In Vitro Study on Green Propolis as a Potential Ingredient of Oral Health Care Products
Abstract
1. Introduction
2. Results
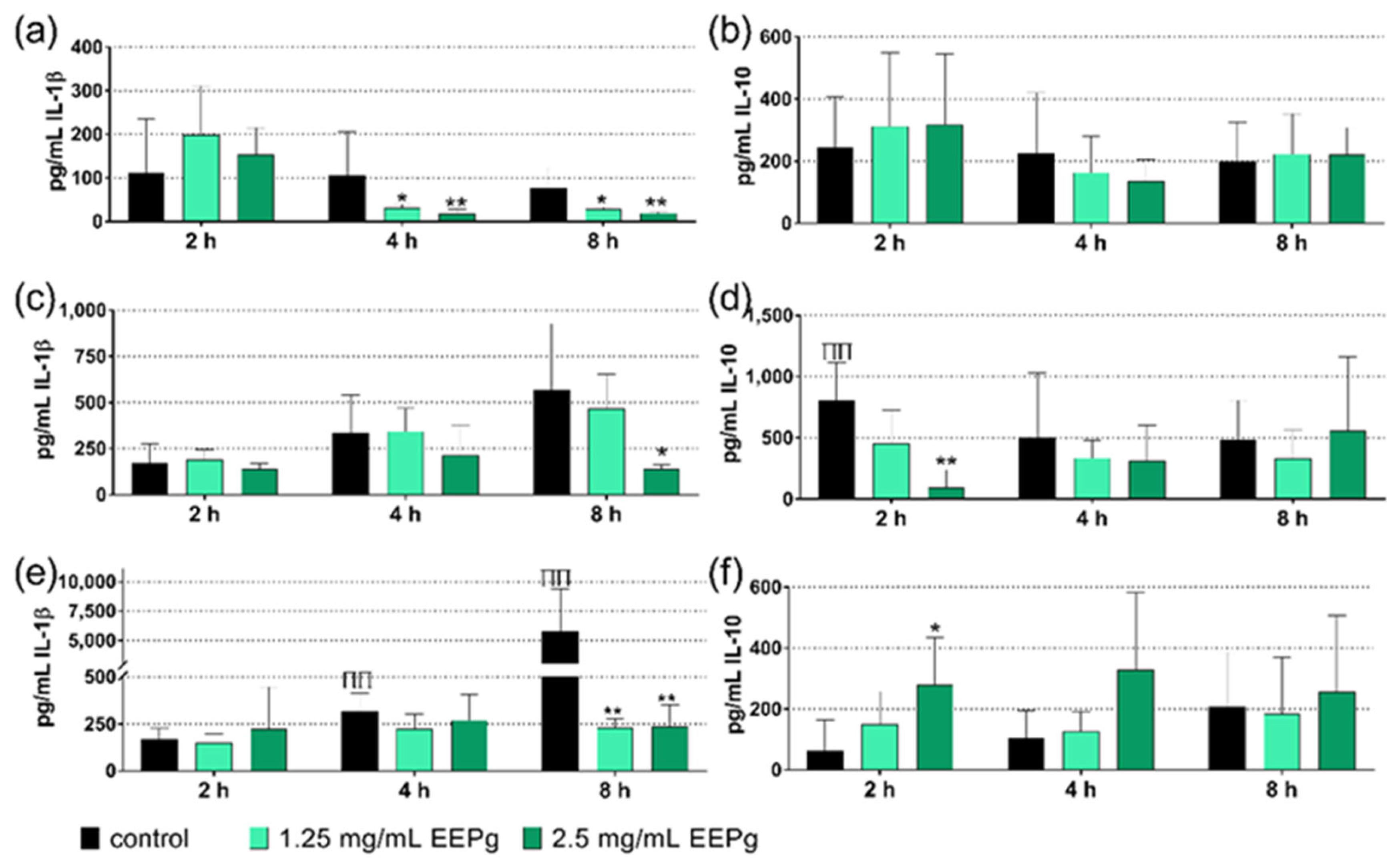
2.1. First Series: Interaction with MONO-MAC-6 Cells
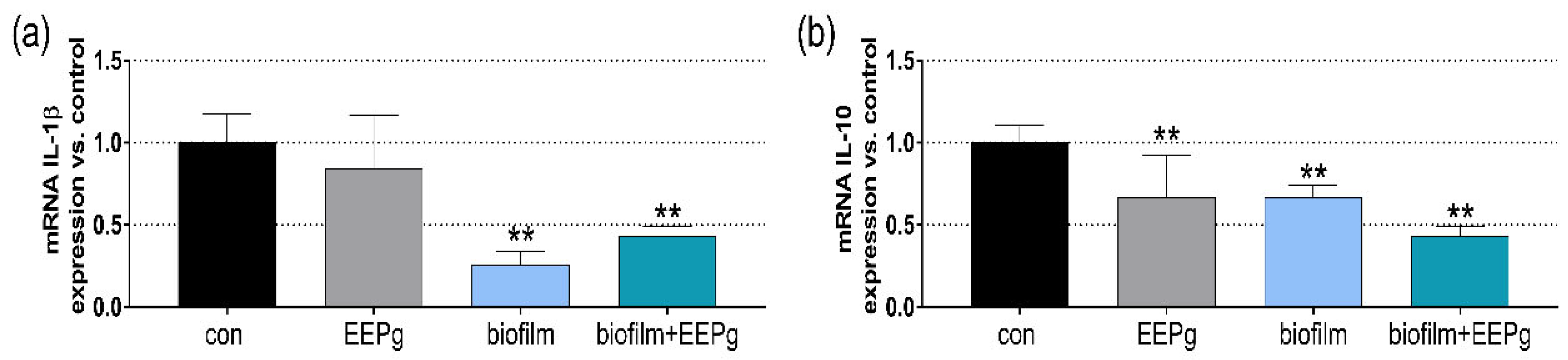
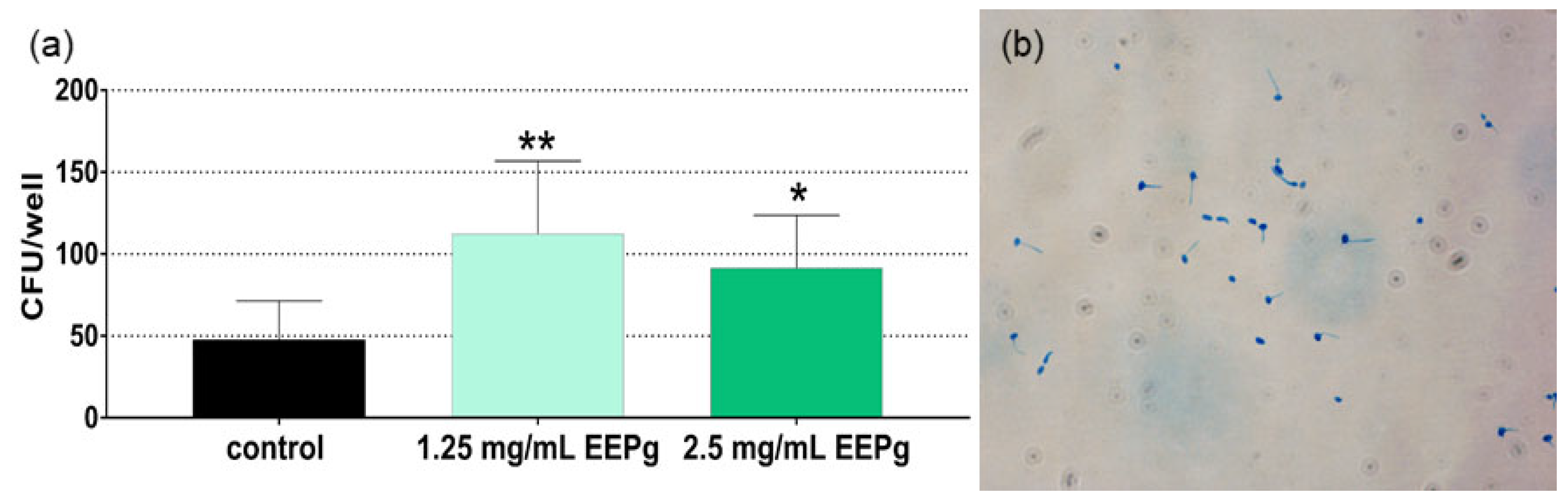
2.2. Second Series: Interaction with TIGK Cells
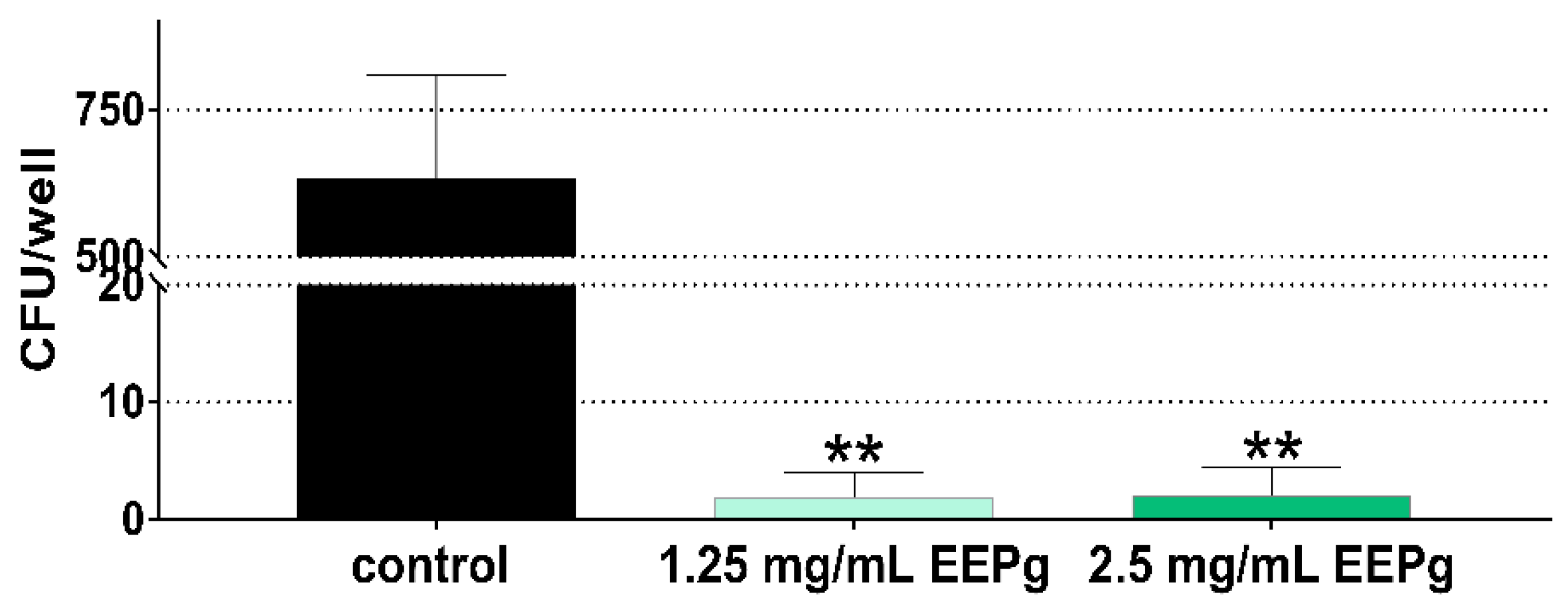
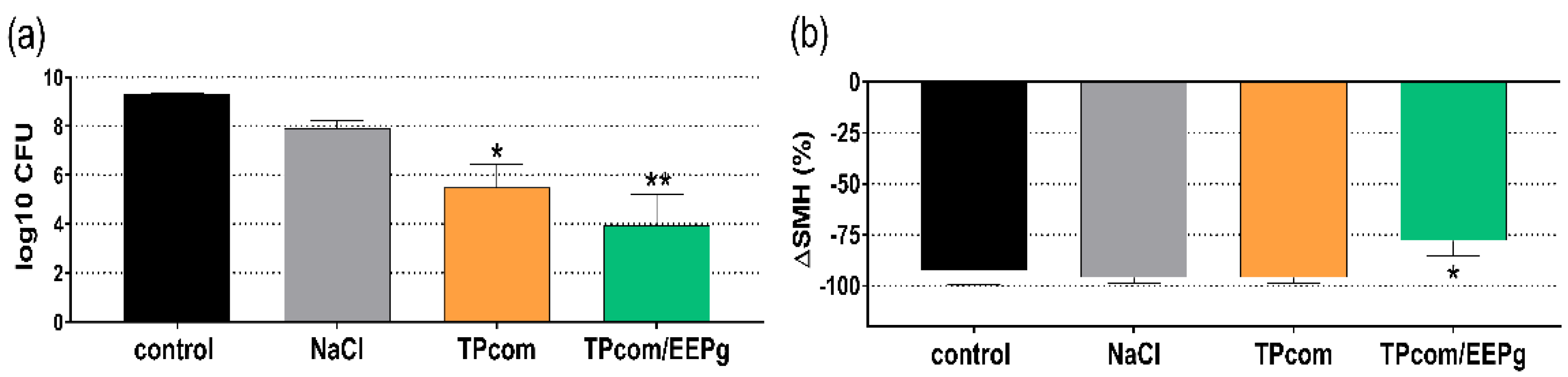
2.3. Third Series: Brushing, Biofilm Removal and Surface Microhardness Loss
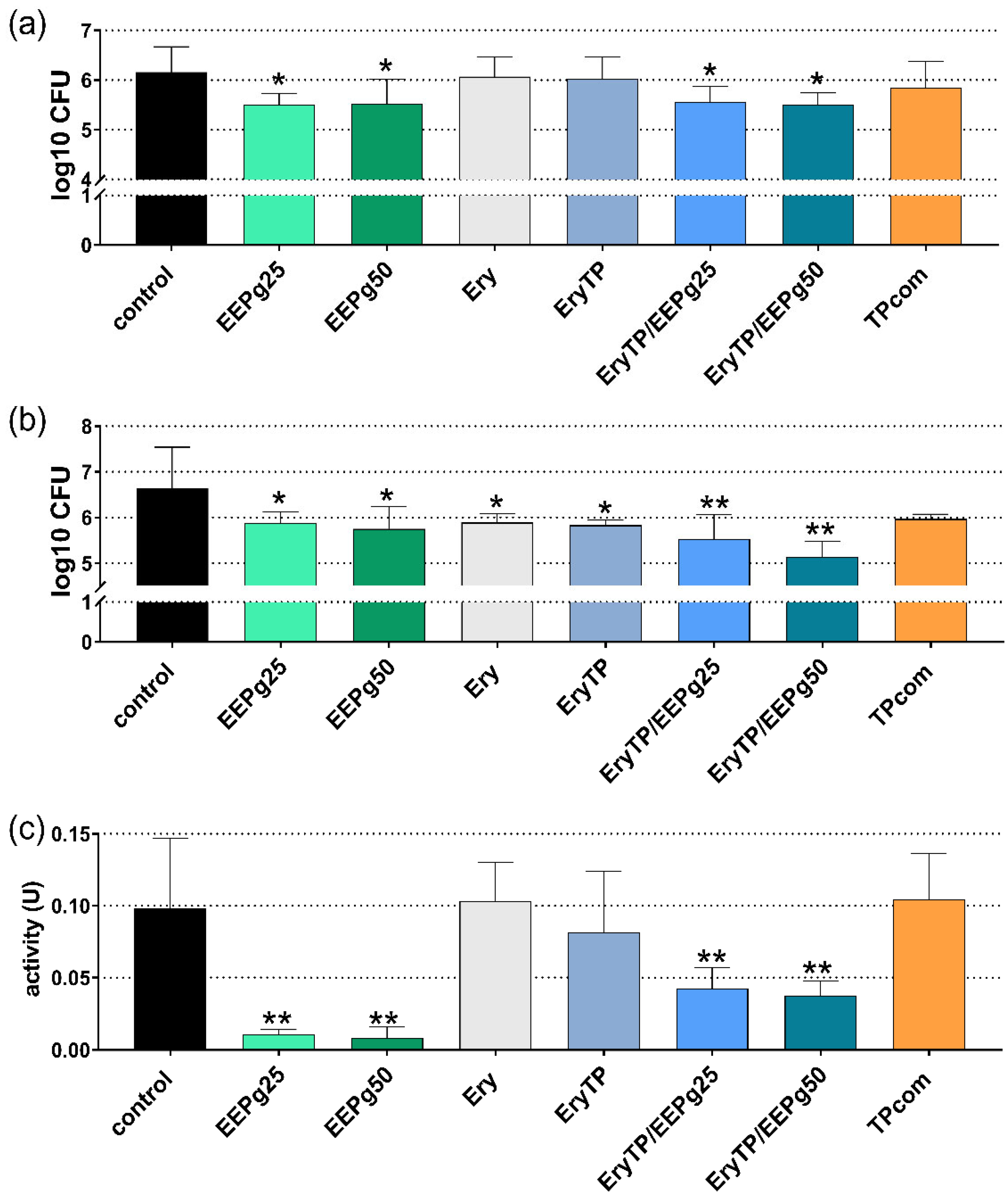
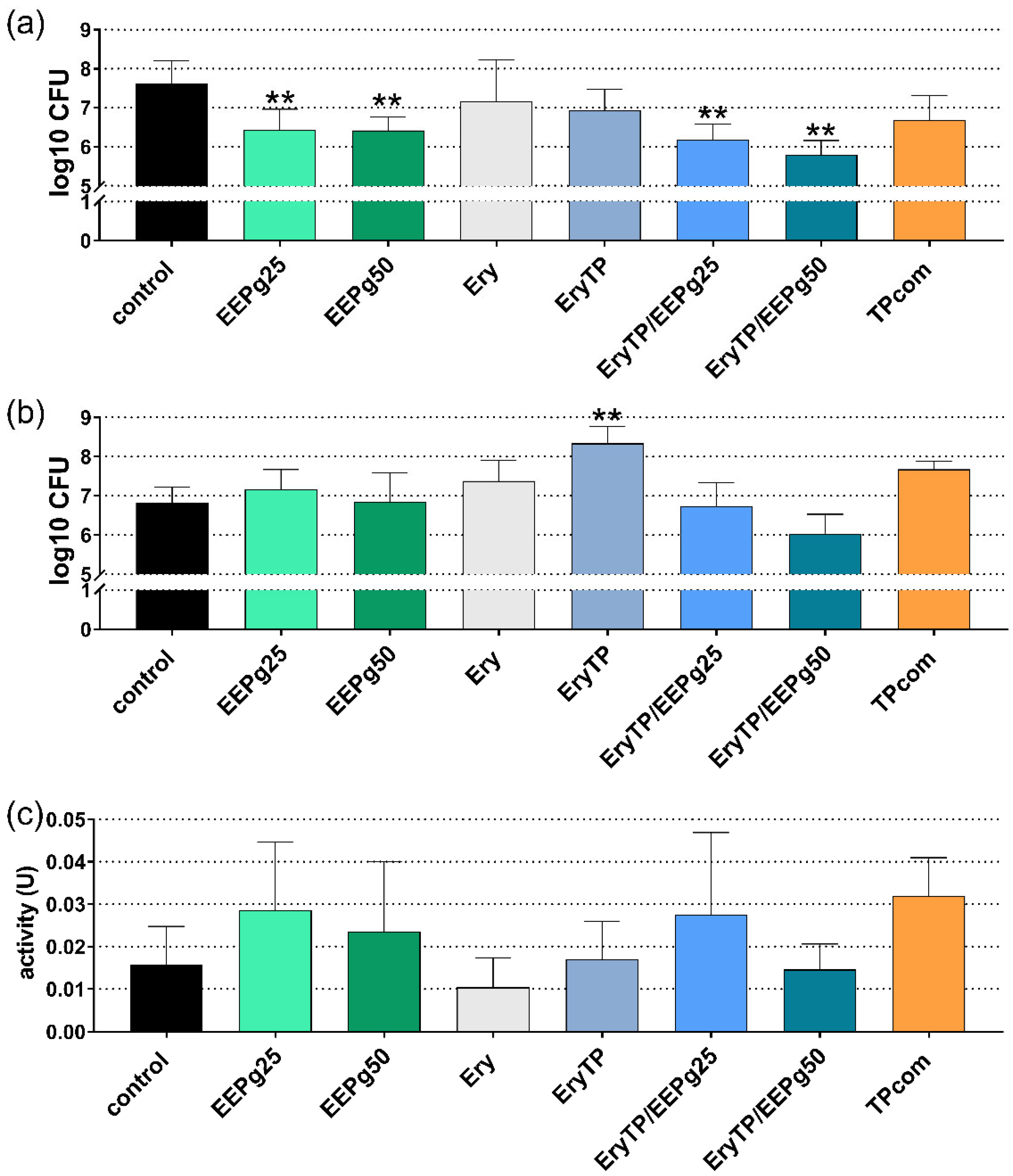
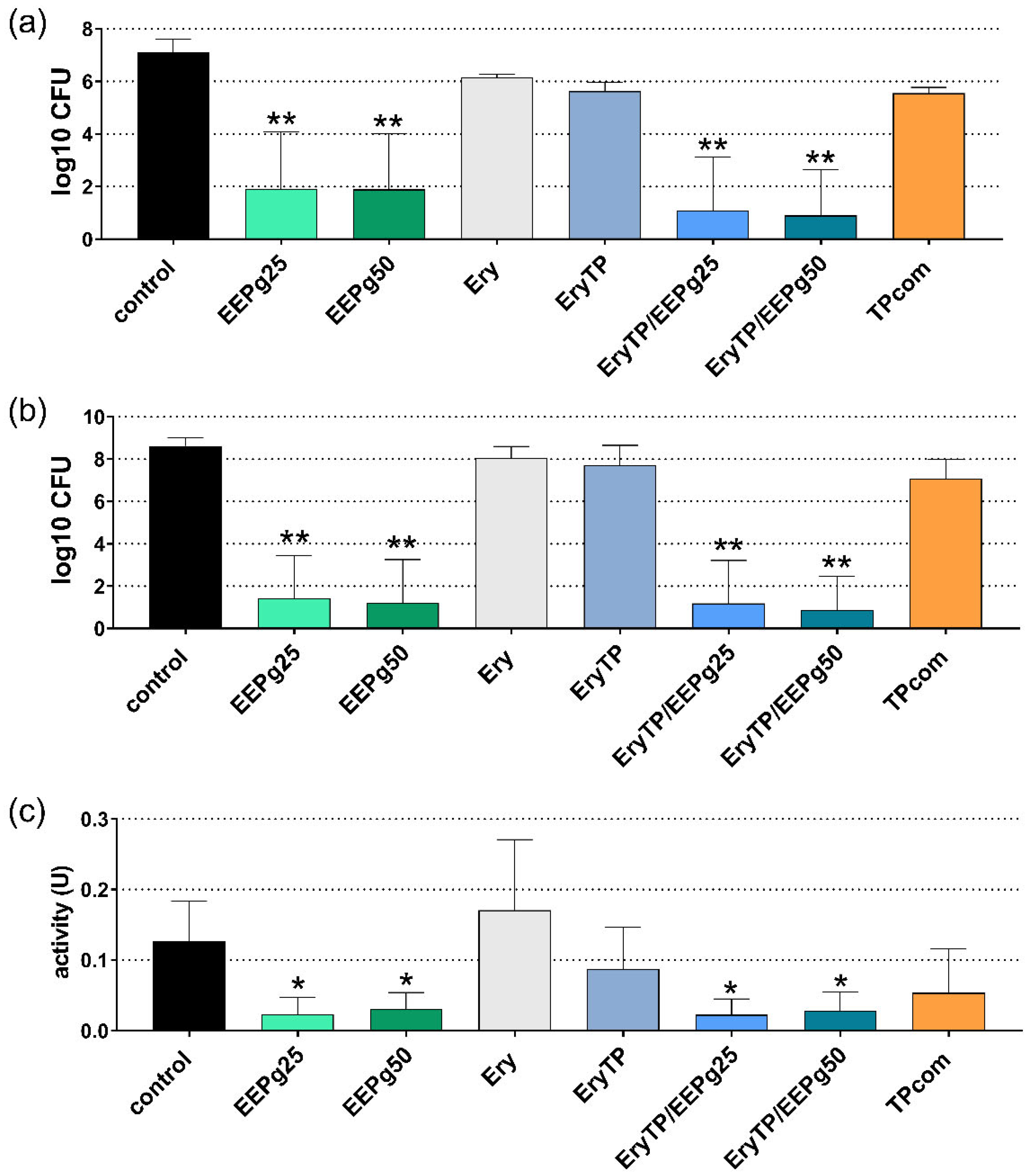
2.4. Fourth Series: Erythritol/Propolis Toothpaste
3. Discussion
4. Materials and Methods
4.1. Propolis, Toothpastes
4.2. Microorganisms
4.3. Cells
4.4. Methods First Series: Interaction with MONO-MAC-6 Cells
4.5. Methods Second Series: Microbial Adhesion to TIGK Cells
4.6. Methods Third Series: Substance Microhardness Loss
4.7. Methods Forth Series: Experimental Toothpaste
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical diversity and challenges in quality control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A. Perspectives for Uses of Propolis in Therapy against Infectious Diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research Progress on Therapeutic Effect and Mechanism of Propolis on Wound Healing. Evidence-Based Complement. Altern. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef] [PubMed]
