Cutibacterium acnes Prosthetic Joint Infections: Is Rifampicin-Combination Therapy Beneficial?
Abstract
1. Introduction
2. Results
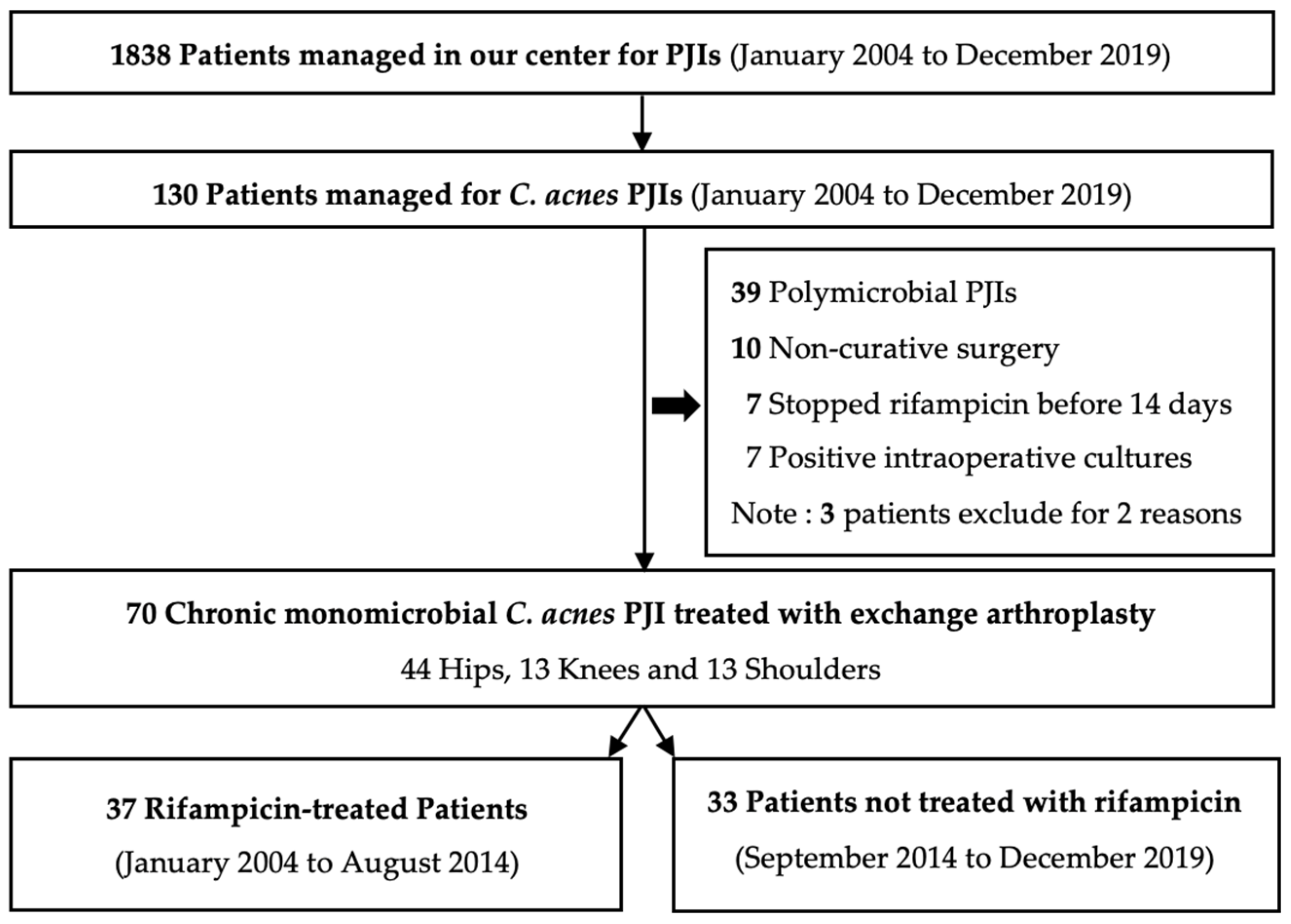
2.1. Population
2.2. Antibiotics and Surgery
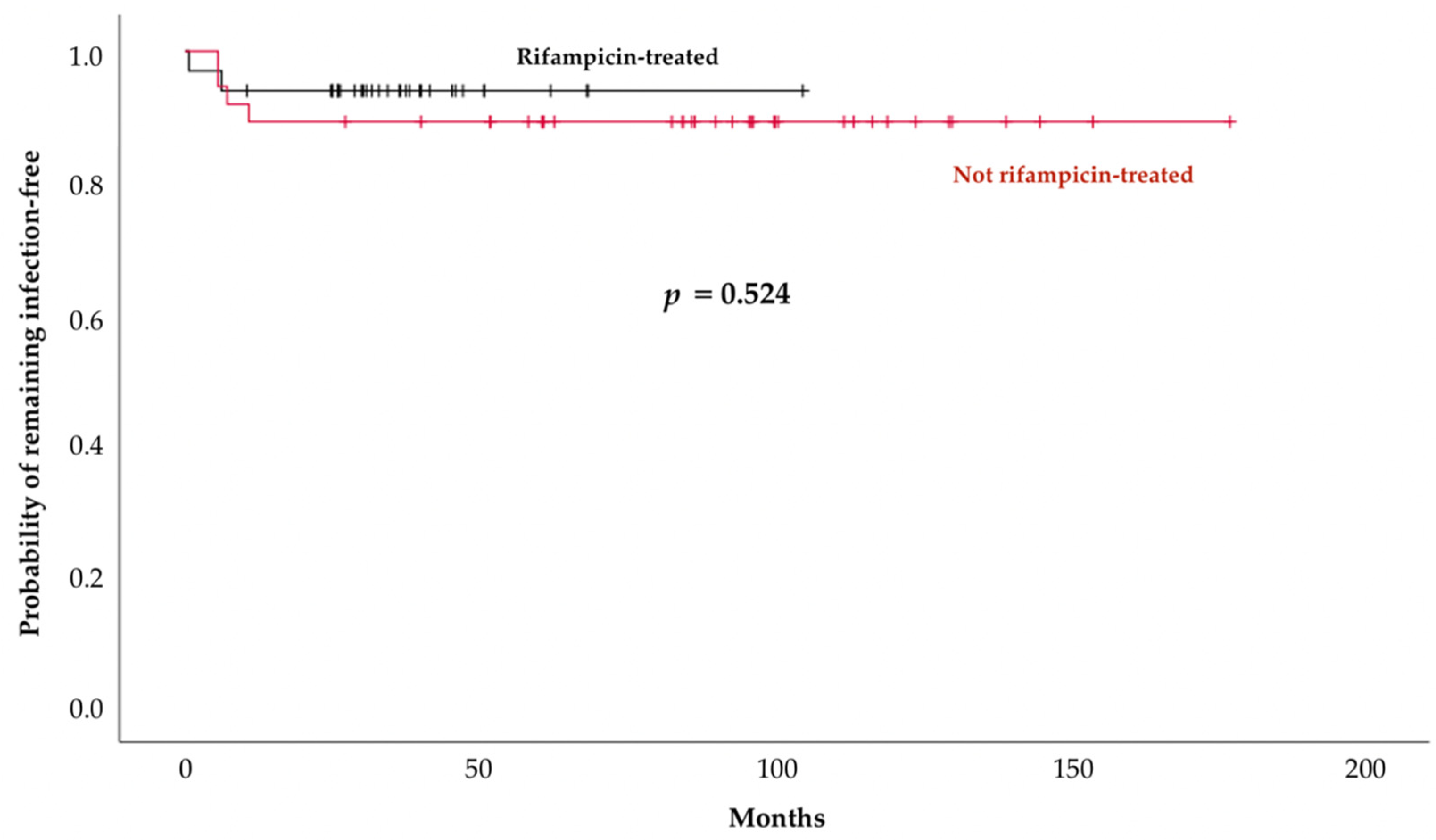
2.3. Outcomes
2.4. Treatment-Related Adverse Events
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Medical and Surgical Treatments
4.3. Outcome Measures
4.4. Statistical Analyses
4.5. Ethics Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholz, C.F.P.; Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef]
- Aubin, G.G.; Portillo, M.E.; Trampuz, A.; Corvec, S. Propionibacterium acnes, an emerging pathogen: From acne to implant-infections, from phylotype to resistance. Med. Et Mal. Infect. 2014, 44, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Banzon, J.M.; Rehm, S.J.; Gordon, S.M.; Hussain, S.T.; Pettersson, G.B.; Shrestha, N.K. Propionibacterium acnes endocarditis: A case series. Clin. Microbiol. Infect. 2017, 23, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Tattevin, P.; Watt, G.; Revest, M.; Arvieux, C.; Fournier, P.E. Update on blood culture-negative endocarditis. Med. Et Mal. Infect. 2015, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Lien, C.Y.; Chien, C.C.; Huang, C.R.; Tsai, N.W.; Chang, C.C.; Lu, C.H.; Chang, W.N. Anaerobic bacterial meningitis in adults. J. Clin. Neurosci. 