In Vitro Antimycobacterial Activity of Human Lactoferrin-Derived Peptide, D-hLF 1-11, against Susceptible and Drug-Resistant Mycobacterium tuberculosis and Its Synergistic Effect with Rifampicin
Abstract
1. Introduction
2. Results
2.1. The Antimycobacterial Activity of L-and D-hLF 1-11 against Mycobacteria
2.2. The Antimycobacterial Activity of Antimicrobial Peptides against M. Tuberculosis H37Rv
2.3. Antibiofilm Activity of D-hLF 1-11 against Biofilm-Forming M. abscessus
2.4. The Analysis of the Synergistic Interaction of D-hLF 1-11 and Anti-TB Drugs Using the Checkerboard Assay
2.5. The RBC Hemolytic Assay for the Toxicity Determination of Antimicrobial Peptides
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Peptides
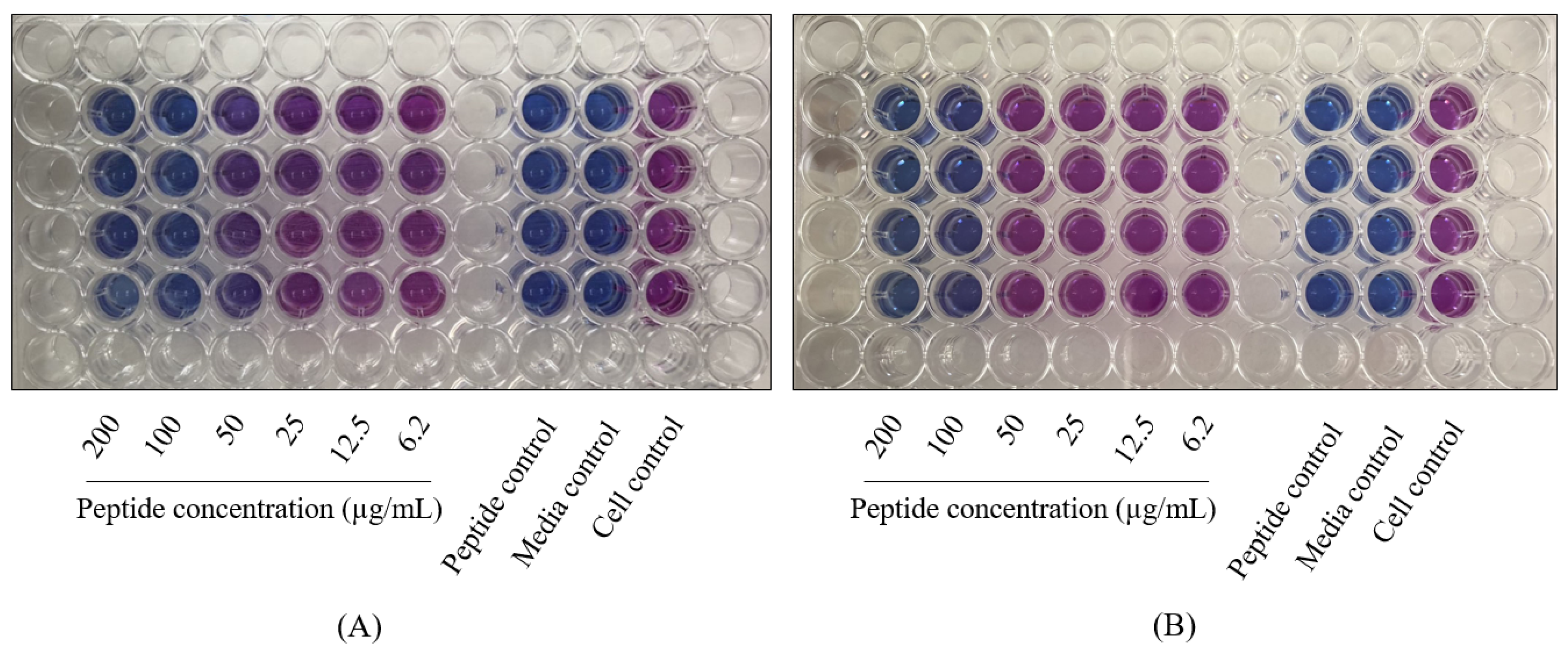
4.3. MTT Assay
4.4. Resazurin Microplate Assay (REMA)
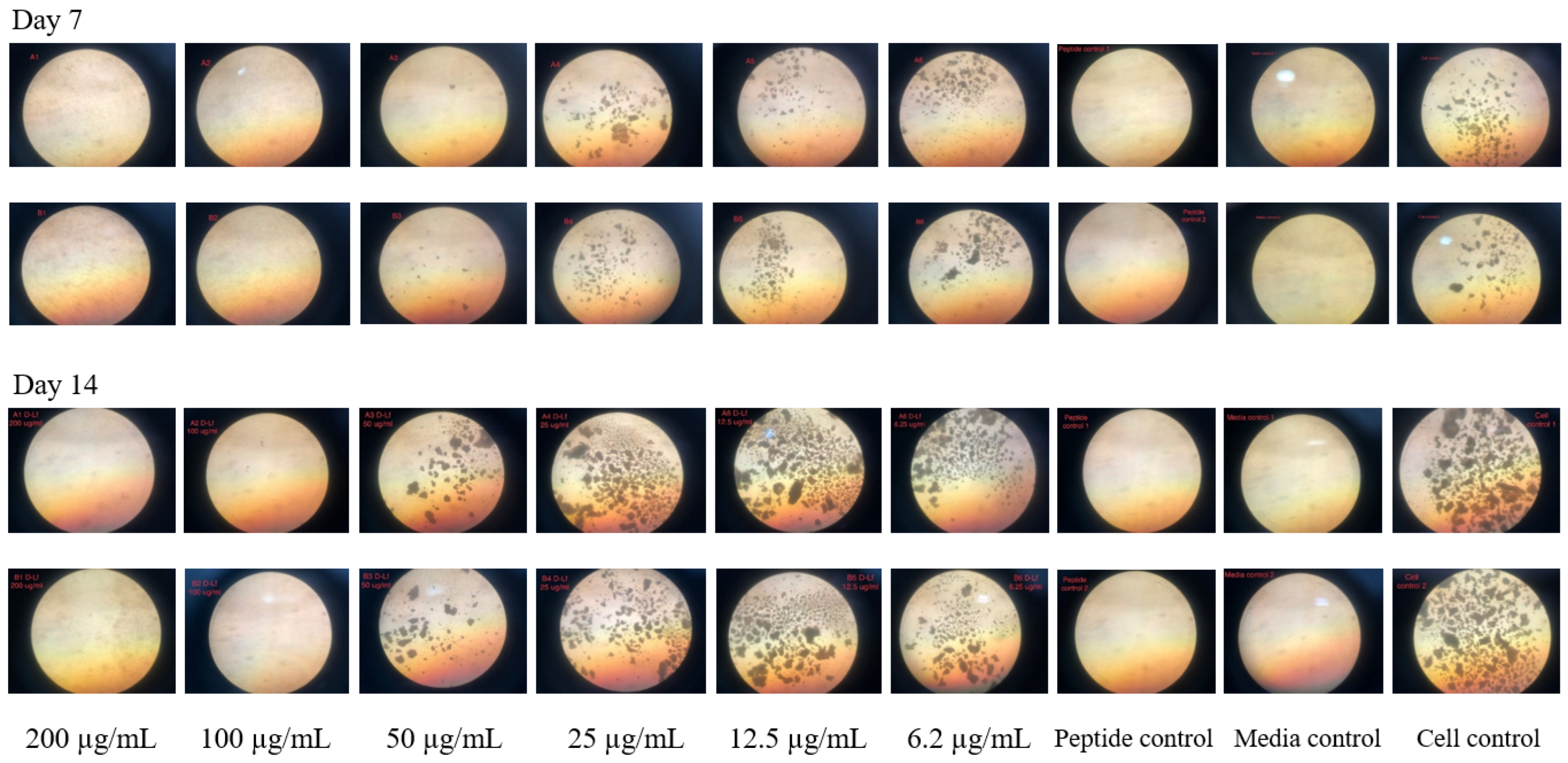
4.5. Microscopic Observation Drug Susceptibility (MODS) Assay
4.6. Crystal Violet Staining
4.7. Checkerboard Assay
4.8. Red Blood Cells Hemolytic Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2020 (accessed on 31 March 2020).
- Rivas-Santiago, B.; Rivas Santiago, E.C.; Castañeda-Delgado, J.E.; León–Contreras, J.C.; Hancock, R.E.; Hernandez-Pando, R. Activity of LL-37, CRAMP and antimicrobial peptide-derived compounds E2, E6 and CP26 against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 2013, 41, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Gennaro, R.; Zanetti, M.; Tan, D.; Brunori, L.; Giannoni, F.; Pardini, M.; Orefici, G. In vitro activity of protegrin-1 and deta-defensin-1, alone and in combination with isoniazid, against Mycobacterium tuberculosis. Peptides 2004, 25, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Garcia, L.; Ortiz, E.; Castañeda-Delgado, J.; Rivas-Santiago, B.; Corzo, G. Bacterial expression and antibiotic activities of recombinant variants of human β-defensins on pathogenic bacteria and M. tuberculosis. Protein Expr. Purif. 2013, 89, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.P.J.M.; Rahman, M.; Welling, M.M. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides 2011, 32, 1953–1963. [Google Scholar] [CrossRef]
- Lupetti, A.; Paulusma-Annema, A.; Welling, M.M.; Dogterom-Ballering, H.; Brouwer, C.P.J.M.; Senesi, S.; van Dissel, J.T.; Nibbering, P.H. Synergistic Activity of the N-Terminal Peptide of Human Lactoferrin and Fluconazole against Candida Species. Antimicrob. Agents Chemother. 2003, 47, 262–267. [Google Scholar] [CrossRef]
