Days of Antibiotic Spectrum Coverage Trends and Assessment in Patients with Bloodstream Infections: A Japanese University Hospital Pilot Study
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics, DASC, and DOT Metrics in This Study
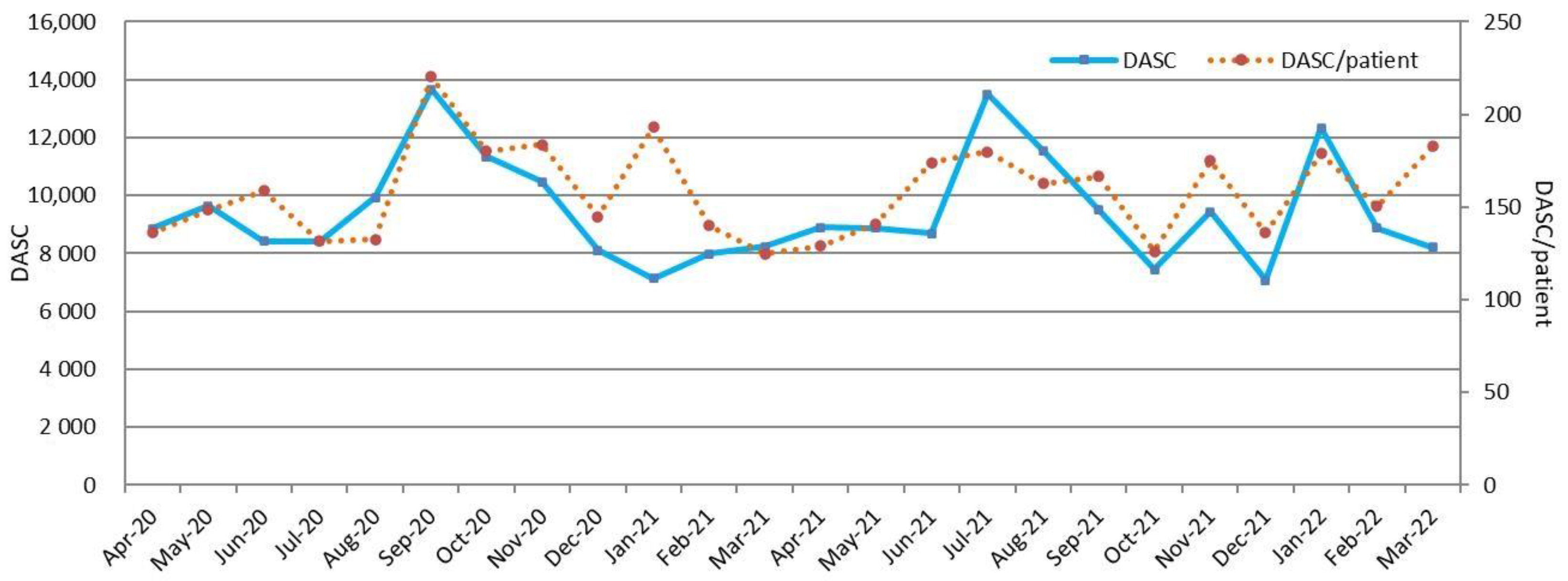
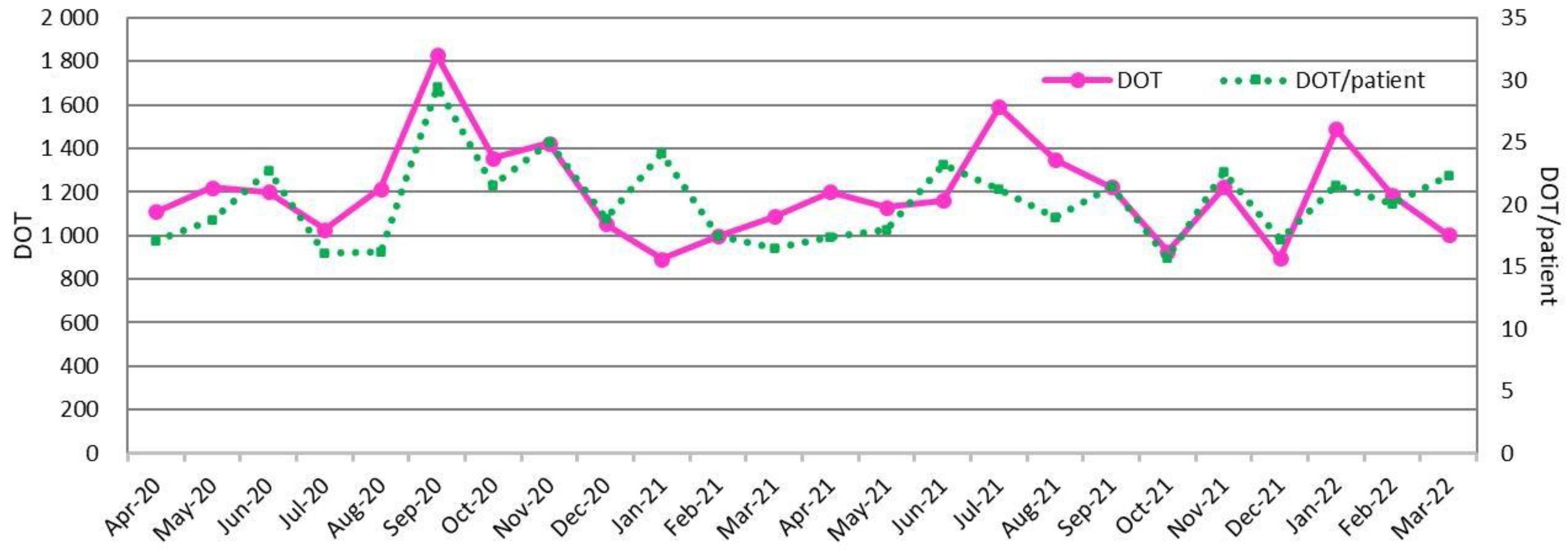
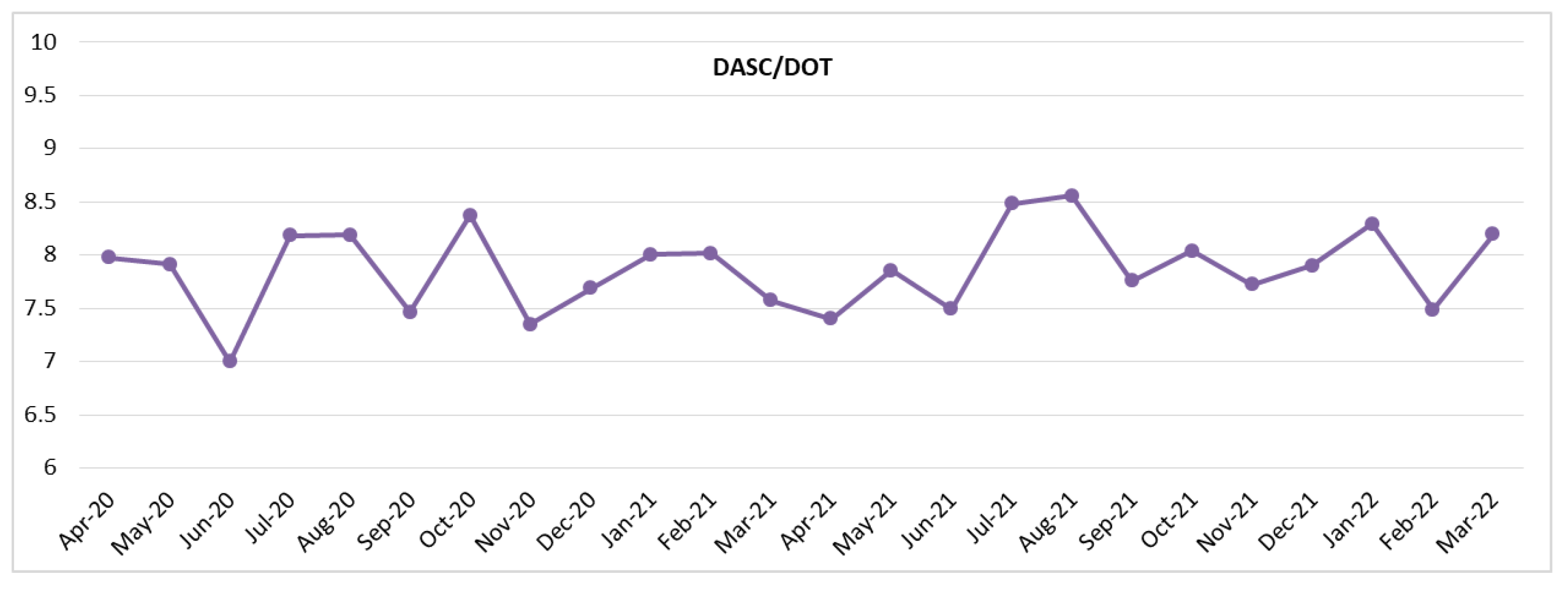
2.2. DASC, DOT, and DASC/DOT Trends in BSI Patients by Month
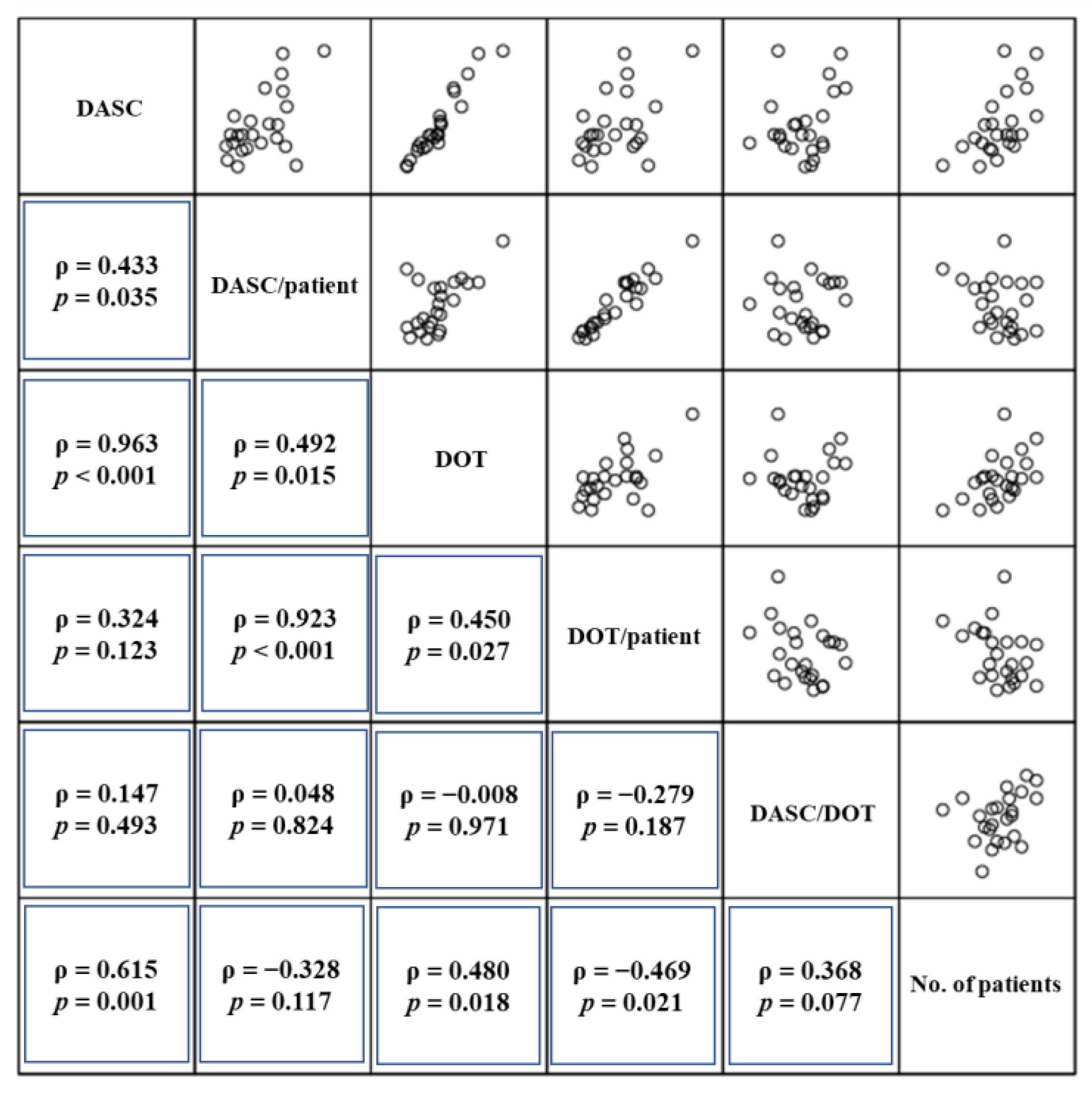
2.3. Relationship between DASC, DOT, DASC/DOT, and BSI Patient Number by Month
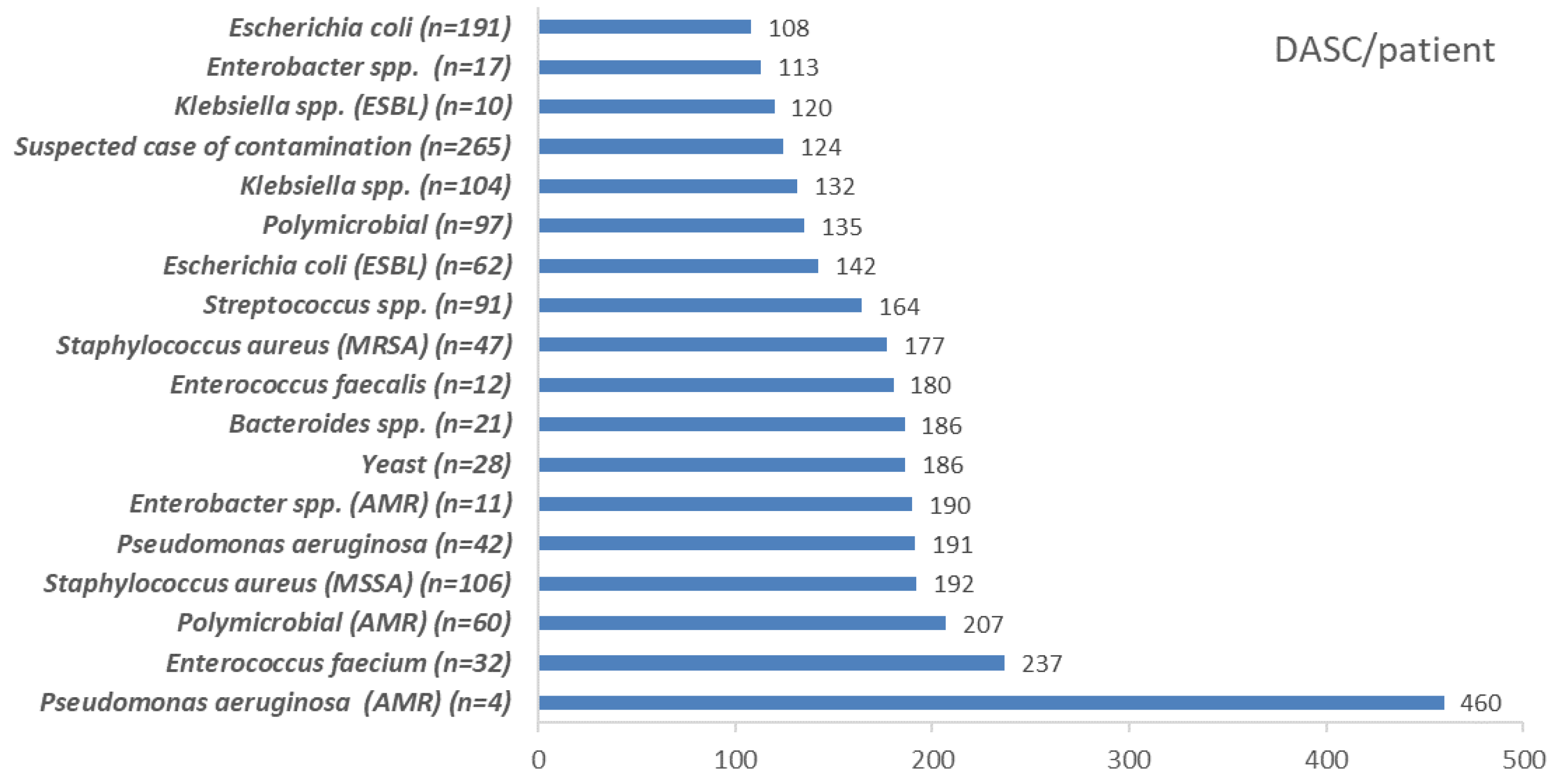
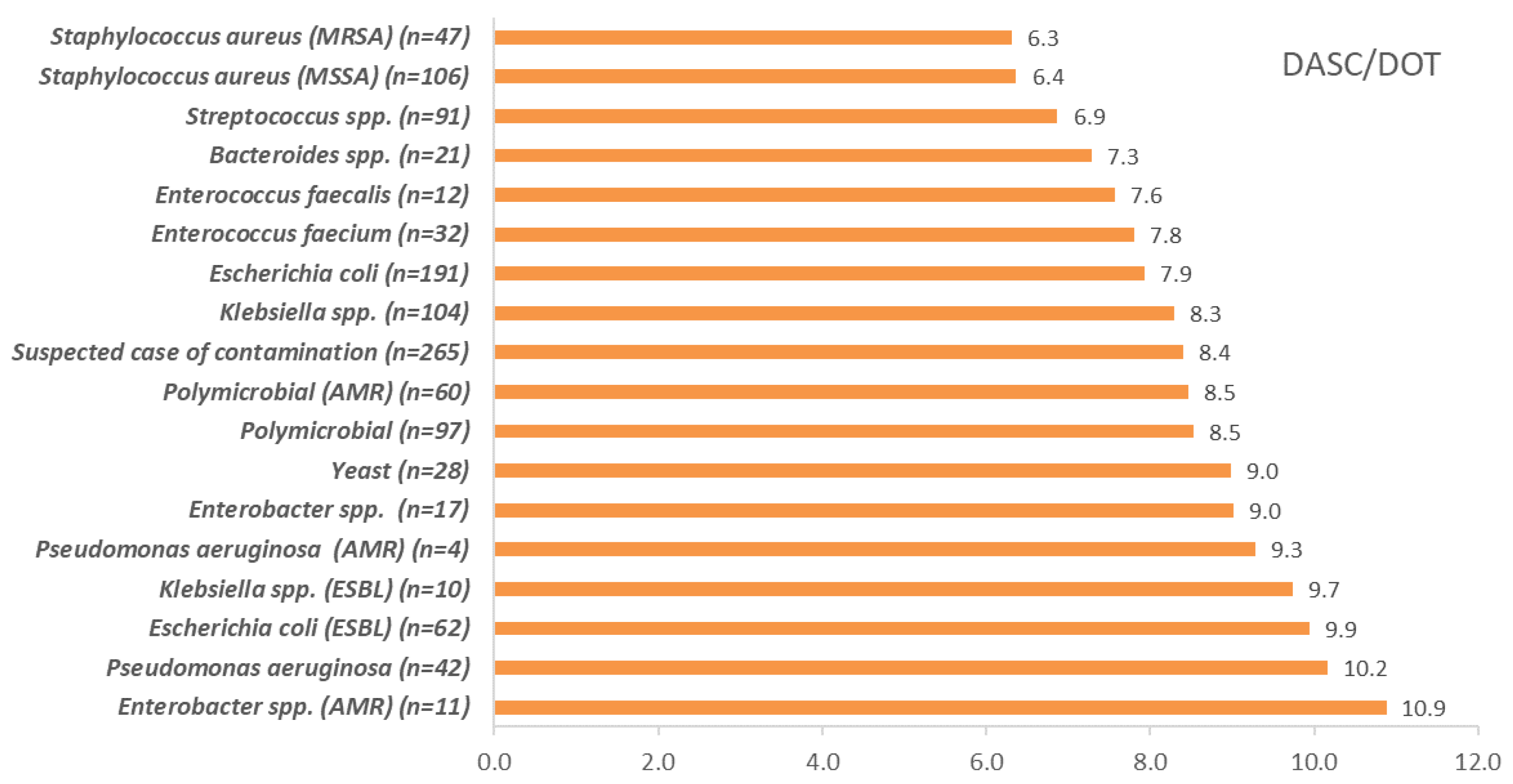
2.4. DASC/Patient, DOT/Patient, and DASC/DOT Comparisons Which Were Stratified by Pathogens
3. Discussion
4. Materials and Methods
4.1. Study Setting and Design
4.2. Calculation of Antimicrobial Use Metrics
4.3. Microbiologic Results of Blood Culture and AMR Definitions
4.4. Statistical Analysis
4.5. Ethics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuster, S.P.; Ruef, C.; Ledergerber, B.; Hintermann, A.; Deplazes, C.; Neuber, L.; Weber, R. Quantitative antibiotic use in hospitals: Comparison of measurements, literature review, and recommendations for a standard of reporting. Infection 2008, 36, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Gravatt, L.A.; Pakyz, A.L. Challenges in measuring antibiotic consumption. Curr. Infect. Dis. Rep. 2013, 15, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Bitterman, R.; Hussein, K.; Leibovici, L.; Carmeli, Y.; Paul, M. Systematic review of antibiotic consumption in acute care hospitals. Clin. Microbiol. Infect. 2016, 22, 561.e7–561.e19. [Google Scholar] [CrossRef]