- González-Serrano, J.; López-Pintor, R.M.; Serrano, J.; Torres, J.; Hernández, G.; Sanz, M. Short-term efficacy of a gel containing propolis extract, nanovitamin C and nanovitamin E on peri-implant mucositis: A double-blind, randomized, clinical trial. J. Periodontal Res. 2021, 56, 897–906. [Google Scholar] [CrossRef]
- Kiani, S.; Birang, R.; Jamshidian, N. Effect of Propolis mouthwash on clinical periodontal parameters in patients with gingivitis: A double-blinded randomized clinical trial. Int. J. Dent. Hyg. 2022, 20, 434–440. [Google Scholar] [CrossRef]
- Nakao, R.; Senpuku, H.; Ohnishi, M.; Takai, H.; Ogata, Y. Effect of topical administration of propolis in chronic periodontitis. Odontology 2020, 108, 704–714. [Google Scholar] [CrossRef]
- El-Allaky, H.S.; Wahba, A.N.; Talaat, D.M.; Zakaria, A.S. Antimicrobial Effect of Propolis Administered through Two Different Vehicles in High Caries Risk Children: A Randomized Clinical Trial. J. Clin. Pediatr. Dent. 2020, 44, 289–295. [Google Scholar] [CrossRef]
- Nyvad, B.; Crielaard, W.; Mira, A.; Takahashi, N.; Beighton, D. Dental Caries from a Molecular Microbiological Perspective. Caries Res. 2013, 47, 89–102. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S162–S170. [Google Scholar] [CrossRef]
- Lombardi, A.; Ouanounou, A. Fungal infections in dentistry: Clinical presentations, diagnosis, and treatment alternatives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 533–546. [Google Scholar] [CrossRef]