2018, 50, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Conen, A.; Walti, L.N.; Merlo, A.; Fluckiger, U.; Battegay, M.; Trampuz, A. Characteristics and Treatment Outcome of Cerebrospinal Fluid Shunt–Associated Infections in Adults: A Retrospective Analysis over an 11-Year Period. Clin. Infect. Dis. 2008, 47, 73–82. [Google Scholar] [CrossRef]
- Vafidis, G. Propionibacterium acnes endophthalmitis. Br. J. Ophthalmol. 1991, 75, 706. [Google Scholar] [CrossRef][Green Version]
- Putman, S.; Girier, N.; Girard, J.; Pasquier, G.; Migaud, H.; Chazard, E. Épidémiologie des prothèses de hanche en France: Analyse de la base nationale du PMSI de 2008 à 2014. Rev. De Chir. Orthop. Et Traumatol. 2017, 103, S90. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic Burden of Periprosthetic Joint Infection in the United States. J. Arthroplast. 2012, 27, 61–65.e1. [Google Scholar] [CrossRef]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorlí, L.; Fresco, G.; Fernández-Sampedro, M.; Del Toro, M.D.; Guío, L.; et al. Time trends in the aetiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.E1–732.E8. [Google Scholar] [CrossRef]
- Triffault-Fillit, C.; Ferry, T.; Laurent, F.; Pradat, P.; Dupieux, C.; Conrad, A.; Becker, A.; Lustig, S.; Fessy, M.H.; Chidiac, C.; et al. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: A prospective cohort study. Clin. Microbiol. Infect. 2019, 25, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Kerroumi, Y.; Meyssonnier, V.; Heym, B.; Metten, M.A.; Desplaces, N.; Marmor, S. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J. Infect. 