- Silva, T.; Magalhães, B.; Maia, S.; Gomes, P.; Nazmi, K.; Bolscher, J.G.M.; Rodrigues, P.N.; Bastos, M.; Gomes, M.S. Killing of Mycobacterium avium by Lactoferricin Peptides: Improved Activity of Arginine- and D-Amino-Acid-Containing Molecules. Antimicrob. Agents Chemother. 2014, 58, 3461–3467. [Google Scholar] [CrossRef]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The Roles of Antimicrobial Peptides in Innate Host Defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef]
- Van der Does, A.M.; Hiemstra, P.S.; Mookherjee, N. Antimicrobial host defense peptides: Immunomodulatory functions and translational prospects. Adv. Exp. Med. Biol. 2019, 117, 149–171. [Google Scholar] [CrossRef]
- Linde, C.M.A.; Hoffner, S.E.; Refai, E.; Andersson, M. In vitro activity of PR-39, a proline-arginine-rich peptide, against susceptible and multi-drug-resistant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2001, 47, 575–580. [Google Scholar] [CrossRef]
- Carroll, J.; Field, D.; O’ Conner, P.M.; Cotter, P.D.; Coffey, A.; Hill, C.; O’Mahony, J. The gene encoded antimicrobial peptides, a template for the design of novel anti-mycobacterial drugs. Bioeng. Bugs 2010, 1, 408–412. [Google Scholar] [CrossRef]
- Silva, J.P.; Gonçalves, C.; Costa, C.; Sousa, J.; Silva-Gomes, R.; Castro, A.G.; Pedrosa, J.; Appelberg, R.; Gama, F.M. Delivery of LLKKK18 loaded into self-assembling hyaluronic acid nanogel for tuberculosis treatment. J. Control. Release 2016, 235, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Coyotl, E.A.P.; Palacios, J.B.; Muciño, G.; Moreno-Blas, D.; Costas, M.; Montes, T.M.; Diener, C.; Uribe-Carvajal, S.; Massieu, L.; Castro-Obregón, S.; et al. Antimicrobial Peptide against Mycobacterium Tuberculosis That Activates Autophagy Is an Effective Treatment for Tuberculosis. Pharmaceutics 2020, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Kapil, S.; Sharma, V. D-amino acids in antimicrobial peptides: A potential approach to treat and combat antimicrobial resistance. Can. J. Microbiol. 2021, 67, 119–137. [Google Scholar] [CrossRef]
- McGillivray, S.M.; Tran, D.N.; Ramadoss, N.S.; Alumasa, J.N.; Okumura, C.Y.; Sakoulas, G.; Vaughn, M.M.; Zhang, D.X.; Keiler, K.C.; Nizet, V. Pharmacological Inhibition of the ClpXP Protease Increases Bacterial Susceptibility to Host Cathelicidin Antimicrobial Peptides and Cell Envelope-Active Antibiotics. Antimicrob. Agents Chemother. 2012, 56, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.D.; Tekinay, A.B.; Guler, M.O.; Tekin, E.D. Effects of temperature, pH and counterions on the stability of peptide amphiphile nanofiber structures. RSC Adv. 2016, 6, 104201. [Google Scholar] [CrossRef]
