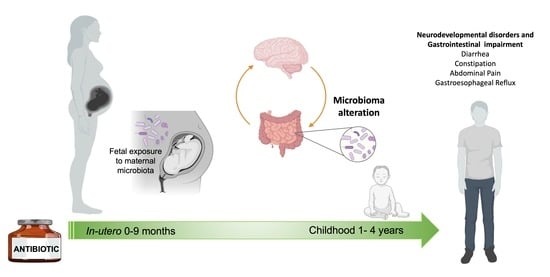
Exposure to Antibiotics and Neurodevelopmental Disorders: Could Probiotics Modulate the Gut–Brain Axis?
Abstract
1. Introduction: Neurodevelopmental Disorders
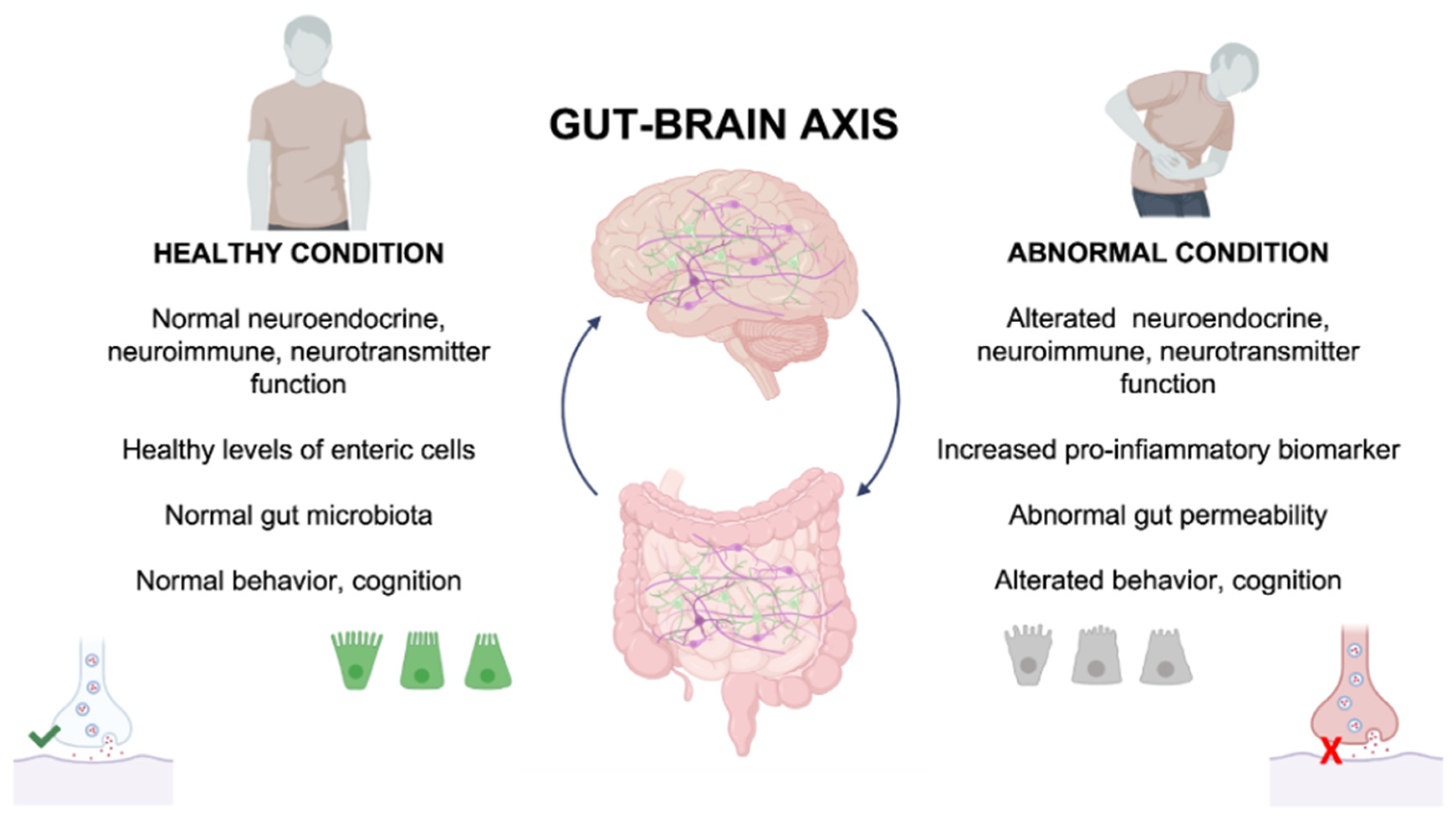
2. Gut–Brain Axis
3. NDD-Associated Gastrointestinal Symptoms
4. Prenatal Antibiotics Exposure and the Risk of ASD
5. Early Antibiotics Exposure and the Risk of ASD
6. Probiotics and Use of Probiotics in NDDs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Boyce, W.T.; Sokolowski, M.B.; Robinson, G.E. Genes and environments, development and time. Proc. Natl. Acad. Sci. USA 2020, 117, 23235–23241. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, T.; Molander, F.; Taylor, M.J.; Jonsson, U.; Bölte, S. Early environmental risk factors for neurodevelopmental disorders—A systematic review of twin and sibling studies. Dev. Psychopathol. 2021, 33, 1448–1495. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.E.K.; Martin, H.C.; Rice, D.L.; Gallone, G.; Gordon, S.; Kelemen, M.; McAloney, K.; McRae, J.; Radford, E.J.; Yu, S.; et al. Common genetic variants contribute to risk of rare severe neurodevelopmental disorders. Nature 2018, 562, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental disorders: From genetics to functional pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef]
- Francés, L.; Quintero, J.; Fernández, A.; Ruiz, A.; Caules, J.; Fillon, G.; Hervás, A.; Soler, C.V. Current state of knowledge on the prevalence of neurodevelopmental disorders in childhood according to the DSM-5: A systematic review in accordance with the PRISMA criteria. Child Adolesc. Psychiatry Ment. Health 2022, 16, 27. [Google Scholar] [CrossRef]
- Antshel, K.M.; Barkley, R. Attention deficit hyperactivity disorder. Handb. Clin. Neurol. 2020, 174, 37–45. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef]
- Davidovitch, M.; Shmueli, D.; Rotem, R.S.; Bloch, A.M. Diagnosis despite clinical ambiguity: Physicians’ perspectives on the rise in Autism Spectrum disorder incidence. BMC Psychiatry 2021, 21, 150. [Google Scholar] [CrossRef]
- DuPaul, G.J.; Gormley, M.J.; Laracy, S.D. Comorbidity of LD and ADHD: Implications of DSM-5 for assessment and treatment. J. Learn. Disabil. 2013, 46, 43–51. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Schmidt, R.J.; Walker, C.K.; Bennett, D.H.; Oliver, M.; Shedd-Wise, K.M.; LaSalle, J.M.; Giulivi, C.; Puschner, B.; Thomas, J.; et al. A Prospective Study of Environmental Exposures and Early Biomarkers in Autism Spectrum Disorder: Design, Protocols, and Preliminary Data from the MARBLES Study. Environ. Health Perspect. 2018, 126, 117004. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Lopes-Marques, M.; Silva, R.M.; Serrano, C.; Amorim, A.; Prata, M.J.; Azevedo, L. Essential genetic findings in neurodevelopmental disorders. Hum. Genom. 2019, 13, 31. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.-Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Gandal, M.J.; Zhang, P.; Hadjimichael, E.; Walker, R.L.; Chen, C.; Liu, S.; Won, H.; van Bakel, H.; Varghese, M.; Wang, Y.; et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018, 362. [Google Scholar] [CrossRef]
- An, J.-Y.; Lin, K.; Zhu, L.; Werling, D.M.; Dong, S.; Brand, H.; Wang, H.Z.; Zhao, X.; Schwartz, G.B.; Collins, R.L.; et al. Genome-wide de novo risk score implicates promoter variation in autism spectrum disorder. Science 2018, 362. [Google Scholar] [CrossRef]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef]
- de la Torre-Ubieta, L.; Won, H.; Stein, J.L.; Geschwind, D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016, 22, 345–361. [Google Scholar] [CrossRef]
- Rasheed, M.; Khan, V.; Harripaul, R.; Siddiqui, M.; Malik, M.A.; Ullah, Z.; Zahid, M.; Vincent, J.B.; Ansar, M. Exome sequencing identifies novel and known mutations in families with intellectual disability. BMC Med. Genom. 2021, 14, 211. [Google Scholar] [CrossRef]
- Hamanaka, K.; Miyake, N.; Mizuguchi, T.; Miyatake, S.; Uchiyama, Y.; Tsuchida, N.; Sekiguchi, F.; Mitsuhashi, S.; Tsurusaki, Y.; Nakashima, M.; et al. Large-scale discovery of novel neurodevelopmental disorder-related genes through a unified analysis of single-nucleotide and copy number variants. Genome Med. 2022, 14, 40. [Google Scholar] [CrossRef]
- Leblond, C.S.; Cliquet, F.; Carton, C.; Huguet, G.; Mathieu, A.; Kergrohen, T.; Buratti, J.; Lemière, N.; Cuisset, L.; Bienvenu, T.; et al. Both rare and common genetic variants contribute to autism in the Faroe Islands. NPJ Genom. Med. 2019, 4, 1. [Google Scholar] [CrossRef]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar] [CrossRef]
- Rahnama, M.; Tehrani, H.A.; Mirzaie, M.; Ziaee, V. Identification of key genes and convergent pathways disrupted in autism spectrum disorder via comprehensive bioinformatic analysis. Inform. Med. Unlocked 2021, 24, 100589. [Google Scholar] [CrossRef]
- Bonsi, P.; De Jaco, A.; Fasano, L.; Gubellini, P. Postsynaptic autism spectrum disorder genes and synaptic dysfunction. Neurobiol. Dis. 2022, 162, 105564. [Google Scholar] [CrossRef]
- Sacai, H.; Sakoori, K.; Konno, K.; Nagahama, K.; Suzuki, H.; Watanabe, T.; Watanabe, M.; Uesaka, N.; Kano, M. Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat. Commun. 2020, 11, 5140. [Google Scholar] [CrossRef] [PubMed]
- Trobiani, L.; Meringolo, M.; Diamanti, T.; Bourne, Y.; Marchot, P.; Martella, G.; Dini, L.; Pisani, A.; De Jaco, A.; Bonsi, P. The neuroligins and the synaptic pathway in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2020, 119, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Masi, A.; Dale, R.C.; Whitehouse, A.J.O.; Pokorski, I.; Alvares, G.A.; Hickie, I.B.; Breen, E.; Guastella, A.J. Social impairments in autism spectrum disorder are related to maternal immune history profile. Mol. Psychiatry 2018, 23, 1794–1797. [Google Scholar] [CrossRef]