2018, 76, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.; Metcalf, S.; Clark, B.; Robinson, J.O.; Huggan, P.; Luey, C.; McBride, S.; Aboltins, C.; Nelson, R.; Campbell, D. Clinical Characteristics, Etiology, and Initial Management Strategy of Newly Diagnosed Periprosthetic Joint Infection: A Multicenter, Prospective Observational Cohort Study of 783 Patients. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2020; Volume 7, p. ofaa068. [Google Scholar]
- Flurin, L.; Greenwood-Quaintance, K.E.; Patel, R. Microbiology of polymicrobial prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2019, 94, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Ghorbani, A.; Strady, C.; Leonard, P.; Mamoudy, P.; Desplaces, N. Propionibacterium acnes: An agent of prosthetic joint infection and colonization. J. Infect. 2007, 55, 119–124. [Google Scholar] [CrossRef]
- Renz, N.; Mudrovcic, S.; Perka, C.; Trampuz, A. Orthopedic implant-associated infections caused by Cutibacterium spp.—A remaining diagnostic challenge. PLoS ONE 2018, 13, e0202639. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Executive Summary: Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1–10. [Google Scholar] [CrossRef]
- Societé de Pathologie Infectieuse de Langue Française. Recommandations de Pratique Clinique Infections ostéo-articulaires sur matériel (Prothèse, Implant, Ostéo-Synthèse). Available online: https://www.infectiologie.com/UserFiles/File/spilf/recos/inf-osseuse-long.pdf (accessed on 13 May 2009).