- Maeda, M.; Muraki, Y.; Kosaka, T.; Yamada, T.; Aoki, Y.; Kaku, M.; Seki, M.; Tanabe, Y.; Fujita, N.; Niki, Y.; et al. Impact of health policy on structural requisites for antimicrobial stewardship: A nationwide survey conducted in Japanese hospitals after enforcing the revised reimbursement system for antimicrobial stewardship programs. J. Infect. Chemother. 2021, 27, 1–6. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Polk, R.E. Antimicrobial use metrics and benchmarking to improve stewardship outcomes: Methodology, opportunities, and challenges. Infect. Dis. Clin. N. Am. 2014, 28, 195–214. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Paul, M.; Dickstein, Y.; Raz-Pasteur, A. Antibiotic de-escalation for bloodstream infections and pneumonia: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2016, 22, 960–967. [Google Scholar] [CrossRef]
- Hogan, C.A.; Ebunji, B.; Watz, N.; Kapphahn, K.; Rigdon, J.; Mui, E.; Meng, L.; Alegria, W.; Holubar, M.; Deresinski, S.; et al. Impact of rapid antimicrobial susceptibility testing in Gram-negative rod bacteremia: A quasi-experimental study. J. Clin. Microbiol. 2020, 58, e00360-20. [Google Scholar] [CrossRef]
- De Waele, J.J.; Schouten, J.; Beovic, B.; Tabah, A.; Leone, M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: No simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020, 46, 236–244. [Google Scholar] [CrossRef]