- Vyas, Y.; Cheyne, J.E.; Lee, K.; Jung, Y.; Cheung, P.Y.; Montgomery, J.M. Shankopathies in the developing brain in autism spectrum disorders. Front. Neurosci. 2021, 15, 775431. [Google Scholar] [CrossRef]
- Pohl, T.T.; Hörnberg, H. Neuroligins in neurodevelopmental conditions: How mouse models of de novo mutations can help us link synaptic function to social behavior. Neuronal Signal. 2022, 6, NS20210030. [Google Scholar] [CrossRef]
- Scattolin, M.A.d.A.; Resegue, R.M.; do Rosário, M.C. The impact of the environment on neurodevelopmental disorders in early childhood. J. Pediatr. 2022, 98 (Suppl. 1), S66–S72. [Google Scholar] [CrossRef]
- De Felice, A.; Ricceri, L.; Venerosi, A.; Chiarotti, F.; Calamandrei, G. Multifactorial origin of neurodevelopmental disorders: Approaches to understanding complex etiologies. Toxics 2015, 3, 89–129. [Google Scholar] [CrossRef]
- Mazina, V.; Gerdts, J.; Trinh, S.; Ankenman, K.; Ward, T.; Dennis, M.Y.; Girirajan, S.; Eichler, E.E.; Bernier, R. Epigenetics of autism-related impairment: Copy number variation and maternal infection. J. Dev. Behav. Pediatr. 2015, 36, 61–67. [Google Scholar] [CrossRef]
- Schaafsma, S.M.; Gagnidze, K.; Reyes, A.; Norstedt, N.; Månsson, K.; Francis, K.; Pfaff, D.W. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, 1383–1388. [Google Scholar] [CrossRef]
- Wang, C.; Geng, H.; Liu, W.; Zhang, G. Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine 2017, 96, e6696. [Google Scholar] [CrossRef]
- Cheroni, C.; Caporale, N.; Testa, G. Autism spectrum disorder at the crossroad between genes and environment: Contributions, convergences, and interactions in ASD developmental pathophysiology. Mol. Autism 2020, 11, 69. [Google Scholar] [CrossRef]
- Pham, C.; Symeonides, C.; O’Hely, M.; Sly, P.D.; Knibbs, L.D.; Thomson, S.; Vuillermin, P.; Saffery, R.; Ponsonby, A.-L.; Barwon Infant Study Investigator Group. Early life environmental factors associated with autism spectrum disorder symptoms in children at age 2 years: A birth cohort study. Autism 2022, 26, 1864–1881. [Google Scholar] [CrossRef]
- Khogeer, A.A.; AboMansour, I.S.; Mohammed, D.A. The role of genetics, epigenetics, and the environment in ASD: A mini review. Epigenomes 2022, 6, 15. [Google Scholar] [CrossRef]
- Aversa, Z.; Atkinson, E.J.; Schafer, M.J.; Theiler, R.N.; Rocca, W.A.; Blaser, M.J.; LeBrasseur, N.K. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 2021, 96, 66–77. [Google Scholar] [CrossRef]
- Manco, M. Gut microbiota and developmental programming of the brain: From evidence in behavioral endophenotypes to novel perspective in obesity. Front. Cell. Infect. Microbiol. 2012, 2, 109. [Google Scholar] [CrossRef]
- Patrick, D.M.; Sbihi, H.; Dai, D.L.Y.; Al Mamun, A.; Rasali, D.; Rose, C.; Marra, F.; Boutin, R.C.T.; Petersen, C.; Stiemsma, L.T.; et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: Evidence from population-based and prospective cohort studies. Lancet Respir. Med. 2020, 8, 1094–1105. [Google Scholar] [CrossRef]
- Trasande, L.; Blustein, J.; Liu, M.; Corwin, E.; Cox, L.M.; Blaser, M.J. Infant antibiotic exposures and early-life body mass. Int. J. Obes. 2013, 37, 16–23. [Google Scholar] [CrossRef]
- Fan, H.; Gilbert, R.; O’Callaghan, F.; Li, L. Associations between macrolide antibiotics prescribing during pregnancy and adverse child outcomes in the UK: Population based cohort study. BMJ 2020, 368, m331. [Google Scholar] [CrossRef]
- Muanda, F.T.; Sheehy, O.; Bérard, A. Use of antibiotics during pregnancy and risk of spontaneous abortion. CMAJ 2017, 189, E625–E633. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Weiner, H.L. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics 2018, 15, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain neurotransmitter modulation by gut microbiota in anxiety and depression. Front. Cell Dev. Biol. 2021, 9, 649103. [Google Scholar] [CrossRef]
- Rastelli, M.; Cani, P.D.; Knauf, C. The gut microbiome influences host endocrine functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Lee, J.E.; Walton, D.; O’Connor, C.P.; Wammes, M.; Burton, J.P.; Osuch, E.A. Drugs, guts, brains, but not rock and roll: The need to consider the role of gut microbiota in contemporary mental health and wellness of emerging adults. Int. J. Mol. Sci. 2022, 23, 6643. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Abdalkareem Jasim, S.; Jade Catalan Opulencia, M.; Alexis Ramírez-Coronel, A.; Kamal Abdelbasset, W.; Hasan Abed, M.; Markov, A.; Raheem Lateef Al-Awsi, G.; Azamatovich Shamsiev, J.; Thaeer Hammid, A.; Nader Shalaby, M.; et al. The emerging role of microbiota-derived short-chain fatty acids in immunometabolism. Int. Immunopharmacol. 2022, 110, 108983. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Fung, T.C. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol. Dis. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef]
- Welcome, M.O. Gut Microbiota Disorder, Gut Epithelial and Blood-Brain Barrier Dysfunctions in Etiopathogenesis of Dementia: Molecular Mechanisms and Signaling Pathways. Neuromolecular Med. 2019, 21, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Omenetti, S.; Bussi, C.; Metidji, A.; Iseppon, A.; Lee, S.; Tolaini, M.; Li, Y.; Kelly, G.; Chakravarty, P.; Shoaie, S.; et al. The Intestine Harbors Functionally Distinct Homeostatic Tissue-Resident and Inflammatory Th17 Cells. Immunity 2019, 51, 77–89.e6. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.-A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Kim, J.A.; Park, M.S.; Kang, S.A.; Ji, G.E. Production of γ-aminobutyric acid during fermentation of Gastrodia elata Bl. by co-culture of Lactobacillus brevis GABA 100 with Bifidobacterium bifidum BGN4. Food Sci. Biotechnol. 2014, 23, 459–466. [Google Scholar] [CrossRef]