- Philip, N.; Suneja, B.; Walsh, L. Beyond Streptococcus mutans: Clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Br. Dent. J. 2018, 224, 219–225. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Hu, L.; He, C.; Zhao, C.; Chen, X.; Hua, H.; Yan, Z. Characterization of oral candidiasis and the Candida species profile in patients with oral mucosal diseases. Microb. Pathog. 2019, 134, 103575. [Google Scholar] [CrossRef]
- Calderone, R.A.; Fonzi, W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Stähli, A.; Schröter, H.; Bullitta, S.; Serralutzu, F.; Dore, A.; Nietzsche, S.; Milia, E.; Sculean, A.; Eick, S. In Vitro Activity of Propolis on Oral Microorganisms and Biofilms. Antibiotics 2021, 10, 1045. [Google Scholar] [CrossRef]
- Moise, A.R.; Bobiş, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, Main Plant Sources for Bioactive Properties in Green and Red Brazilian Propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Reales-Calderón, J.A.; Aguilera-Montilla, N.; Corbí, L.; Molero, G.; Gil, C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics 2014, 14, 1503–1518. [Google Scholar] [CrossRef]
- Shimada, Y.; Tabeta, K.; Sugita, N.; Yoshie, H. Profiling biomarkers in gingival crevicular fluid using multiplex bead immunoassay. Arch. Oral Biol. 2013, 58, 724–730. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Y.; Duan, D.; Wang, P.; Xin, Y.; Bai, L.; Liu, Y.; Xu, Y. Enhanced activity of macrophage M1/M2 phenotypes in periodontitis. Arch. Oral Biol. 2018, 96, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Asgharpour, F.; Moghadamnia, A.A.; Motallebnejad, M.; Nouri, H.R. Propolis attenuates lipopolysaccharide-induced inflammatory responses through intracellular ROS and NO levels along with downregulation of IL-1β and IL-6 expressions in murine RAW 264.7 macrophages. J. Food Biochem. 2019, 43, e12926. [Google Scholar] [CrossRef] [PubMed]
- Blonska, M.; Bronikowska, J.; Pietsz, G.; Czuba, Z.; Scheller, S.; Krol, W. Effects of ethanol extract of propolis (EEP) and its flavones on inducible gene expression in J774A.1 macrophages. J. Ethnopharmacol. 2004, 91, 25–30. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, F.; Tecca, M.; Scazzocchio, F.; Renzini, V.; Strippoli, V. Effect of Propolis on Virulence Factors of Candida albicans. J. Chemother. 2003, 15, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.; Araujo, G.; Gomes, R.; Iwanaga, S.; Barbosa, M.; Abdo, E.; e Ferreira, E.; Viana, A.; Souza, A.; Abreu, S.; et al. Mucoadhesive Propolis Gel for Prevention of Radiation-Induced Oral Mucositis. Curr. Clin. Pharmacol. 2014, 9, 359–364. [Google Scholar] [CrossRef]
