 * |
| Abnormal D-Dimer | 432 (67.9) | 51 (82.3) | 381 (66.4) | 0.010 * |
| Abnormal Serum ferritin | 320 (50.3) | 46 (74.2) | 274 (47.7) | <0.001 * |
| Oxygen therapy | 0.150* | |||
| Yes | 582 (91.5) | 60 (96.8) | 522 (90.9) | |
| No †† | 54 (8.5) | 2 (3.2) | 52 (9.1) | |
| Medications | ||||
| Antipyretic ** | 636 (100.0) | 62 (100.0) | 574 (100.0) | -- |
| Antihistamine | 476 (74.8) | 51 (82.3) | 425 (74.0) | 0.169 * |
| Anticoagulant | 506 (79.6) | 58 (93.5) | 448 (78.0) | 0.003 * |
| Antithrombotic | 92 (14.5) | 30 (48.4) | 62 (10.8) | <0.001 * |
| Antitussive | 377 (59.3) | 39 (62.9) | 338 (58.9) | 0.588 * |
| Corticosteroid ** | 636 (100.0) | 62 (100.0) | 574 (100.0) | -- |
| Antibiotic ** | 636 (100.0) | 62 (100.0) | 574 (100.0) | -- |
| Antiviral (remdesivir) | 364 (57.2) | 52 (83.9) | 312 (54.4) | <0.001 * |
| IL-6 inhibitor (tocilizumab) | 73 (11.5) | 50 (80.6) | 23 (4.0) | <0.001 * |
| Vitamin | 362 (56.9) | 37 (59.7) | 325 (56.6) | 0.687 * |
| Devices used in the ICU | ||||
| Invasive mechanical ventilation ††† | 111 (17.5) | 37 (59.7) | 74 (12.9) | <0.001 * |
| Central venous catheter | 7 (1.1) | 3 (4.8) | 4 (0.7) | 0.023 * |
| Urinary catheter | 157 (24.7) | 28 (45.2) | 129 (22.5) | <0.001 * |
| Orotracheal tube | 117 (18.4) | 17 (27.4) | 100 (17.4) | 0.59 * |
| Covariates | B | SE | Wald | df | p-Value | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Age * | −0.589 | 0.623 | 0.894 | 1 | 0.345 | 0.555 | 0.163 | 1.882 |
| Heart disease | −1.031 | 0.629 | 2.686 | 1 | 0.101 | 0.357 | 0.104 | 1.224 |
| COVID Severity † | ||||||||
| Moderate (Reference) | ||||||||
| Severe | −0.423 | 1.171 | 0.131 | 1 | 0.718 | 0.655 | 0.066 | 6.503 |
| Critical | 1.772 | 1.290 | 1.887 | 1 | 0.169 | 5.884 | 0.469 | 73.748 |
| Anticoagulant use | −0.303 | 0.813 | 0.139 | 1 | 0.710 | 0.739 | 0.150 | 3.633 |
| Antithrombotic agents | −0.332 | 0.562 | 0.350 | 1 | 0.554 | 0.717 | 0.238 | 2.158 |
| Antiviral | 0.002 | 0.694 | 0.000 | 1 | 0.998 | 1.002 | 0.257 | 3.905 |
| Tocilizumab †† | 4.437 | 0.482 | 84.920 | 1 | <0.001 | 84.559 | 32.906 | 217.291 |
| Invasive mechanical ventilation | 0.600 | 0.655 | 0.839 | 1 | 0.360 | 1.822 | 0.505 | 6.576 |
| Central venous line | 1.066 | 1.565 | 0.464 | 1 | 0.496 | 2.904 | 0.135 | 62.450 |
| Urinary catheter | 1.124 | 0.451 | 6.223 | 1 | 0.013 | 3.077 | 1.272 | 7.443 |
| Outcome | N (%) | p-Value | ||
|---|---|---|---|---|
| Total | HAI (N = 62) | No HAI (N = 574) | ||
| Length of ICU stay (days) | <0.001 | |||
| ≤7 | 44 (6.9) | 1 (1.6) | 43 (7.5) | |
| 8–14 | 69 (10.8) | 3 (4.8) | 66 (11.5) | |
| 15–21 | 226 (35.5) | 9 (14.5) | 217 (37.8) | |
| 22–29 | 159 (25.0) | 13 (21.0) | 146 (25.4) | |
| ≥30 | 138 (21.7) | 36 (58.1) | 102 (17.8) | |
| Outcome † | <0.001 * | |||
| Discharged alive | 613 (96.4) | 46 (74.2) | 587 (98.8) | |
| Deceased | 23 (3.6) | 16 (25.8) | 7 (1.2) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, Z.U.; Tariq, S.; Iftikhar, Z.; Meyer, J.C.; Salman, M.; Mallhi, T.H.; Khan, Y.H.; Godman, B.; Seaton, R.A. Predictors and Outcomes of Healthcare-Associated Infections among Patients with COVID-19 Admitted to Intensive Care Units in Punjab, Pakistan; Findings and Implications. Antibiotics 2022, 11, 1806. https://doi.org/10.3390/antibiotics11121806
Mustafa ZU, Tariq S, Iftikhar Z, Meyer JC, Salman M, Mallhi TH, Khan YH, Godman B, Seaton RA. Predictors and Outcomes of Healthcare-Associated Infections among Patients with COVID-19 Admitted to Intensive Care Units in Punjab, Pakistan; Findings and Implications. Antibiotics. 2022; 11(12):1806. https://doi.org/10.3390/antibiotics11121806
Chicago/Turabian StyleMustafa, Zia Ul, Sania Tariq, Zobia Iftikhar, Johanna C. Meyer, Muhammad Salman, Tauqeer Hussain Mallhi, Yusra Habib Khan, Brian Godman, and R. Andrew Seaton. 2022. "Predictors and Outcomes of Healthcare-Associated Infections among Patients with COVID-19 Admitted to Intensive Care Units in Punjab, Pakistan; Findings and Implications" Antibiotics 11, no. 12: 1806. https://doi.org/10.3390/antibiotics11121806
APA StyleMustafa, Z. U., Tariq, S., Iftikhar, Z., Meyer, J. C., Salman, M., Mallhi, T. H., Khan, Y. H., Godman, B., & Seaton, R. A. (2022). Predictors and Outcomes of Healthcare-Associated Infections among Patients with COVID-19 Admitted to Intensive Care Units in Punjab, Pakistan; Findings and Implications. Antibiotics, 11(12), 1806. https://doi.org/10.3390/antibiotics11121806