- Diez-Gutiérrez, L.; San Vicente, L.; Barrón, L.J.R.; Villarán, M.d.C.; Chávarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Mele, M.; Costa, R.O.; Duarte, C.B. Alterations in GABAA-Receptor Trafficking and Synaptic Dysfunction in Brain Disorders. Front. Cell. Neurosci. 2019, 13, 77. [Google Scholar] [CrossRef]
- Golofast, B.; Vales, K. The connection between microbiome and schizophrenia. Neurosci. Biobehav. Rev. 2020, 108, 712–731. [Google Scholar] [CrossRef]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the role of the gut microbiome in brain development and its association with neurodevelopmental psychiatric disorders. Front. Cell Dev. Biol. 2022, 10, 880544. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Dovgan, K.; Takahashi, N.; Beversdorf, D.Q. The relationship among gastrointestinal symptoms, problem behaviors, and internalizing symptoms in children and adolescents with autism spectrum disorder. Front. Psychiatry 2019, 10, 194. [Google Scholar] [CrossRef]
- Lefter, R.; Ciobica, A.; Timofte, D.; Stanciu, C.; Trifan, A. A descriptive review on the prevalence of gastrointestinal disturbances and their multiple associations in autism spectrum disorder. Medicina 2019, 56, 11. [Google Scholar] [CrossRef]
- Kim, A.; Zisman, C.R.; Holingue, C. Influences of the immune system and microbiome on the etiology of ASD and GI symptomology of autistic individuals. In Current Topics in Behavioral Neurosciences; Springer: Berlin, Heidelberg, 2022. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef]
- Vuong, H.E.; Hsiao, E.Y. Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry 2017, 81, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, X.; Li, J.; Li, F. Association Between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef] [PubMed]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current evidence on the role of the gut microbiome in ADHD pathophysiology and therapeutic implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Richarte, V.; Sánchez-Mora, C.; Corrales, M.; Fadeuilhe, C.; Vilar-Ribó, L.; Arribas, L.; Garcia, E.; Rosales-Ortiz, S.K.; Arias-Vasquez, A.; Soler-Artigas, M.; et al. Gut microbiota signature in treatment-naïve attention-deficit/hyperactivity disorder. Transl. Psychiatry 2021, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Lacorte, E.; Gervasi, G.; Bacigalupo, I.; Vanacore, N.; Raucci, U.; Parisi, P. A systematic review of the microbiome in children with neurodevelopmental disorders. Front. Neurol. 2019, 10, 727. [Google Scholar] [CrossRef]
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Yeh, T.-C.; Bai, Y.-M.; Tsai, S.-J.; Chen, T.-J.; Liang, C.-S.; Chen, M.-H. Risks of Major Mental Disorders and Irritable Bowel Syndrome among the Offspring of Parents with Irritable Bowel Syndrome: A Nationwide Study. Int. J. Environ. Res. Public Health 2021, 18, 4679. [Google Scholar] [CrossRef]
- Shah, E.; Rezaie, A.; Riddle, M.; Pimentel, M. Psychological disorders in gastrointestinal disease: Epiphenomenon, cause or consequence? Ann. Gastroenterol. 2014, 27, 224–230. [Google Scholar]
- Klein-Petersen, A.W.; Köhler-Forsberg, O.; Benros, M.E. Infections, antibiotic treatment and the Microbiome in relation to schizophrenia. Schizophr. Res. 2021, 234, 71–77. [Google Scholar] [CrossRef]
- Severance, E.G.; Yolken, R.H.; Eaton, W.W. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: More than a gut feeling. Schizophr. Res. 2016, 176, 23–35. [Google Scholar] [CrossRef]
- Patrono, E.; Svoboda, J.; Stuchlík, A. Schizophrenia, the gut microbiota, and new opportunities from optogenetic manipulations of the gut-brain axis. Behav. Brain Funct. 2021, 17, 7. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Zhu, F.; Ju, Y.; Wang, W.; Wang, Q.; Guo, R.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 2020, 11, 1612. [Google Scholar] [CrossRef]
- Castro-Nallar, E.; Bendall, M.L.; Pérez-Losada, M.; Sabuncyan, S.; Severance, E.G.; Dickerson, F.B.; Schroeder, J.R.; Yolken, R.H.; Crandall, K.A. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 2015, 3, e1140. [Google Scholar] [CrossRef]
- Bojović, K.; Ignjatović, Ð.-D.I.; Soković Bajić, S.; Vojnović Milutinović, D.; Tomić, M.; Golić, N.; Tolinački, M. Gut microbiota dysbiosis associated with altered production of short chain fatty acids in children with neurodevelopmental disorders. Front. Cell. Infect. Microbiol. 2020, 10, 223. [Google Scholar] [CrossRef]
- Jolanta Wasilewska, J.; Klukowski, M. Gastrointestinal symptoms and autism spectrum disorder: Links and risks—A possible new overlap syndrome. PHMT 2015, 2015, 153–166. [Google Scholar] [CrossRef]
- Zhang, D.-M.; Ye, J.-X.; Mu, J.-S.; Cui, X.-P. Efficacy of Vitamin B Supplementation on Cognition in Elderly Patients With Cognitive-Related Diseases. J. Geriatr. Psychiatry Neurol. 2017, 30, 50–59. [Google Scholar] [CrossRef]
- Sarubbo, F.; Cavallucci, V.; Pani, G. The influence of gut microbiota on neurogenesis: Evidence and hopes. Cells 2022, 11, 382. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef] [PubMed]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The human gut microbiome as a potential factor in autism spectrum disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Balasuriya, G.K.; Franks, A.E.; Hill-Yardin, E.L. Using Animal Models to Study the Role of the Gut-Brain Axis in Autism. Curr. Dev. Disord. Rep. 2017, 4, 28–36. [Google Scholar] [CrossRef]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef]