- Braga, A.S.; Abdelbary, M.M.H.; Kim, R.R.; Melo, F.P.D.S.R.D.; Saldanha, L.L.; Dokkedal, A.L.; Conrads, G.; Esteves-Oliveira, M.; Magalhães, A.C. The Effect of Toothpastes Containing Natural Extracts on Bacterial Species of a Microcosm Biofilm and on Enamel Caries Development. Antibiotics 2022, 11, 414. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A.; Gruber, R. The in vitro impact of toothpaste extracts on cell viability. Eur. J. Oral Sci. 2015, 123, 179–185. [Google Scholar] [CrossRef]
- Tadin, A.; Gavic, L.; Govic, T.; Galic, N.; Vladislavic, N.Z.; Zeljezic, D. In vivo evaluation of fluoride and sodium lauryl sulphate in toothpaste on buccal epithelial cells toxicity. Acta Odontol. Scand. 2019, 77, 386–393. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A. The Biocompatibility of a New Erythritol-and Xyltol-Containing Fluoride Toothpaste. Healthcare 2021, 9, 935. [Google Scholar] [CrossRef]
- Moon, H.-J.; Jeya, M.; Kim, I.-W.; Lee, J.-K. Biotechnological production of erythritol and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 1017–1025. [Google Scholar] [CrossRef]
- Woelnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am. J. Physiol. Metab. 2016, 310, E1053–E1061. [Google Scholar] [CrossRef]
- De Cock, P.; Mäkinen, K.; Honkala, E.; Saag, M.; Kennepohl, E.; Eapen, A. Erythritol Is More Effective Than Xylitol and Sorbitol in Managing Oral Health Endpoints. Int. J. Dent. 2016, 2016, 9868421. [Google Scholar] [CrossRef]