- Weiss, E.; Zahar, J.-R.; Lesprit, P.; Ruppe, E.; Leone, M.; Chastre, J.; Lucet, J.-C.; Paugam-Burtz, C.; Brun-Buisson, C.; Timsit, J.-F.; et al. Elaboration of a consensual definition of de-escalation allowing a ranking of β-lactams. Clin. Microbiol. Infect. 2015, 21, 649.e1–649.e10. [Google Scholar] [CrossRef]
- Silva, B.N.; Andriolo, R.B.; Atallah, A.N.; Salomão, R. De-escalation of antimicrobial treatment for adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst. Rev. 2013, 3, CD007934. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.; Bassetti, M.; Kollef, M.H.; Zahar, J.R.; Paiva, J.A.; Timsit, J.F.; Roberts, J.A.; Schouten, J.; Giamarellou, H.; Rello, J.; et al. Antimicrobial de-escalation in critically ill patients: A position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020, 46, 245–265. [Google Scholar] [PubMed]
- Al-Qahtani, S.M.; Baffoe-Bonnie, H.; El-Saed, A.; Alshamrani, M.; Algwizani, A.; Alaklabi, A.; AlJoudi, K.; Albaalharith, N.; Mohammed, A.; Hussain, S.; et al. Appropriateness of antimicrobial use among septic patients managed by the critical care response team: An opportunity for improvement through de-escalation. Antimicrob. Resist. Infect. Control 2019, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Maeda, M.; Yokoe, T.; Hashiguchi, M.; Togashi, M.; Ishino, K. Impact of the multidisciplinary antimicrobial stewardship team intervention focusing on carbapenem de-escalation: A single-centre and interrupted time series analysis. Int. J. Clin. Pract. 2021, 75, e13693. [Google Scholar] [CrossRef]
- Moehring, R.W.; Ashley, E.S.D.; Davis, A.E.; Dyer, A.P.; Parish, A.; Ren, X.; Lokhnygina, Y.; Hicks, L.A.; Srinivasan, A.; Anderson, D.J. Development of an electronic definition for de-escalation of antibiotics in hospitalized patients. Clin. Infect. Dis. 2021, 73, e4507–e4514. [Google Scholar] [CrossRef]