- Hosie, S.; Ellis, M.; Swaminathan, M.; Ramalhosa, F.; Seger, G.O.; Balasuriya, G.K.; Gillberg, C.; Råstam, M.; Churilov, L.; McKeown, S.J.; et al. Gastrointestinal dysfunction in patients and mice expressing the autism-associated R451C mutation in neuroligin-3. Autism Res. 2019, 12, 1043–1056. [Google Scholar] [CrossRef]
- Herath, M.; Bornstein, J.C.; Hill-Yardin, E.L.; Franks, A.E. The autism-associated Neuroligin-3 R451C mutation alters mucus density and the spatial distribution of bacteria in the mouse gastrointestinal tract. BioRxiv 2022. [Google Scholar] [CrossRef]
- Leembruggen, A.J.L.; Balasuriya, G.K.; Zhang, J.; Schokman, S.; Swiderski, K.; Bornstein, J.C.; Nithianantharajah, J.; Hill-Yardin, E.L. Colonic dilation and altered ex vivo gastrointestinal motility in the neuroligin-3 knockout mouse. Autism Res. 2020, 13, 691–701. [Google Scholar] [CrossRef]
- Sauer, A.K.; Bockmann, J.; Steinestel, K.; Boeckers, T.M.; Grabrucker, A.M. Altered intestinal morphology and microbiota composition in the autism spectrum disorders associated SHANK3 mouse model. Int. J. Mol. Sci. 2019, 20, 2134. [Google Scholar] [CrossRef]
- Tabouy, L.; Getselter, D.; Ziv, O.; Karpuj, M.; Tabouy, T.; Lukic, I.; Maayouf, R.; Werbner, N.; Ben-Amram, H.; Nuriel-Ohayon, M.; et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav. Immun. 2018, 73, 310–319. [Google Scholar] [CrossRef]
- Alotaibi, M.; Ramzan, K. A de novo variant of CHD8 in a patient with autism spectrum disorder. Discoveries 2020, 8, e107. [Google Scholar] [CrossRef]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Vulto-van Silfhout, A.T.; et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, B.; Ji, P.; Zuo, Z.; Huang, Y.; Wang, N.; Liu, C.; Liu, S.-J.; Zhao, F. Changes to gut amino acid transporters and microbiome associated with increased E/I ratio in Chd8+/- mouse model of ASD-like behavior. Nat. Commun. 2022, 13, 1151. [Google Scholar] [CrossRef]
- Katayama, Y.; Nishiyama, M.; Shoji, H.; Ohkawa, Y.; Kawamura, A.; Sato, T.; Suyama, M.; Takumi, T.; Miyakawa, T.; Nakayama, K.I. CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature 2016, 537, 675–679. [Google Scholar] [CrossRef]
- Chatterjee, I.; Getselter, D.; Ghaneem, N.; Bel, S.; Elliott, E. CHD8 regulates gut epithelial cell function and affects autism-related behaviours through the gut-brain axis. BioRxiv 2021. [Google Scholar] [CrossRef]
- Gaudissard, J.; Ginger, M.; Premoli, M.; Memo, M.; Frick, A.; Pietropaolo, S. Behavioral abnormalities in the Fmr1-KO2 mouse model of fragile X syndrome: The relevance of early life phases. Autism Res. 2017, 10, 1584–1596. [Google Scholar] [CrossRef]
- Altimiras, F.; Garcia, J.A.; Palacios-García, I.; Hurley, M.J.; Deacon, R.; González, B.; Cogram, P. Altered gut microbiota in a fragile X syndrome mouse model. Front. Neurosci. 2021, 15, 653120. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Blanchard, D.C. The BTBR mouse model of idiopathic autism—Current view on mechanisms. Neurosci. Biobehav. Rev. 2017, 76, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Coretti, L.; Cristiano, C.; Florio, E.; Scala, G.; Lama, A.; Keller, S.; Cuomo, M.; Russo, R.; Pero, R.; Paciello, O.; et al. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci. Rep. 2017, 7, 45356. [Google Scholar] [CrossRef] [PubMed]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Di Gesù, C.M.; Matz, L.M.; Buffington, S.A. Diet-induced dysbiosis of the maternal gut microbiome in early life programming of neurodevelopmental disorders. Neurosci. Res. 2021, 168, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.C.; Montgomery, J.M.; Taylor, M.W. The Gut-Microbiota-Brain Axis in Autism Spectrum Disorder. In Autism Spectrum Disorders; Grabrucker, A.M., Ed.; Exon Publications: Brisbane, Australia, 2021; ISBN 9780645001785. [Google Scholar]
- Lammert, C.R.; Lukens, J.R. Modeling Autism-Related Disorders in Mice with Maternal Immune Activation (MIA). Methods Mol. Biol. 2019, 1960, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Feng, X.; Song, M.; Shao, M.; Yang, Y.; Zhang, L.; Liu, Q.; Lv, L.; Su, X. Maternal immune activation alters adult behavior, intestinal integrity, gut microbiota and the gut inflammation. Brain Behav. 2021, 11, e02133. [Google Scholar] [CrossRef]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast milk microbiota is shaped by mode of delivery and intrapartum antibiotic exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Daliry, A.; Pereira, E.N.G.d.S. Role of Maternal Microbiota and Nutrition in Early-Life Neurodevelopmental Disorders. Nutrients 2021, 13, 3533. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Doi, M.; Usui, N.; Shimada, S. Prenatal environment and neurodevelopmental disorders. Front. Endocrinol. 2022, 13, 860110. [Google Scholar] [CrossRef]
- Stokholm, J.; Schjørring, S.; Eskildsen, C.E.; Pedersen, L.; Bischoff, A.L.; Følsgaard, N.; Carson, C.G.; Chawes, B.L.K.; Bønnelykke, K.; Mølgaard, A.; et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin. Microbiol. Infect. 2014, 20, 629–635. [Google Scholar] [CrossRef]
- Persaud, R.R.; Azad, M.B.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Kozyrskyj, A.L.; CHILD Study Investigators. Perinatal antibiotic exposure of neonates in Canada and associated risk factors: A population-based study. J. Matern. Fetal Neonatal Med. 2015, 28, 1190–1195. [Google Scholar] [CrossRef]
- Koebnick, C.; Tartof, S.Y.; Sidell, M.A.; Rozema, E.; Chung, J.; Chiu, V.Y.; Taylor, Z.W.; Xiang, A.H.; Getahun, D. Effect of In-Utero Antibiotic Exposure on Childhood Outcomes: Methods and Baseline Data of the Fetal Antibiotic EXposure (FAX) Cohort Study. JMIR Res. Protoc. 2019, 8, e12065. [Google Scholar] [CrossRef]