- De Andrade, R.G.; Junqueira-Kipnis, A.P.; Kipnis, A. Proteases Associated with Mycobacterium tuberculosis Infection. Rev. Patol. Trop. 2019, 48, 1–12. [Google Scholar] [CrossRef]
- Jiang, Z.; Higgins, M.P.; Whitehurst, J.; Kisich, K.O.; Voskuil, M.I.; Hodges, R.S. Anti-Tuberculosis Activity of α-Helical Antimicrobial Peptides: De Novo Designed L- and D-Enantiomers Versus L- and D-LL37. Protein Pept. Lett. 2011, 18, 241–252. [Google Scholar] [CrossRef]
- Lan, Y.; Lam, J.T.; Siu, G.K.; Yam, W.C.; Mason, A.J.; Lam, J.K. Cationic amphipathic D-enantiomeric antimicrobial peptides with in vitro and ex vivo activity against drug-resistant Mycobacterium tuberculosis. Tuberculosis 2014, 94, 678–689. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D- and Unnatural Amino Acid Substituted Antimicrobial Peptides With Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front. Microbiol. 2020, 11, 563030. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, I.; Khuller, G.K. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: In vitro and ex vivo study. Eur. Respir. J. 2000, 16, 112–117. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; He, X.; Yang, J.; Wu, J.; Yang, H.; Li, M.; Qian, Q.; Lai, R.; Xu, W.; et al. A small mycobacteriophage-derived peptide and its improved isomer restrict mycobacterial infection via dual mycobactericidal-immunoregulatory activities. J. Biol. Chem. 2019, 294, 7615–7631. [Google Scholar] [CrossRef] [PubMed]
- Portell-Buj, E.; Vergara, A.; Alejo, I.; López-Gavín, A.; Monté, M.R.; Nicolás, L.S.; González-Martín, J.; Tudó, G. In vitro activity of 12 antimicrobial peptides against Mycobacterium tuberculosis and Mycobacterium avium clinical isolates. J. Med. Microbiol. 2019, 68, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.; Dalcolmo, M.P.; Daley, C.L.; Eather, G.; Gayoso, R.; Hasegawa, N.; Jhun, B.W.; Koh, W.-J.; Namkoong, H.; Park, J.; et al. Mycobacterium abscessus pulmonary disease: Individual patient data meta-analysis. Eur. Respir. J. 2019, 54, 1801991. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, E.; Cheretakis, A.; Modarressi, A.; Harbarth, S.; Pittet-Cuénod, B. Multisite infection with Mycobacterium abscessus after replacement of breast implants and gluteal lipofilling. Case Rep. Infect. Dis. 2015, 2015, 361340. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; García-Coca, M. Mycobacterium Biofilms. Front. Microbiol. 2018, 8, 2651. [Google Scholar] [CrossRef]
- Kulka, K.; Hatfull, G.; Ojha, A.K. Growth of Mycobacterium tuberculosis Biofilms. J. Vis. Exp. 2012, 15, 3820. [Google Scholar] [CrossRef]