- Teh, H.L.; Abdullah, S.; Ghazali, A.K.; Khan, R.A.; Ramadas, A.; Leong, C.L. Impact of extended and restricted antibiotic deescalation on mortality. Antibiotics 2021, 11, 22. [Google Scholar] [CrossRef]
- Naito, Y.; Maeda, M.; Nagatomo, Y.; Ugajin, K.; Akima, E.; Tanaka, M.; Tokimatsu, I.; Sasaki, T. Impact of the antimicrobial stewardship team intervention focusing on changes in prescribing trends and the rate of carbapenem-resistant P. aeruginosa. Yakugaku Zasshi 2022, 142, 527–534. [Google Scholar] [CrossRef]
- Kakiuchi, S.; Livorsi, D.J.; Perencevich, E.N.; Diekema, D.J.; Ince, D.; Prasidthrathsint, K.; Kinn, P.; Percival, K.; Heintz, B.H.; Goto, M. Days of antibiotic spectrum coverage (DASC): A novel metric for inpatient antibiotic consumption. Clin. Infect. Dis. 2021, 75, ciab1034. [Google Scholar]
- Hattori, H.; Maeda, M.; Nagatomo, Y.; Takuma, T.; Niki, Y.; Naito, Y.; Sasaki, T.; Ishino, K. Epidemiology and risk factors for mortality in bloodstream infections: A single-center retrospective study in Japan. Am. J. Infect. Control 2018, 46, e75–e79. [Google Scholar] [CrossRef]
- Gerber, J.S.; Hersh, A.L.; Kronman, M.P.; Newland, J.G.; Ross, R.K.; Metjian, T.A. Development and application of an antibiotic spectrum index for benchmarking antibiotic selection patterns Across hospitals. Infect. Control Hosp. Epidemiol. 2017, 38, 993–997. [Google Scholar] [CrossRef]