- Petersen, I.; Gilbert, R.; Evans, S.; Ridolfi, A.; Nazareth, I. Oral antibiotic prescribing during pregnancy in primary care: UK population-based study. J. Antimicrob. Chemother. 2010, 65, 2238–2246. [Google Scholar] [CrossRef]
- Kenyon, S.; Pike, K.; Jones, D.R.; Brocklehurst, P.; Marlow, N.; Salt, A.; Taylor, D.J. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 2008, 372, 1319–1327. [Google Scholar] [CrossRef]
- Andersen, J.T.; Petersen, M.; Jimenez-Solem, E.; Rasmussen, J.N.; Andersen, N.L.; Afzal, S.; Broedbaek, K.; Hjelvang, B.R.; Køber, L.; Torp-Pedersen, C.; et al. Trimethoprim Use prior to Pregnancy and the Risk of Congenital Malformation: A Register-Based Nationwide Cohort Study. Obstet. Gynecol. Int. 2013, 2013, 364526. [Google Scholar] [CrossRef]
- Hamad, A.F.; Alessi-Severini, S.; Mahmud, S.; Brownell, M.; Kuo, I.F. Prenatal antibiotic exposure and risk of attention-deficit/hyperactivity disorder: A population-based cohort study. CMAJ 2020, 192, E527–E535. [Google Scholar] [CrossRef]
- Wimberley, T.; Agerbo, E.; Pedersen, C.B.; Dalsgaard, S.; Horsdal, H.T.; Mortensen, P.B.; Thompson, W.K.; Köhler-Forsberg, O.; Yolken, R.H. Otitis media, antibiotics, and risk of autism spectrum disorder. Autism Res. 2018, 11, 1432–1440. [Google Scholar] [CrossRef]
- Atladóttir, H.Ó.; Henriksen, T.B.; Schendel, D.E.; Parner, E.T. Autism after infection, febrile episodes, and antibiotic use during pregnancy: An exploratory study. Pediatrics 2012, 130, e1447–e1454. [Google Scholar] [CrossRef] [PubMed]
- Lavebratt, C.; Yang, L.L.; Giacobini, M.; Forsell, Y.; Schalling, M.; Partonen, T.; Gissler, M. Early exposure to antibiotic drugs and risk for psychiatric disorders: A population-based study. Transl. Psychiatry 2019, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Tochitani, S.; Ikeno, T.; Ito, T.; Sakurai, A.; Yamauchi, T.; Matsuzaki, H. Administration of Non-Absorbable Antibiotics to Pregnant Mice to Perturb the Maternal Gut Microbiota Is Associated with Alterations in Offspring Behavior. PLoS ONE 2016, 11, e0138293. [Google Scholar] [CrossRef] [PubMed]
- Harshaw, C.; Kojima, S.; Wellman, C.L.; Demas, G.E.; Morrow, A.L.; Taft, D.H.; Kenkel, W.M.; Leffel, J.K.; Alberts, J.R. Maternal antibiotics disrupt microbiome, behavior, and temperature regulation in unexposed infant mice. Dev. Psychobiol. 2022, 64, e22289. [Google Scholar] [CrossRef]
- Degroote, S.; Hunting, D.J.; Baccarelli, A.A.; Takser, L. Maternal gut and fetal brain connection: Increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 76–82. [Google Scholar] [CrossRef]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. The impact of studying brain plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef]
- Smith, K.E.; Pollak, S.D. Early life stress and development: Potential mechanisms for adverse outcomes. J. Neurodev. Disord. 2020, 12, 34. [Google Scholar] [CrossRef]
- Sturman, D.A.; Moghaddam, B. The neurobiology of adolescence: Changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci. Biobehav. Rev. 2011, 35, 1704–1712. [Google Scholar] [CrossRef]
- Duong, Q.A.; Pittet, L.F.; Curtis, N.; Zimmermann, P. Antibiotic exposure and adverse long-term health outcomes in children: A systematic review and meta-analysis. J. Infect. 2022, 85, 213–300. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Łukasik, J.; Patro-Gołąb, B.; Horvath, A.; Baron, R.; Szajewska, H.; SAWANTI Working Group. Early life exposure to antibiotics and autism spectrum disorders: A systematic review. J. Autism Dev. Disord. 2019, 49, 3866–3876. [Google Scholar] [CrossRef]
- Neuman, H.; Forsythe, P.; Uzan, A.; Avni, O.; Koren, O. Antibiotics in early life: Dysbiosis and the damage done. FEMS Microbiol. Rev. 2018, 42, 489–499. [Google Scholar] [CrossRef]
- Volker, E.; Tessier, C.; Rodriguez, N.; Yager, J.; Kozyrskyj, A. Pathways of atopic disease and neurodevelopmental impairment: Assessing the evidence for infant antibiotics. Expert Rev. Clin. Immunol. 2022, 18, 901–922. [Google Scholar] [CrossRef]
- Bittker, S.S.; Bell, K.R. Acetaminophen, antibiotics, ear infection, breastfeeding, vitamin D drops, and autism: An epidemiological study. Neuropsychiatr. Dis. Treat. 2018, 14, 1399–1414. [Google Scholar] [CrossRef]
- Fallon, J. Could one of the most widely prescribed antibiotics amoxicillin/clavulanate “augmentin” be a risk factor for autism? Med. Hypotheses 2005, 64, 312–315. [Google Scholar] [CrossRef]
- Niehus, R.; Lord, C. Early medical history of children with autism spectrum disorders. J. Dev. Behav. Pediatr. 2006, 27, S120–S127. [Google Scholar] [CrossRef]
- Köhler, O.; Petersen, L.; Mors, O.; Mortensen, P.B.; Yolken, R.H.; Gasse, C.; Benros, M.E. Infections and exposure to anti-infective agents and the risk of severe mental disorders: A nationwide study. Acta Psychiatr. Scand. 2017, 135, 97–105. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Petersen, L.; Gasse, C.; Mortensen, P.B.; Dalsgaard, S.; Yolken, R.H.; Mors, O.; Benros, M.E. A nationwide study in denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents. JAMA Psychiatry 2019, 76, 271–279. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Thompson, J.; Waldie, K.E.; Murphy, R.; Wall, C.; Mitchell, E.A. Antibiotics in the first year of life and subsequent neurocognitive outcomes. Acta Paediatr. 2017, 106, 87–94. [Google Scholar] [CrossRef]
- Hamad, A.F.; Alessi-Severini, S.; Mahmud, S.M.; Brownell, M.; Kuo, I.F. Antibiotic Exposure in the First Year of Life and the Risk of Attention-Deficit/Hyperactivity Disorder: A Population-Based Cohort Study. Am. J. Epidemiol. 2019, 188, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.L.; Nelson, C.A. Brain development and the role of experience in the early years. Zero Three 2009, 30, 9–13. [Google Scholar] [PubMed]