- Fais, R.; Di Luca, M.; Rizzato, C.; Morici, P.; Bottai, D.; Tavanti, A.; Lupetti, A. The N-terminus of human lactoferrin displays anti-biofilm activity on Candida parapsilosis in lumen catherters. Front. Microbiol. 2017, 8, 2218. [Google Scholar] [CrossRef]
- Ramamourthy, G.; Vogel, H.J. Antibiofilm activity of lactoferrin-derived synthetic peptides against Pseudomonas aeruginosa PAO1. Biochem. Cell Biol. 2021, 99, 138–148. [Google Scholar] [CrossRef]
- Schön, T.; Werngren, J.; Machado, D.; Borroni, E.; Wijkander, M.; Lina, G.; Mouton, J.; Matuschek, E.; Kahlmeter, G.; Giske, C.; et al. Antimicrobial susceptibility testing of Mycobacterium tuberculosis complex isolates—The EUCAST broth microdilution reference method for MIC determination. Clin. Microbiol. Infect. 2020, 26, 1488–1492. [Google Scholar] [CrossRef]
- Heinrichs, M.T.; May, R.J.; Heider, F.; Reimers, T.; Sy, S.K.B.; Peloquin, C.A.; Derendorf, H. Mycobacterium tuberculosis Strains H37ra and H37rv have equivalent minimum inhibitory concentrations to most antituberculosis drugs. Int. J. Mycobacteriol. 2018, 7, 156–161. [Google Scholar] [CrossRef]
- Khara, J.S.; Wang, Y.; Ke, X.-Y.; Liu, S.; Newton, S.M.; Langford, P.R.; Yang, Y.Y.; Ee, P.L.R. Anti-mycobacterial activities of synthetic cationic α-helical peptides and their synergism with rifampicin. Biomaterials 2014, 35, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, W.J.F.M.; van Iersel, T.M.P.; Blijlevens, N.M.A.; Donnelly, J.P. Safety and tolerability of the antimicrobial peptide human lactoferricin 1-11 (hLF 1-11). BMC Med. 2009, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, M.A.; Myers, M.H.; Sathe, Y.S.; Koffsky, P. Toxicity, the Therapeutic Index, and the Ranking of Drugs. Science 1964, 144, 1212–1213. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Carter, D.E. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Mshana, R.N.; Miörner, H. Evaluation of a colorimetric assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) for rapid detection of rifampicin resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 1998, 2, 1011–1016. [Google Scholar] [PubMed]
- Sirgel, F.A.; Wiid, I.J.F.; van Helden, P.D. Measuring minimum inhibitory concentrations in mycobacteria. Methods Mol. Biol. 2009, 465, 173–186. [Google Scholar] [CrossRef]
- Palomino, J.-C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin Microtiter Assay Plate: Simple and Inexpensive Method for Detection of Drug Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef]