- Boyle, K.K.; Kuo, F.C.; Horcajada, J.P.; Hughes, H.; Cavagnaro, L.; Marculescu, C.; McLaren, A.; Nodzo, S.R.; Riccio, G.; Sendi, P.; et al. General Assembly, Treatment, Antimicrobials: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S225–S237. [Google Scholar] [CrossRef]
- Achermann, Y.; Goldstein, E.J.C.; Coenye, T.; Shirtliff, M.E. Propionibacterium acnes: From Commensal to Opportunistic Biofilm-Associated Implant Pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef]
- Oprica, C.; Nord, C.E. European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin. Microbiol. Infect. 2005, 11, 204–213. [Google Scholar] [CrossRef]
- Khassebaf, J.; Hellmark, B.; Davidsson, S.; Unemo, M.; Nilsdotter-Augustinsson, Å.; Söderquist, B. Antibiotic susceptibility of Propionibacterium acnes isolated from orthopaedic implant-associated infections. Anaerobe 2015, 32, 57–62. [Google Scholar] [CrossRef]
- Furustrand Tafin, U.; Trampuz, A.; Corvec, S. In vitro emergence of rifampicin resistance in Propionibacterium acnes and molecular characterization of mutations in the rpoB gene. J. Antimicrob. Chemother. 2013, 68, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Furustrand Tafin, U.; Corvec, S.; Betrisey, B.; Zimmerli, W.; Trampuz, A. Role of Rifampin against Propionibacterium acnes Biofilm In Vitro and in an Experimental Foreign-Body Infection Model. Antimicrob. Agents Chemother. 2012, 56, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.M.E.; Van Hooff, M.L.; Meis, J.F.; Vos, F.; Goosen, J.H.M. Treatment of prosthetic joint infections due to Propionibacterium: Similar results in 60 patients treated with and without rifampicin. Acta Orthop. 2016, 87, 60–66. [Google Scholar] [CrossRef]
- Piggott, D.A.; Higgins, Y.M.; Melia, M.T.; Ellis, B.; Carroll, K.C.; McFarland, E.G.; Auwaerter, P.G. Characteristics and Treatment Outcomes of Propionibacterium acnes Prosthetic Shoulder Infections in Adults. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2015; Volume 3, p. ofv191. [Google Scholar]
- Vilchez, H.; Escudero-Sanchez, R.; Fernandez-Sampedro, M.; Murillo, O.; Auñón, Á.; Rodríguez-Pardo, D.; Jover-Sáenz, A.; Dolores del Tolo, M.; Rico, A.; Falgueras, L.; et al. Prosthetic Shoulder Joint Infection by Cutibacterium acnes: Does Rifampin Improve Prognosis? A Retrospective, Multicenter, Observational Study. Antibiotics 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Kusejko, K.; Auñón, Á.; Jost, B.; Natividad, B.; Strahm, C.; Thurnheer, C.; Pablo-Marcos, D.; Slama, D.; Scanferla, G.; Uckay, I.; et al. The Impact of Surgical Strategy and Rifampin on Treatment Outcome in Cutibacterium Periprosthetic Joint Infections. Clin. Infect. Dis. 2021, 72, e1064–e1073. [Google Scholar] [CrossRef]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections: A review. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Zimmerli, W.; Widmer, A.F.; Blatter, M.; Frei, R.; Ochsner, P.E. Role of Rifampin for Treatment of Orthopedic Implant–Related Staphylococcal Infections A Randomized Controlled Trial. JAMA 1998, 279, 1537–1541. [Google Scholar] [CrossRef]
- Renz, N.; Trampuz, A.; Zimmerli, W. Controversy about the Role of Rifampin in Biofilm Infections: Is It Justified? Antibiotics 2021, 10, 165. [Google Scholar] [CrossRef]
- Grosset, J.; Leventis, S. Adverse Effects of Rifampin. Clin. Infect. Dis. 1983, 5 (Suppl. S3), S440–S446. [Google Scholar] [CrossRef]