- Slykerman, R.F.; Coomarasamy, C.; Wickens, K.; Thompson, J.M.D.; Stanley, T.V.; Barthow, C.; Kang, J.; Crane, J.; Mitchell, E.A. Exposure to antibiotics in the first 24 months of life and neurocognitive outcomes at 11 years of age. Psychopharmacology 2019, 236, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Slob, E.M.A.; Brew, B.K.; Vijverberg, S.J.H.; Dijs, T.; van Beijsterveldt, C.E.M.; Koppelman, G.H.; Bartels, M.; Dolan, C.V.; Larsson, H.; Lundström, S.; et al. Early-life antibiotic use and risk of attention-deficit hyperactivity disorder and autism spectrum disorder: Results of a discordant twin study. Int. J. Epidemiol. 2021, 50, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, P.B.; Clausen, T.D.; Petersen, A.H.; Hageman, I.; Pinborg, A.; Kessing, L.V.; Bergholt, T.; Rasmussen, S.C.; Keiding, N.; Løkkegaard, E.C.L. Investigating the effects of cesarean delivery and antibiotic use in early childhood on risk of later attention deficit hyperactivity disorder. J. Child Psychol. Psychiatry 2019, 60, 151–159. [Google Scholar] [CrossRef]
- Karlsson, H.; Sjöqvist, H.; Brynge, M.; Gardner, R.; Dalman, C. Childhood infections and autism spectrum disorders and/or intellectual disability: A register-based cohort study. J. Neurodev. Disord. 2022, 14, 12. [Google Scholar] [CrossRef]
- Nudel, R.; Wang, Y.; Appadurai, V.; Schork, A.J.; Buil, A.; Agerbo, E.; Bybjerg-Grauholm, J.; Børglum, A.D.; Daly, M.J.; Mors, O.; et al. A large-scale genomic investigation of susceptibility to infection and its association with mental disorders in the Danish population. Transl. Psychiatry 2019, 9, 283. [Google Scholar] [CrossRef]
- Gau, S.S.-F.; Chang, L.-Y.; Huang, L.-M.; Fan, T.-Y.; Wu, Y.-Y.; Lin, T.-Y. Attention-deficit/hyperactivity-related symptoms among children with enterovirus 71 infection of the central nervous system. Pediatrics 2008, 122, e452–e458. [Google Scholar] [CrossRef]
- Singh, G.; Prabhakar, S. The association between central nervous system (CNS) infections and epilepsy: Epidemiological approaches and microbiological and epileptological perspectives. Epilepsia 2008, 49, 2–7. [Google Scholar] [CrossRef]
- Koponen, H.; Rantakallio, P.; Veijola, J.; Jones, P.; Jokelainen, J.; Isohanni, M. Childhood central nervous system infections and risk for schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 9–13. [Google Scholar] [CrossRef]
- Sabourin, K.R.; Reynolds, A.; Schendel, D.; Rosenberg, S.; Croen, L.A.; Pinto-Martin, J.A.; Schieve, L.A.; Newschaffer, C.; Lee, L.-C.; DiGuiseppi, C. Infections in children with autism spectrum disorder: Study to Explore Early Development (SEED). Autism Res. 2019, 12, 136–146. [Google Scholar] [CrossRef]
- Pedersen, E.M.J.; Köhler-Forsberg, O.; Nordentoft, M.; Christensen, R.H.B.; Mortensen, P.B.; Petersen, L.; Benros, M.E. Infections of the central nervous system as a risk factor for mental disorders and cognitive impairment: A nationwide register-based study. Brain Behav. Immun. 2020, 88, 668–674. [Google Scholar] [CrossRef]
- Adams, D.J.; Susi, A.; Erdie-Lalena, C.R.; Gorman, G.; Hisle-Gorman, E.; Rajnik, M.; Elrod, M.; Nylund, C.M. Otitis Media and Related Complications Among Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2016, 46, 1636–1642. [Google Scholar] [CrossRef]
- Sadaka, Y.; Freedman, J.; Ashkenazi, S.; Vinker, S.; Golan-Cohen, A.; Green, I.; Israel, A.; Eran, A.; Merzon, E. The Effect of Antibiotic Treatment of Early Childhood Shigellosis on Long-Term Prevalence of Attention Deficit/Hyperactivity Disorder. Children 2021, 8, 880. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef]
- Schmidtner, A.K.; Slattery, D.A.; Gläsner, J.; Hiergeist, A.; Gryksa, K.; Malik, V.A.; Hellmann-Regen, J.; Heuser, I.; Baghai, T.C.; Gessner, A.; et al. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Transl. Psychiatry 2019, 9, 223. [Google Scholar] [CrossRef]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017, 8, 15062. [Google Scholar] [CrossRef]
- Bistoletti, M.; Caputi, V.; Baranzini, N.; Marchesi, N.; Filpa, V.; Marsilio, I.; Cerantola, S.; Terova, G.; Baj, A.; Grimaldi, A.; et al. Antibiotic treatment-induced dysbiosis differently affects BDNF and TrkB expression in the brain and in the gut of juvenile mice. PLoS ONE 2019, 14, e0212856. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Perna, J.; Lu, J.; Mullen, B.; Liu, T.; Tjia, M.; Weiser, S.; Ackman, J.; Zuo, Y. Perinatal penicillin exposure affects cortical development and sensory processing. Front. Mol. Neurosci. 2021, 14, 704219. [Google Scholar] [CrossRef] [PubMed]
- Helaly, A.M.N.; El-Attar, Y.A.; Khalil, M.; Ahmed Ghorab, D.S.E.-D.; El-Mansoury, A.M. Antibiotic abuse induced histopathological and neurobehavioral disorders in mice. Curr. Drug Saf. 2019, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Atli, O.; Demir-Ozkay, U.; Ilgin, S.; Aydin, T.H.; Akbulut, E.N.; Sener, E. Evidence for neurotoxicity associated with amoxicillin in juvenile rats. Hum. Exp. Toxicol. 2016, 35, 866–876. [Google Scholar] [CrossRef]
- Volkova, A.; Ruggles, K.; Schulfer, A.; Gao, Z.; Ginsberg, S.D.; Blaser, M.J. Effects of early-life penicillin exposure on the gut microbiome and frontal cortex and amygdala gene expression. iScience 2021, 24, 102797. [Google Scholar] [CrossRef]
- Liu, G.; Yu, Q.; Tan, B.; Ke, X.; Zhang, C.; Li, H.; Zhang, T.; Lu, Y. Gut dysbiosis impairs hippocampal plasticity and behaviors by remodeling serum metabolome. Gut Microbes 2022, 14, 2104089. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The effect of antibiotics on the composition of the intestinal microbiota—A systematic review. J. Infect. 2019, 79, 471–489. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef]