- Mello, F.C.Q.; Arias, M.S.; Rosales, S.; Marsico, A.G.; Pavón, A.; Alvarado-Gálvez, C.; Pessôa, C.L.C.; Pérez, M.; Andrade, M.K.; Kritski, A.L.; et al. Clinical Evaluation of the Microscopic Observation Drug Susceptibility Assay for Detection of Mycobacterium tuberculosis Resistance to Isoniazid or Rifampin. J. Clin. Microbiol. 2007, 45, 3387–3389. [Google Scholar] [CrossRef][Green Version]
- Agarwal, A.; Katoch, C.D.S.; Kumar, M.; Dhole, T.N.; Sharma, Y.K. Evaluation of Microscopic observation drug susceptibility (MODS) assay as a rapid, sensitive and inexpensive test for detection of tuberculosis and multidrug resistant tuberculosis. Med. J. Armed Forces India 2019, 75, 58–64. [Google Scholar] [CrossRef]
- Belardinelli, J.M.; Li, W.; Avanzi, C.; Angala, S.K.; Lian, E.; Wiersma, C.J.; Palčeková, Z.; Martin, K.H.; Angala, B.; de Moura, V.C.N.; et al. Unique Features of Mycobacterium abscessus Biofilms Formed in Synthetic Cystic Fibrosis Medium. Front. Microbiol. 2021, 12, 743126. [Google Scholar] [CrossRef]
- Dokic, A.; Peterson, E.; Arrieta-Ortiz, M.L.; Pan, M.; Di Maio, A.; Baliga, N.; Bhatt, A. Mycobacterium abscessus biofilms produce an extracellular matrix and have a distinct mycolic acid profile. Cell Surf. 2021, 7, 100051. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Gonçalves, S.; Felício, M.R.; Maturana, P.; Santos, N.C.; Semorile, L.; Hollmann, A.; Maffía, P.C. Synergistic and antibiofilm activity of the antimicrobial peptide P5 against carbapenem-resistant Pseudomonas aeruginosa. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Caleffi-Ferracioli, K.R.; Maltempe, F.G.; Siqueira, V.L.D.; Cardoso, R.F. Fast detection of drug interaction in Mycobacterium tuberculosis by a checkerboard resazurin method. Tuberculosis 2013, 93, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.U.; Henderson, D.I.; Krishnan, N.; Puthia, M.; Glegola-Madejska, I.; Brive, L.; Bjarnemark, F.; Fureby, A.M.; Hjort, K.; Andersson, D.I.; et al. A broad spectrum anti-bacterial peptide with an adjunct potential for tuberculosis chemotherapy. Sci. Rep. 2021, 11, 4201. [Google Scholar] [CrossRef]
- Feng, W.; Yang, J.; Ma, Y.; Xi, Z.; Ji, Y.; Ren, Q.; Ning, H.; Wang, S. Cotreatment with Aspirin and Azole Drugs Increases Sensitivity of Candida albicans in vitro. Infect. Drug Resist. 2021, 14, 2027–2038. [Google Scholar] [CrossRef] [PubMed]



| Peptide Concentration (µg/mL) | Mean of Relative Growth ± SD (%) | |||
|---|---|---|---|---|
| 7 Days of Incubation | 14 Days of Incubation | |||
| L-hLF 1-11 | D-hLF 1-11 | L-hLF 1-11 | D-hLF 1-11 | |