- Nguyen, S.; Robineau, O.; Titecat, M.; Blondiaux, N.; Valette, M.; Loiez, C.; Beltrand, E.; Migaud, H.; Senneville, E. Influence of daily dosage and frequency of administration of rifampicin–levofloxacin therapy on tolerance and effectiveness in 154 patients treated for prosthetic joint infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1675–1682. [Google Scholar] [CrossRef]
- Finch, C.K.; Chrisman, C.R.; Baciewicz, A.M.; Self, T.H. Rifampin and Rifabutin Drug Interactions: An Update. Arch. Intern. Med. 2002, 162, 985. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Dzeing-Ella, A.; Kitzis, M.D.; Ziza, J.M.; Mamoudy, P.; Desplaces, N. Continuous Clindamycin Infusion, an Innovative Approach to Treating Bone and Joint Infections. Antimicrob. Agents Chemother. 2010, 54, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Magreault, S.; Heym, B.; Salmon, D.; Kitzis, M.D.; Billaud, E.; Marmor, S.; Jannot, A.S.; Salomon, L.; Jullien, V. Influence of the clindamycin administration route on the magnitude of clindamycin–rifampicin interaction: A prospective pharmacokinetic study. Clin. Microbiol. Infect. 2021, 27, e1–e1857. [Google Scholar] [CrossRef]
- Zeller, V.; Lhotellier, L.; Marmor, S.; Leclerc, P.; Krain, A.; Graff, W.; Ducroquet, F.; Biau, D.; Leonard, P.; Desplaces, N. One-Stage Exchange Arthroplasty for Chronic Periprosthetic Hip Infection: Results of a Large Prospective Cohort Study. J. Bone Jt. Surg. 2014, 96, e1. [Google Scholar] [CrossRef]
- Available online: https://solidarites-sante.gouv.fr/systeme-de-sante-et-medico-social/innovation-et-recherche/l-innovation-et-la-recherche-clinique/appels-a-projets/programmes-recherche? (accessed on 21 July 2022).
- Boselli, E.; Allaouchiche, B. Diffusion in bone tissue of antibiotics. Presse Med. 1999, 28, 2265–2276. [Google Scholar] [PubMed]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef]
- Vollmer, N.J.; Rivera, C.G.; Stevens, R.W.; Oravec, C.P.; Mara, K.C.; Suh, G.A.; Osmon, D.R.; Beam, E.N.; Abdel, M.P.; Virk, A. Safety and Tolerability of Fluoroquinolones in Patients with Staphylococcal Periprosthetic Joint Infections. Clin. Infect. Dis. 2021, 73, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Seng, P.; Mainard, D.; Jenny, J.Y.; Laurent, F.; Senneville, E.; Grare, M.; Jolivet-Gougeon, A.; Bernard, L.; Marmor, S. The CRIOAc healthcare network in France: A nationwide Health Ministry program to improve the management of bone and joint infection. Orthop. Traumatol. Surg. Res. 2019, 105, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef]
- Obremskey, W.T.; Metsemakers, W.J.; Schlatterer, D.R.; Tetsworth, K.; Egol, K.; Kates, S.; McNally, M. Musculoskeletal Infection in Orthopaedic Trauma: Assessment of the 2018 International Consensus Meeting on Musculoskeletal Infection. J. Bone Jt. Surg. 2020, 102, e44. [Google Scholar] [CrossRef]
- Zeller, V.A.; Letembet, V.A.; Meyssonnier, V.A.; Heym, B.; Ziza, J.M.; Marmor, S.D. Cutibacterium (Formerly Propionibacterium) avidum: A Rare but Avid Agent of Prosthetic Hip Infection. J. Arthroplast. 2018, 33, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Societé Francaise de Microbiologie. Comité de l’antibiogramme de la Société Française de Microbiologie. Available online: https://www.sfm-microbiologie.org/wp-content/uploads/2022/05/CASFM2022_V1.0.pdf? (accessed on 30 May 2022).