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordonii and Porphyromonas gingivalis. Mol. Oral Microbiol. 2013, 28, 435–451. [Google Scholar] [CrossRef]
- Janus, M.M.; Volgenant, C.M.C.; Brandt, B.W.; Buijs, M.J.; Keijser, B.J.F.; Crielaard, W.; Zaura, E.; Krom, B.P. Effect of erythritol on microbial ecology of in vitro gingivitis biofilms. J. Oral Microbiol. 2017, 9, 1337477. [Google Scholar] [CrossRef]
- Li, W.; Wang, R.E.; Finger, M.; Lang, N.P. Evaluation of the antigingivitis effect of a chlorhexidine mouthwash with or without an antidiscoloration system compared to placebo during experimental gingivitis. J. Investig. Clin. Dent. 2014, 5, 15–22. [Google Scholar] [CrossRef]
- Eick, S.; Goltz, S.; Nietzsche, S.; Jentsch, H.; Pfister, W. Efficacy of chlorhexidine digluconate-containing formulations and other mouthrinses against periodontopathogenic microorganisms. Quintessence Int. 2011, 42, 687–700. [Google Scholar]
- Smolarek, P.D.C.; Esmerino, L.A.; Chibinski, A.C.; Bortoluzzi, M.C.; Dos Santos, E.B.; Junior, V.A.K.; Kozlowski, V.A. In vitro antimicrobial evaluation of toothpastes with natural compounds. Eur. J. Dent. 2015, 9, 580–586. [Google Scholar] [CrossRef]
- Attin, T.; Hornecker, E. Tooth brushing and oral health: How frequently and when should tooth brushing be performed? Oral. Health Prev. Dent. 2005, 3, 135–140. [Google Scholar]