| 0 * | 100.00 ± 5.92 | 100.00 ± 5.92 | 100.00 ± 5.35 | 100.00 ± 5.35 |
| 6.2 | 83.51 ± 3.66 | 95.62 ± 13.39 | 112.52 ± 3.40 | 96.89 ± 1.40 |
| 12.5 | 67.66 ± 5.21 | 64.11 ± 7.47 | 101.49 ± 3.64 | 102.70 ± 0.97 |
| 25 | 46.23 ± 14.93 | 57.55 ± 14.81 | 92.49 ± 5.42 | 72.09 ± 1.84 |
| 50 | 43.40 ± 9.82 | 41.71 ± 15.71 | 107.15 ± 4.61 | 67.57 ± 11.59 |
| 100 | 11.77 ± 2.33 | 2.09 ± 0.19 | 64.20 ± 6.16 | 31.25 ± 0.21 |
| 200 | 5.18 ± 0.66 | 0.97 ± 0.19 | 99.81 ± 5.68 | 0.56 ± 0.05 |
| Peptide Concentration (µg/mL) | Mean of Relative Growth ± SD (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| M. tuberculosis H37Ra | M. tuberculosis H37Rv | INH-Resistant M. tuberculosis | RF-Resistant M. tuberculosis | MDR M. tuberculosis | M. bovis | M. microti | M. avium | M. intracellulare | |
| 0 * | 100.00 ± 0.54 | 100.00 ± 17.34 | 100.00 ± 11.69 | 100.00 ± 7.02 | 100.00 ± 15.04 | 100.00 ± 4.32 | 100.00 ± 3.34 | 100.00 ± 3.91 | 100.00 ± 6.74 |
| 6.2 | 78.32 ± 6.20 | 119.92 ± 6.75 | 114.64 ± 3.84 | 165.75 ± 21.00 | 93.90 ± 10.40 | 104.63 ± 6.09 | 116.24 ± 4.13 | 104.73 ± 2.63 | 112.24 ± 7.25 |
| 12.5 | 83.19 ± 5.38 | 97.32 ± 23.52 | 114.03 ± 7.87 | 94.93 ± 20.70 | 113.35 ± 16.62 | 113.62 ± 2.35 | 95.12 ± 10.80 | 108.50 ± 3.66 | 117.28 ± 8.14 |
| 25 | 82.52 ± 8.52 | 86.26 ± 19.18 | 70.55 ± 16.30 | 133.33 ± 6.41 | 73.16 ± 12.24 | 118.13 ± 5.34 | 84.73 ± 10.26 | 95.52 ± 9.42 | 122.60 ± 6.05 |
| 50 | 64.36 ± 3.42 | 37.78 ± 15.62 | 53.58 ± 17.38 | 130.72 ± 11.90 | 44.93 ± 8.48 | 90.07 ± 5.96 | 34.51 ± 5.72 | 90.72 ± 6.53 | 84.16 ± 4.58 |
| 100 | 0.68 ± 0.96 | 0.91 ± 0.23 | 4.96 ± 0.07 | 9.81 ± 4.67 | 5.51 ± 3.00 | 43.25 ± 0.98 | 0.72 ± 0.21 | 13.58 ± 0.62 | 9.47 ± 1.73 |
| 200 | 0.46 ± 0.79 | 0.36 ± 0.00 | 0.21 ± 0.05 | 0.51 ± 0.21 | 0.47 ± 0.27 | 16.56 ± 0.44 | 0.64 ± 0.00 | 2.82 ± 0.71 | 5.37 ± 0.86 |
| Strains of Mycobacteria | MIC (µg/mL) | ||
|---|---|---|---|
| MTT Assay | REMA | MODS Assay | |
| M. tuberculosis H37Ra | 100 | 100 | 100 |
| M. tuberculosis H37Rv | 100 | 100 | 100 |
| INH-monoresistant M. tuberculosis | 200 | 100 | 200 |
| RF-monoresistant M. tuberculosis | 200 | 100 | 200 |
| MDR M. tuberculosis | 200 | 100 | 200 |
| M. bovis | >200 | 100 | >200 |
| M. microti | 100 | 100 | 200 |
| M. avium | >200 | 100 | ND * |
| M. intracellulare | >200 | 100 | ND |
| Drug-Peptide Combination | MIC (µg/mL) | FIC | FICI | |
|---|---|---|---|---|
| Alone | Combined | |||
| INH | 0.025 | 0.012 | 0.48 | 0.730 |
| D-hLF 1-11 | 100 | 25 | 0.25 | |
| RF D-hLF 1-11 | 0.5 100 | 0.031 25 | 0.062 0.25 | 0.312 |
| Strain | Phenotype | Mutation Identification | ||