- Tsukayama, D.T.; Estrada, R.; Gustilo, R.B. Infection after Total Hip Arthroplasty. A Study of the Treatment of One Hundred and Six Infections. J. Bone Jt. Surg. 1996, 78, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Durand, F.; Kitzis, M.D.; Lhotellier, L.; Ziza, J.M.; Mamoudy, P.; Desplaces, N. Continuous Cefazolin Infusion To Treat Bone and Joint Infections: Clinical Efficacy, Feasibility, Safety, and Serum and Bone Concentrations. Antimicrob. Agents Chemother. 2009, 53, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Dubée, V.; Zeller, V.; Lhotellier, L.; Kitzis, M.D.; Ziza, J.M.; Mamoudy, P.; Desplaces, N. Continuous high-dose vancomycin combination therapy for methicillin-resistant staphylococcal prosthetic hip infection: A prospective cohort study. Clin. Microbiol. Infect. 2013, 19, E98–E105. [Google Scholar] [CrossRef]
- Trotti, A.; Colevas, A.; Setser, A.; Rusch, V.; Jaques, D.; Budach, V.; Langer, C.; Murphy, B.; Cumberlin, R.; Coleman, C.N.; et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. In Seminars in Radiation Oncology; WB Saunders: Philadelphia, PA, USA, 2003; Volume 13, pp. 176–181. [Google Scholar]
| All | Rifampicin-Treated | Not Rifampicin-Treated | p-Value | |
|---|---|---|---|---|
| Characteristics | n = 70 | n = 37 | n = 33 | |
| Age, years, median [IQR] | 69 (62–76) | 70 (59–77) | 69 (66–76) | 0.937 |
| Male, n (%) | 50 (71) | 27 (73) | 23 (70) | 0.796 |
| Female, n (%) | 20 (29) | 10 (27) | 10 (30) | 0.796 |
| Body mass index, kg/m2, median [IQR] | 25 (23–29) | 25 (22–29) | 26 (23–29) | 0.676 |
| ASA score >2, n (%) | 22 (31) | 7 (19) | 15 (45) | 0.022 |
| Comorbidities, n (%) | ||||
| Immunosuppressive treatment | 2 (3) | 0 | 2 (6) | 0.219 |
| Active neoplasia | 3 (4) | 3 (8) | 0 | 0.242 |
| Diabetes mellitus | 10 (14) | 4 (11) | 6 (18) | 0.499 |
| Renal insufficiency (CrCl <60 mL/min) | 2 (3) | 1 (3) | 1 (3) | 1.000 |
| PJI characteristics, n (%) | ||||
| Hip | 44 (63) | 23 (62) | 21 (64) | 1.000 |
| Knee | 13 (19) | 10 (27) | 3 (9) | 0.069 |
| Shoulder | 13 (19) | 4 (11) | 9 (27) | 0.123 |
| Initial classification, n (%) | ||||
| Early post-operative | 6 (9) | 4 (11) | 2 (6) | 0.677 |
| Late chronic | 56 (80) | 27 (73) | 29 (88) | 0.144 |
| Not determined | 8 (11) | 6 (16) | 2 (6) | 0.266 |
| Prior surgeries, n (%) | ||||
| 1 | 39 (56) | 16 (43) | 23 (70) | 0.032 |
| ≥2 | 31 (44) | 21 (57) | 10 (30) | 0.032 |
| Previous on-joint PJI, n (%) | 6 (9) | 4 (11) | 2 (6) | 0.677 |
| Symptom duration before admission to our center, months, median (IQR) | 12 (5–30) | 14 (5–36) | 12 (5–26) | 0.824 |
| All | Rifampicin- Treated | Not Rifampicin-Treated | p-Value | |
|---|---|---|---|---|
| Treatments and Outcomes | n = 70 | n = 37 | n = 33 | |
| Antibiotic therapy | ||||
| IV administration * | ||||
| Rifampicin, n (%) | 37 (53) | 37 (100) | — | NA |
| Duration, days, median [IQR] | 28 (25–41) | 28 (25–41) | — | NA |
| Clindamycin, n (%) | 49 (70) | 19 (51) | 30 (91) | <0.0001 |
| Duration, days, median [IQR] | 20 (14–28) | 28 (26–30) | 14 (11–21) | 0.583 |
| Cefazolin, n (%) | 34 (49) | 21 (57) | 13 (39) | 0.161 |
| Duration, days, median [IQR] | 29 (10–42) | 40 (29–43) | 7 (6–13) | <0.0001 |