- Madaras-Kelly, K.; Jones, M.; Remington, R.; Hill, N.; Huttner, B.; Samore, M. Development of an antibiotic spectrum score based on Veterans Affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: A modified Delphi approach. Infect. Control Hosp. Epidemiol. 2014, 35, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Madaras-Kelly, K.; Jones, M.; Remington, R.; Caplinger, C.M.; Huttner, B.; Jones, B.; Samore, M. Antimicrobial de-escalation of treatment for healthcare-associated pneumonia within the Veterans Healthcare Administration. J. Antimicrob. Chemother. 2016, 71, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Ilges, D.; Ritchie, D.J.; Krekel, T.; Neuner, E.A.; Hampton, N.; Kollef, M.H.; Micek, S. Assessment of antibiotic de-escalation by spectrum score in patients With nosocomial pneumonia: A single-center, retrospective cohort study. Open Forum. Infect. Dis. 2021, 8, ofab508. [Google Scholar] [CrossRef]
- Sullivan, B.A.; Panda, A.; Wallman-Stokes, A.; Sahni, R.; Fairchild, K.D.; Newland, J.G.; McPherson, C.C.; Vesoulis, Z.A. Antibiotic spectrum index: A new tool comparing antibiotic use in three NICUs. Infect. Control Hosp. Epidemiol. 2021, 43, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Doern, G.V.; Carroll, K.C.; Diekema, D.J.; Garey, K.W.; Rupp, M.E.; Weinstein, M.P.; Sexton, D.J. Practical guidance for clinical microbiology laboratories: A comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin. Microbiol. Rev. 2019, 33, e00009-19. [Google Scholar] [CrossRef]