|---|---|---|---|---|
| rpoB | katG | inhA | ||
| Isoniazid monoresistant M. tuberculosis | INH-resistant | - | C-15T | |
| Rifampicin monoresistant M. tuberculosis | RF-resistant | H526D | - | - |
| MDR M. tuberculosis | INH- & RF-resistant | D516V | S315T | - |
| Antimicrobial Peptide | Amino Acid Sequence | Length | Source | MIC (µg/mL) | Test | Reference |
|---|---|---|---|---|---|---|
| PG-1 | RGGRLCYCRR RFCVCVGR | 18 | Sus domesticus | 128 | Plate count assay | [3] |
| AK 15-6 | AVKKLLRWWS RWWKK | 15 | Mycobac teriophage | 37.5 | Kinetic killing assay | [22] |
| AM-mel | GIGAVLKVLT TGLPALISWI KRKRQQ | 26 | Apis mellifera | 32–64 | REMA | [23] |
| hLF 1-11 (D & L-form) | GRRRRSVQWC A | 11 | Homo sapiens | - | - | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intorasoot, S.; Intorasoot, A.; Tawteamwong, A.; Butr-Indr, B.; Phunpae, P.; Tharinjaroen, C.S.; Wattananandkul, U.; Sangboonruang, S.; Khantipongse, J. In Vitro Antimycobacterial Activity of Human Lactoferrin-Derived Peptide, D-hLF 1-11, against Susceptible and Drug-Resistant Mycobacterium tuberculosis and Its Synergistic Effect with Rifampicin. Antibiotics 2022, 11, 1785. https://doi.org/10.3390/antibiotics11121785
Intorasoot S, Intorasoot A, Tawteamwong A, Butr-Indr B, Phunpae P, Tharinjaroen CS, Wattananandkul U, Sangboonruang S, Khantipongse J. In Vitro Antimycobacterial Activity of Human Lactoferrin-Derived Peptide, D-hLF 1-11, against Susceptible and Drug-Resistant Mycobacterium tuberculosis and Its Synergistic Effect with Rifampicin. Antibiotics. 2022; 11(12):1785. https://doi.org/10.3390/antibiotics11121785
Chicago/Turabian StyleIntorasoot, Sorasak, Amornrat Intorasoot, Arocha Tawteamwong, Bordin Butr-Indr, Ponrut Phunpae, Chayada Sitthidet Tharinjaroen, Usanee Wattananandkul, Sirikwan Sangboonruang, and Jiaranai Khantipongse. 2022. "In Vitro Antimycobacterial Activity of Human Lactoferrin-Derived Peptide, D-hLF 1-11, against Susceptible and Drug-Resistant Mycobacterium tuberculosis and Its Synergistic Effect with Rifampicin" Antibiotics 11, no. 12: 1785. https://doi.org/10.3390/antibiotics11121785
APA StyleIntorasoot, S., Intorasoot, A., Tawteamwong, A., Butr-Indr, B., Phunpae, P., Tharinjaroen, C. S., Wattananandkul, U., Sangboonruang, S., & Khantipongse, J. (2022). In Vitro Antimycobacterial Activity of Human Lactoferrin-Derived Peptide, D-hLF 1-11, against Susceptible and Drug-Resistant Mycobacterium tuberculosis and Its Synergistic Effect with Rifampicin. Antibiotics, 11(12), 1785. https://doi.org/10.3390/antibiotics11121785