- Vanni, R.; Waldner-Tomic, N.M.; Belibasakis, G.N.; Attin, T.; Schmidlin, P.R.; Thurnheer, T. Antibacterial Efficacy of a Propolis Toothpaste and Mouthrinse Against a Supragingival Multispecies Biofilm. Oral Health Prev Dent 2015, 13, 531–535. [Google Scholar] [CrossRef]
- De Figueiredo, K.A.; Da Silva, H.D.P.; Miranda, S.L.F.; Gonçalves, F.J.D.S.; De Sousa, A.P.; De Figueiredo, L.C.; Feres, M.; Bueno-Silva, B. Brazilian Red Propolis Is as Effective as Amoxicillin in Controlling Red-Complex of Multispecies Subgingival Mature Biofilm In Vitro. Antibiotics 2020, 9, 432. [Google Scholar] [CrossRef]
- Machorowska-Pieniążek, A.; Morawiec, T.; Olek, M.; Mertas, A.; Aebisher, D.; Bartusik-Aebisher, D.; Cieślar, G.; Kawczyk-Krupka, A. Advantages of using toothpaste containing propolis and plant oils for gingivitis prevention and oral cavity hygiene in cleft lip/palate patients. Biomed. Pharmacother. 2021, 142, 111992. [Google Scholar] [CrossRef] [PubMed]
- Botushanov, I.P.; Grigorov, I.G.; Aleksandrov, A.G. A clinical study of a silicate toothpaste with extract from propolis. Folia Medica 2001, 43, 28–30. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Botran, R.; Vetvicka, V. Analysis of cytokine or cytokine receptor mRNA. In Advanced Methods in Cellular Immunology; CRC Press: Boca Raton, FL, USA, 2000; pp. 101–114. Available online: https://www.taylorfrancis.com/chapters/mono/10.1201/9781420039238-12/analysis-cytokine-cytokine-receptor-mrna-rafael-fernandez-botran-vaclav-vetvicka (accessed on 8 November 2022).