- Hao, W.-Z.; Li, X.-J.; Zhang, P.-W.; Chen, J.-X. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 2020, 284, 112691. [Google Scholar] [CrossRef]
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial neuroactive compounds produced by psychobiotics. Adv. Exp. Med. Biol. 2014, 817, 221–239. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive mechanisms of probiotics and their therapeutic potential. Front. Neurosci. 2019, 13, 1361. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-Promoting Role of Lactiplantibacillus plantarum Isolated from Fermented Foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Liu, G.; Chong, H.-X.; Chung, F.Y.-L.; Li, Y.; Liong, M.-T. Lactobacillus plantarum DR7 Modulated Bowel Movement and Gut Microbiota Associated with Dopamine and Serotonin Pathways in Stressed Adults. Int. J. Mol. Sci. 2020, 21, 4608. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Marler, S.; Ferguson, B.J.; Lee, E.B.; Peters, B.; Williams, K.C.; McDonnell, E.; Macklin, E.A.; Levitt, P.; Gillespie, C.H.; Anderson, G.M.; et al. Brief report: Whole blood serotonin levels and gastrointestinal symptoms in autism spectrum disorder. J. Autism Dev. Disord. 2016, 46, 1124–1130. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liu, W.-H.; Wu, C.-C.; Juan, Y.-C.; Wu, Y.-C.; Tsai, H.-P.; Wang, S.; Tsai, Y.-C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, KC01–KC05. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148. [Google Scholar] [CrossRef]
- Grossi, E.; Melli, S.; Dunca, D.; Terruzzi, V. Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Med. Case Rep. 2016, 4, 2050313X16666231. [Google Scholar] [CrossRef]
- Dickerson, F.B.; Stallings, C.; Origoni, A.; Katsafanas, E.; Savage, C.L.G.; Schweinfurth, L.A.B.; Goga, J.; Khushalani, S.; Yolken, R.H. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim. Care Companion CNS Disord. 2014, 16. [Google Scholar] [CrossRef]
- Gómez-Eguílaz, M.; Ramón-Trapero, J.L.; Pérez-Martínez, L.; Blanco, J.R. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: A pilot study. Benef. Microbes 2018, 9, 875–881. [Google Scholar] [CrossRef]
- Kim, B.; Hong, V.M.; Yang, J.; Hyun, H.; Im, J.J.; Hwang, J.; Yoon, S.; Kim, J.E. A Review of Fermented Foods with Beneficial Effects on Brain and Cognitive Function. Prev. Nutr. Food Sci. 2016, 21, 297–309. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism spectrum disorders: A randomized controlled trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef]
- Kalenik, A.; Kardaś, K.; Rahnama, A.; Sirojć, K.; Wolańczyk, T. Gut microbiota and probiotic therapy in ADHD: A review of current knowledge. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110277. [Google Scholar] [CrossRef]
- Arteaga-Henríquez, G.; Rosales-Ortiz, S.K.; Arias-Vásquez, A.; Bitter, I.; Ginsberg, Y.; Ibañez-Jimenez, P.; Kilencz, T.; Lavebratt, C.; Matura, S.; Reif, A.; et al. Treating impulsivity with probiotics in adults (PROBIA): Study protocol of a multicenter, double-blind, randomized, placebo-controlled trial. Trials 2020, 21, 161. [Google Scholar] [CrossRef]
- Ng, Q.X.; Soh, A.Y.S.; Venkatanarayanan, N.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.-S. A systematic review of the effect of probiotic supplementation on schizophrenia symptoms. Neuropsychobiology 2019, 78, 1–6. [Google Scholar] [CrossRef]
- Tomasik, J.; Yolken, R.H.; Bahn, S.; Dickerson, F.B. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark Insights 2015, 10, 47–54. [Google Scholar] [CrossRef]
- Nagamine, T. The potential of probiotics in the treatment of schizophrenia. CNPT 2021, 12, 18–22. [Google Scholar] [CrossRef]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafanas, E.; Schweinfurth, L.A.; Savage, C.L.G.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S.; et al. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav. Immun. 2017, 62, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Okubo, R.; Koga, M.; Katsumata, N.; Odamaki, T.; Matsuyama, S.; Oka, M.; Narita, H.; Hashimoto, N.; Kusumi, I.; Xiao, J.; et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: A proof-of-concept study. J. Affect. Disord. 2019, 245, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; Banafshe, H.R.; Mirhosseini, N.; Moradi, M.; Karimi, M.-A.; Mehrzad, F.; Bahmani, F.; Asemi, Z. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry 2019, 19, 77. [Google Scholar] [CrossRef]
- Munawar, N.; Ahmad, A.; Anwar, M.A.; Muhammad, K. Modulation of Gut Microbial Diversity through Non-Pharmaceutical Approaches to Treat Schizophrenia. Int. J. Mol. Sci. 2022, 23, 2625. [Google Scholar] [CrossRef]
- Bravo, R.; Vicencio, J.M.; Parra, V.; Troncoso, R.; Munoz, J.P.; Bui, M.; Quiroga, C.; Rodriguez, A.E.; Verdejo, H.E.; Ferreira, J.; et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J. Cell Sci. 2011, 124, 2511. [Google Scholar] [CrossRef]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liong, M.T.; Chung, Y.-C.E.; Huang, H.-Y.; Peng, W.-S.; Cheng, Y.-F.; Lin, Y.-S.; Wu, Y.-Y.; Tsai, Y.-C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef]
| Antibiotics | Human Gut Bacteria | |
|---|---|---|
| Penicillin (Amoxicillin, ampicillin, Oxacillin, PenV) | ↑Enterobacteria ↑Bacteroidaceae | ↓Bifidobacteria ↓Lactobacilli ↓Eubacteria |
| Cephalosporins (Cefalor, Cafotaxime, Cefuroxime, Cefepime) | ↑Clostridia ↑Bacteroides spp. | ↓E. coli ↓Bifidobacteria ↓Enterobacteriaceae |
| Macrolides (Azithromycin, Clarithromycin, Erythromycin, Spiramycin) | ↑Enterococci ↑Streptococci ↑Bacteroidetes ↑Enterobacteria | ↓Actinobacteria ↓Clostriales spp. ↓Veillonella spp. |
| Bacteria | Health Condition | Experimental Model | Main Outcomes | Reference |
|---|---|---|---|---|