| Vancomycin, n (%) | 16 (23) | 3 (8) | 13 (39) | 0.003 |
| Duration, days, median [IQR] | 7 (6–11) | 6 (4–9) | 7 (6–11) | 0.306 |
| Oral intake * | ||||
| Rifampicin, n (%) | 5 (7) | 5 (14) | — | NA |
| Duration, days, median [IQR] | 42 (42–63) | 42 (42–63) | — | NA |
| Clindamycin, n (%) | 56 (80) | 29 (78) | 27 (82) | 0.772 |
| Amoxicillin, n (%) | 6 (9) | 1 (3) | 5 (15) | 0.193 |
| Cefalexin, n (%) | 6 (9) | 5 (14) | 1 (3) | 0.203 |
| None, n (%) | 2 (3) | 2 (5) | 0 | NA |
| Total duration | ||||
| Antibiotics, days, median [IQR] | 84 (43–85) | 84 (84–91) | 43 (42–84) | <0.0001 |
| IV antibiotics, days, median [IQR] | 28 (19–34) | 30 (28–42) | 19 (14–26) | <0.0001 |
| Surgery, n (%) | ||||
| 1-stage replacement | 62 (89) | 29 (78) | 33 (100) | 0.006 |
| 2-stage replacement | 8 (11) | 8 (22) | 0 | 0.006 |
| Outcomes | ||||
| Follow-up duration, months, median [IQR] | 60 (35–99) | 95 (71–125) | 36 (26–45) | <0.0001 |
| Patients lost-to-follow-up <24 months, n (%) | 1 (1) | 0 | 1 (3) | NA |
| Reinfections, n (%) | 6 (9) | 4 (11) | 2 (6) | 0.677 |
| Relapses | 0 | 0 | 0 | NA |
| New infections | 6 (9) | 4 (11) | 2 (6) | 0.677 |
| After 1-stage replacement | 3 (4) | 1 (3) | 2 (6) | 0.599 |
| After 2-stage replacement | 3/8 (38) | 3/8 (38) | — | NA |
| PJI History and Comorbidities | C. acnes PJI | New PJI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PJI Site | Prior Surgery | Previous PJI | Comorbidities | Ca-PJI Classification | Last-Clean-To-Curative-Surgery Interval (Months) | PJI-Symptom Duration (Months) | Surgical & Antibiotic Treatments | Time of Onset after C. acnes PJI Treatment (Months) | Microorganism PJI Classification | Surgical & Antibiotic Treatments | |
| Rifampicin-Treated | |||||||||||
| 1 | Knee | ≥2 | Acute hematogenous S. pneumoniae PJI, 6 months earlier Treatment: DAIR and antibiotic therapy | None | Late chronic | 6 | 6 | 1-stage exchange arthroplasty & IV: cefazolin + rifampicin (42 days) No oral treatment | 12 | Methicillin- susceptible S. aureus Acute hematogenous PJI | 2-stage exchange arthroplasty & Antibiotics |
| 2 | Hip | 1 | None | None | Unknown | 249 | 24 | 2-stage exchange arthroplasty & IV: cefazolin + rifampicin (42 days) Oral: cefalexin + rifampicin (42 days) | 6 | Citrobacter freundii Acute hematogenous PJI | DAIR & Antibiotics |
| 3 | Hip | ≥2 | Late chronic Cutibacterium avidum PJI, 7 years earlier Treatment: 1-stage exchange arthroplasty & antibiotics | Active cancer | Late chronic | 81 | 40 | 2-stage exchange arthroplasty & IV: cefazolin + rifampicin (42 days) Oral: clindamycin (41 days) | 8 | Methicillin-susceptible Staphylococcus capitis Late chronic PJI | Suppressive antibiotherapy |
| 4 | Hip | ≥2 | None | None | Late chronic | 6 | 3 | 2-stage exchange arthroplasty & IV: cefazolin + rifampicin (47 days) Oral: cefalexin + rifampicin (74 days) | 7 | Methicillin-resistant Staphylococcus epidermidis Corynebacterium macginleyi Positive intra-operative during new prosthesis implantation cultures | Antibiotics |
| Not Rifampicin-Treated | |||||||||||
| 5 | Knee | 1 | None | None | Late chronic | 17 | 12 | 1-stage exchange arthroplasty & IV: vancomycin followed by clindamycin (20 days) Oral: clindamycin (21 days) | 7 | Streptococcus dysgalactiae Acute hematogenous | DAIR & Antibiotics |