- Esquer Garrigos, Z.; Wingler, M.J.B.; Svoronos, P.A.; Vijayvargiya, P.; Goodman-Meza, D.; O’Horo, J.C.; Navalkele, B.D.; Cretella, D.; Frame, I.J.; Parham, J.; et al. Increased rates of blood culture contamination during the coronavirus disease 2019 pandemic. Infect. Control Hosp. Epidemiol. 2021, 43, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.E.; Nguyen, K.; Wahl, C.K.; Huss, J.L.; Chang, D.; Ager, E.P.; Hamilton, L. Initial Specimen Diversion Device® reduces blood culture contamination and vancomycin use in academic medical centre. J. Hosp. Infect. 2022, 120, 127–133. [Google Scholar] [CrossRef]
- O’Leary, E.N.; Edwards, J.R.; Srinivasan, A.; Neuhauser, M.M.; Webb, A.K.; Soe, M.M.; Hicks, L.A.; Wise, W.; Wu, H.; Pollock, D.A. National healthcare safety network Standardized Antimicrobial Administration ratios (SAARs): A progress report and risk modeling update using 2017 data. Clin. Infect. Dis. 2020, 71, e702–e709. [Google Scholar] [CrossRef]
- Maeda, M.; Muraki, Y.; Anno, Y.; Sawa, A.; Kusama, Y.; Ishikane, M.; Ohmagari, N.; Ohge, H. Development of the predicted and standardized carbapenem usage metric: Analysis of the Japanese Diagnosis Procedure Combination payment system data. J. Infect. Chemother. 2020, 26, 633–635. [Google Scholar] [CrossRef]
- Kim, B.; Ahn, S.V.; Kim, D.S.; Chae, J.; Jeong, S.J.; Uh, Y.; Kim, H.B.; Kim, H.S.; Park, S.H.; Park, Y.S.; et al. Development of the Korean standardized antimicrobial administration ratio as a tool for benchmarking antimicrobial use in each hospital. J. Korean Med. Sci. 2022, 37, e191. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council Infective endocarditis in adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [PubMed]