- Chhabra, A.; Chakraborty, N.G.; Mukherji, B. Silencing of endogenous IL-10 in human dendritic cells leads to the generation of an improved CTL response against human melanoma associated antigenic epitope, MART-127–35. Clin. Immunol. 2008, 126, 251–259. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Ye, F.; Wang, F.; Lu, W.; Xie, X. Identification of suitable reference genes for measurement of gene expression in human cervical tissues. Anal. Biochem. 2010, 405, 224–229. [Google Scholar] [CrossRef]
- Berto, L.A.; Lauener, A.; Carvalho, T.S.; Lussi, A.; Eick, S. In Vitro Effects of Arginine-Containing Toothpastes on Cariogenic Biofilms. Oral. Health Prev. Dent. 2019, 17, 375–383. [Google Scholar] [CrossRef]
- Voronets, J.; Jaeggi, T.; Buergin, W.; Lussi, A. Controlled Toothbrush Abrasion of Softened Human Enamel. Caries Res. 2008, 42, 286–290. [Google Scholar] [CrossRef]
- Pettit, R.K.; Weber, C.A.; Kean, M.J.; Hoffmann, H.; Pettit, G.R.; Tan, R.; Franks, K.S.; Horton, M.L. Microplate Alamar Blue Assay for Staphylococcus epidermidis Biofilm Susceptibility Testing. Antimicrob. Agents Chemother. 2005, 49, 2612–2617. [Google Scholar] [CrossRef]
| Strain | Ery | Ery TP | EEPg | Ery TP + 50 mg/mL EEPg | Ery TP + 25 mg/mL EEPg | TPcom |
|---|---|---|---|---|---|---|
| S. gordonii ATCC 10,558 | 125 | 62.5 | <0.1 | <0.1 | 31.3 | 31.3 |
| A. naeslundii ATCC 12,104 | 15.6 | 15.6 | <0.1 | <0.1 | 7.8 | 15.6 |
| S. mutans ATCC 25,175 | 500 | >500 | 0.2 | 0.2 | 31.3 | 31.3 |
| F. nucleatum ATCC 25,586 | 125 | 250 | 0.2 | 0.2 | 7.8 | 7.8 |
| P. micra ATCC 33,270 | 500 | 62.5 | <0.1 | <0.1 | 7.8 | 7.8 |
| P. intermedia ATCC 25,611 | 15.6 | 7.8 | <0.1 | <0.1 | 7.8 | 7.8 |
| P. gingivalis ATCC 33,277 | 62.5 | 7.8 | 0.2 | 0.2 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluccia, A.; Matti, F.; Zhu, X.; Lussi, A.; Stähli, A.; Sculean, A.; Eick, S. In Vitro Study on Green Propolis as a Potential Ingredient of Oral Health Care Products. Antibiotics 2022, 11, 1764. https://doi.org/10.3390/antibiotics11121764
Coluccia A, Matti F, Zhu X, Lussi A, Stähli A, Sculean A, Eick S. In Vitro Study on Green Propolis as a Potential Ingredient of Oral Health Care Products. Antibiotics. 2022; 11(12):1764. https://doi.org/10.3390/antibiotics11121764
Chicago/Turabian StyleColuccia, Achille, Fabienne Matti, Xilei Zhu, Adrian Lussi, Alexandra Stähli, Anton Sculean, and Sigrun Eick. 2022. "In Vitro Study on Green Propolis as a Potential Ingredient of Oral Health Care Products" Antibiotics 11, no. 12: 1764. https://doi.org/10.3390/antibiotics11121764
APA StyleColuccia, A., Matti, F., Zhu, X., Lussi, A., Stähli, A., Sculean, A., & Eick, S. (2022). In Vitro Study on Green Propolis as a Potential Ingredient of Oral Health Care Products. Antibiotics, 11(12), 1764. https://doi.org/10.3390/antibiotics11121764