| 6 | Shoulder | ≥2 | None | None | Early post-operative | 3 | 3 | 1-stage exchange arthroplasty & IV: vancomycin + clindamycin then cefazolin (14 days) Oral: amoxicillin (23 days) | 1 | Methicillin-resistant Staphylococcus haemolyticus Early post-operative | DAIR & Antibiotics |
| Susceptibility | ||||
|---|---|---|---|---|
| Patient | New Infection Bacterial Strain | Rifampicin | Clindamycin | Macrolide |
| Rifampicin-Treated | ||||
| 1 | Methicillin susceptible Staphylococcus aureus | ND | ND | ND |
| 2 | Citrobacter freundii | / | / | / |
| 3 | Methicillin susceptible Staphylococcus capitis | Yes | Yes | Yes |
| 4 | Methicillin-resistant Staphylococcus epidermidis Corynebacterium macginleyi | No Yes | Yes Yes | Yes Yes |
| Not Rifampicin Treated | ||||
| 5 | Streptococcus dysgalactiae | Yes | Yes | Yes |
| 6 | Methicillin-resistant Staphylococcus haemolyticus | No | No | No |
| All | Rifampicin-Treated | Not Rifampicin-Treated | p-Value | |
|---|---|---|---|---|
| Adverse Events | n = 70 | n = 37 | n = 33 | |
| Total, n (%) | 10 (14) | 6 (16) | 4 (12) | 0.739 |
| Treatment discontinued, n (%) | 7 (10) | 3 (8) | 4 (12) | 0.699 |
| According to antibiotic (IV or oral) | ||||
| Rifampicin, n | 37 | 37 | — | |
| Adverse events, n (%) | 5 (14) | 5 (14) | — | NA |
| Vancomycin, n | 16 | 3 | 13 | |
| Adverse events, n (%) | 1 (6) | 1 (33) | 0 | 0.003 |
| Clindamycin, n | 59 | 29 | 30 | |
| Adverse events, n (%) | 3 (5) | 0 | 3 (10) | 0.197 |
| Cefazolin, n | 34 | 21 | 13 | |
| Adverse events, n (%) | 1 (3) | 0 | 1 (8) | 0.161 |
| Antibiotic | Adverse Event, n | n | Treatment, n | |
|---|---|---|---|---|
| Discontinued | Changed to | |||
| Rifampicin | Rash | 1 | 1 | Monotherapy without rifampicin |
| Gastrointestinal disorders | 4 | 1 | ||
| Vancomycin | Rash | 1 | 1 | Clindamycin |
| Clindamycin | Rash | 2 | 2 | Cefazolin or amoxicillin |
| Gastrointestinal disorders | 1 | 1 | Intravenous clindamycin | |
| Cefazolin | Liver toxicity | 1 | 1 | Clindamycin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saltiel, G.; Meyssonnier, V.; Kerroumi, Y.; Heym, B.; Lidove, O.; Marmor, S.; Zeller, V. Cutibacterium acnes Prosthetic Joint Infections: Is Rifampicin-Combination Therapy Beneficial? Antibiotics 2022, 11, 1801. https://doi.org/10.3390/antibiotics11121801
Saltiel G, Meyssonnier V, Kerroumi Y, Heym B, Lidove O, Marmor S, Zeller V. Cutibacterium acnes Prosthetic Joint Infections: Is Rifampicin-Combination Therapy Beneficial? Antibiotics. 2022; 11(12):1801. https://doi.org/10.3390/antibiotics11121801
Chicago/Turabian StyleSaltiel, Grégoire, Vanina Meyssonnier, Younes Kerroumi, Beate Heym, Olivier Lidove, Simon Marmor, and Valérie Zeller. 2022. "Cutibacterium acnes Prosthetic Joint Infections: Is Rifampicin-Combination Therapy Beneficial?" Antibiotics 11, no. 12: 1801. https://doi.org/10.3390/antibiotics11121801
APA StyleSaltiel, G., Meyssonnier, V., Kerroumi, Y., Heym, B., Lidove, O., Marmor, S., & Zeller, V. (2022). Cutibacterium acnes Prosthetic Joint Infections: Is Rifampicin-Combination Therapy Beneficial? Antibiotics, 11(12), 1801. https://doi.org/10.3390/antibiotics11121801