- Weis, S.; Kesselmeier, M.; Davis, J.S.; Morris, A.M.; Lee, S.; Scherag, A.; Hagel, S.; Pletz, M.W. Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 2019, 25, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Hagel, S.; Bahrs, C.; Schumann, R.; Pletz, M.; Weis, S. Complicated and uncomplicated S. aureus bacteraemia: An international Delphi survey among infectious diseases experts on definitions and treatment. Clin. Microbiol. Infect. 2022, 28, 1026.e7–1026.e11. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P.; Towns, M.L.; Quartey, S.M.; Mirrett, S.; Reimer, L.G.; Parmigiani, G.; Reller, L.B. The clinical significance of positive blood cultures in the 1990s: A prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin. Infect. Dis. 1997, 24, 584–602. [Google Scholar] [CrossRef]
- Hall, K.K.; Lyman, J.A. Updated review of blood culture contamination. Clin. Microbiol. Rev. 2006, 19, 788–802. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. M100-S26; CLSI: Wayne, IN, USA, 2016. [Google Scholar]
- Ministry of Health, Labor and Welfare Japan. Carbapenem-Resistant Enterobacteriaceae Infection. Available online: http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou11/01-05-140912-1.html (accessed on 22 July 2022).

| Age Category | No. of Cases (All, n = 1443) | No. of Cases (Without Contamination, n = 1178) |
|---|---|---|
| 0–5 | 46 (3.2) | 35 (3.0) |
| 6–14 | 9 (0.6) | 8 (0.7) |
| 15–49 | 121 (8.4) | 93 (7.9) |
| 50–64 | 232 (16.1) | 189 (16.0) |
| 65–74 | 324 (22.5) | 264 (22.4) |
| ≥75 | 711 (49.3) | 589 (50.0) |
| Year | DASC | DASC/Patient | DOT | DOT/Patient | DASC/DOT |