| L. rhamnosus JB-1 | Healthy condition | Adult male BALB/c mice | ↓stress-induced corticosterone and anxiety- and depression-related behavior Modulation of GABA expression at the brain level via the vagus nerve | [227] |
| L. plantarum DR7 | Mental stress condition | Adult patients | ↓stress; ↓anxiety; ↑cognitive functions ↑dopamine and norepinephrine ↓plasma cortisol and pro- inflammatory cytokines | [201] |
| L. plantarum DR7 | Mental stress condition | Adult patients | ↓stress; ↓anxiety; ↑cognitive functions Modulation of stress-induced bowel movement and gut microbiota in association with dopamine and serotonin | [202] |
| L. plantarum P8 | Mental stress condition | Adult patients | ↓stress; ↓anxiety; ↑memory and cognitive traits ↓pro-inflammatory markers | [228] |
| L. plantarum MTCC1325 | Neurodegenerative disorders | Albino rats | Behavioral changes; ↓cognitive deficits ↓acetylcholine levels | [209] |
| L. plantarum C29 | Adult with mild cognitive impairments | Adult patients | ↑cognitive functions especially in the attention domain ↑serum BDNF levels | [203] |
| L. casei Shirota | Chronic fatigue syndrome | Adult patients | ↓anxiety symptoms with modulation of gut microbiota | [229] |
| B. infantis 35624 | Maternal separation (MS) model | MS adult rat offsprings | Normalization of the immune response; ↓behavioral deficits ↓noradrenaline in the brain ↓depression-like behavior; ↓5-HT, noradrenaline and dopamine levels; ↑peripheral IL6 | [206] |
| L. helveticus R0052 combined with B. longum R0175 | Induced stress | Adult Wistar rats | ↓anxiety-like behavior; ↓stress-induced gastrointestinal discomfort | [205] |
| L. helveticus R0052 combined with B. longum R0175 | Induced stress | Adult patients | ↓psychological distress; ↓stress-induced gastrointestinal discomfort | [205] |
| L. helveticus NS8 | Chronic restraint induced stress | Sprague-Dawley rats | ↑intestinal barrier and BBB; ↓global inflammation status ↑BDNF; ↓serotonin and noradrenaline in the hippocampus ↑circulating anti-inflammatory cytokines | [210] |
| L. plantarum PS128 | Early life stress condition | Adult C57BL/6J mice | ↓locomotor activities anxiety-like and depression-like behaviors ↓serum corticosterone and inflammatory cytokine levels ↑anti-inflammatory cytokine levels ↑dopamine and serotonin in the prefrontal cortex | [208] |
| L. plantarum PS128 | ASD | Children | ↓Age-dependent autism symptoms; ↓behavioral aspects, such as disruptive and rule-breaking attitudes and hyperactivity/impulsivity | [230] |
| Bacteroides fragilis | ASD | Maternal immune activation murine model | ↓ASD-related defects in communicative, stereotypic, anxiety-like and sensorimotor behaviors Restoration of gut permeability and modulation of gut microbial composition | [128] |
| Vivomixx→ VSL#3 | ASD | 12-year-old boy | ↓severity of abdominal symptoms; ↓Autistic core symptoms Modulation of gut microbiota and positive regulation of intestinal barrier | [212] |
| Vivomixx→ VSL#3 | ASD | Preschool children | ↓GI symptoms; ↑multisensory processing, adaptive functions, and developmental pathways | [216,217] |
| L. acidophilus DSM32241, L. plantarum DSM32244, L. casei DSM32243, L. helveticus DSM32242, L. brevis DSM11988, B. lactis DSM32246, B. lactis DSM32247, and S. salivarius subsp. thermophilus DSM32245. | Drug-resistant Epilepsy | Adult patients | ↓epileptic seizures; ↑quality of life ↓serum IL-6 and sCD14; ↑serum GABA | [214] |
| L. rhamnosus GG | ADHD | Infants | Preventive effect in reducing the risk of developing ADHD | [87] |
| L. rhamnosus GG combined with B. animalis subsp. lactis Bb12 | SCZ | Adult patients | ↓severe bowel difficulty and prevention of common somatic symptoms associated with SCZ Positive effects on digestion and on GI disorders such as chronic constipation | [213] |
| L. rhamnosus GG combined with B. animalis subsp. lactis Bb12 | SCZ | Adult patients | ↑intestinal barrier integrity; ↓bowel difficulties via modulation of inflammatory cytokines belonging to IL17 family no significant impact on positive and negative syndrome scale (PANSS) psychiatric symptom scores | [221] |
| BIO-THREE® | SCZ | Adult patients | ↓constipation; ↓insulin resistance; ↓intestinal inflammation | [222] |
| B. breve A-1 | SCZ | Adult patients | Improved PANSS scores; ↓depression; ↓anxiety ↓symptoms with a modulation of IL22 and tumor necrosis factor-related activation induced cytokines (TRANCE) | [229] |
| B. bifidum, L. acidophilus, L. fermentum and L. reuteri combined with vitamin D | SCZ | Adult patients | Amelioration of PANSS scores ↓inflammation; ↑plasma total antioxidant capacity | [225] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamanti, T.; Prete, R.; Battista, N.; Corsetti, A.; De Jaco, A. Exposure to Antibiotics and Neurodevelopmental Disorders: Could Probiotics Modulate the Gut–Brain Axis? Antibiotics 2022, 11, 1767. https://doi.org/10.3390/antibiotics11121767
Diamanti T, Prete R, Battista N, Corsetti A, De Jaco A. Exposure to Antibiotics and Neurodevelopmental Disorders: Could Probiotics Modulate the Gut–Brain Axis? Antibiotics. 2022; 11(12):1767. https://doi.org/10.3390/antibiotics11121767
Chicago/Turabian StyleDiamanti, Tamara, Roberta Prete, Natalia Battista, Aldo Corsetti, and Antonella De Jaco. 2022. "Exposure to Antibiotics and Neurodevelopmental Disorders: Could Probiotics Modulate the Gut–Brain Axis?" Antibiotics 11, no. 12: 1767. https://doi.org/10.3390/antibiotics11121767
APA StyleDiamanti, T., Prete, R., Battista, N., Corsetti, A., & De Jaco, A. (2022). Exposure to Antibiotics and Neurodevelopmental Disorders: Could Probiotics Modulate the Gut–Brain Axis? Antibiotics, 11(12), 1767. https://doi.org/10.3390/antibiotics11121767