|---|---|---|---|---|---|
| 2020 | 112,235 | 155.9 | 14,403 | 20.0 | 7.8 |
| 2021 | 114,391 | 158.2 | 14,375 | 19.9 | 8.0 |
| Total | 226,626 | 157.1 | 28,778 | 19.9 | 7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, M.; Nakata, M.; Naito, Y.; Yamaguchi, K.; Yamada, K.; Kinase, R.; Takuma, T.; On, R.; Tokimatsu, I. Days of Antibiotic Spectrum Coverage Trends and Assessment in Patients with Bloodstream Infections: A Japanese University Hospital Pilot Study. Antibiotics 2022, 11, 1745. https://doi.org/10.3390/antibiotics11121745
Maeda M, Nakata M, Naito Y, Yamaguchi K, Yamada K, Kinase R, Takuma T, On R, Tokimatsu I. Days of Antibiotic Spectrum Coverage Trends and Assessment in Patients with Bloodstream Infections: A Japanese University Hospital Pilot Study. Antibiotics. 2022; 11(12):1745. https://doi.org/10.3390/antibiotics11121745
Chicago/Turabian StyleMaeda, Masayuki, Mari Nakata, Yuika Naito, Kozue Yamaguchi, Kaho Yamada, Ryoko Kinase, Takahiro Takuma, Rintaro On, and Issei Tokimatsu. 2022. "Days of Antibiotic Spectrum Coverage Trends and Assessment in Patients with Bloodstream Infections: A Japanese University Hospital Pilot Study" Antibiotics 11, no. 12: 1745. https://doi.org/10.3390/antibiotics11121745
APA StyleMaeda, M., Nakata, M., Naito, Y., Yamaguchi, K., Yamada, K., Kinase, R., Takuma, T., On, R., & Tokimatsu, I. (2022). Days of Antibiotic Spectrum Coverage Trends and Assessment in Patients with Bloodstream Infections: A Japanese University Hospital Pilot Study. Antibiotics, 11(12), 1745. https://doi.org/10.3390/antibiotics11121745






