Development and Psychometric Evaluation of the Antibiotic Knowledge and Consumption Tool (AKCT)
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
2.2. Structural Validity—Exploratory Factor Analysis
2.3. Internal Consistency Reliability
2.4. Convergent Validity
3. Discussion
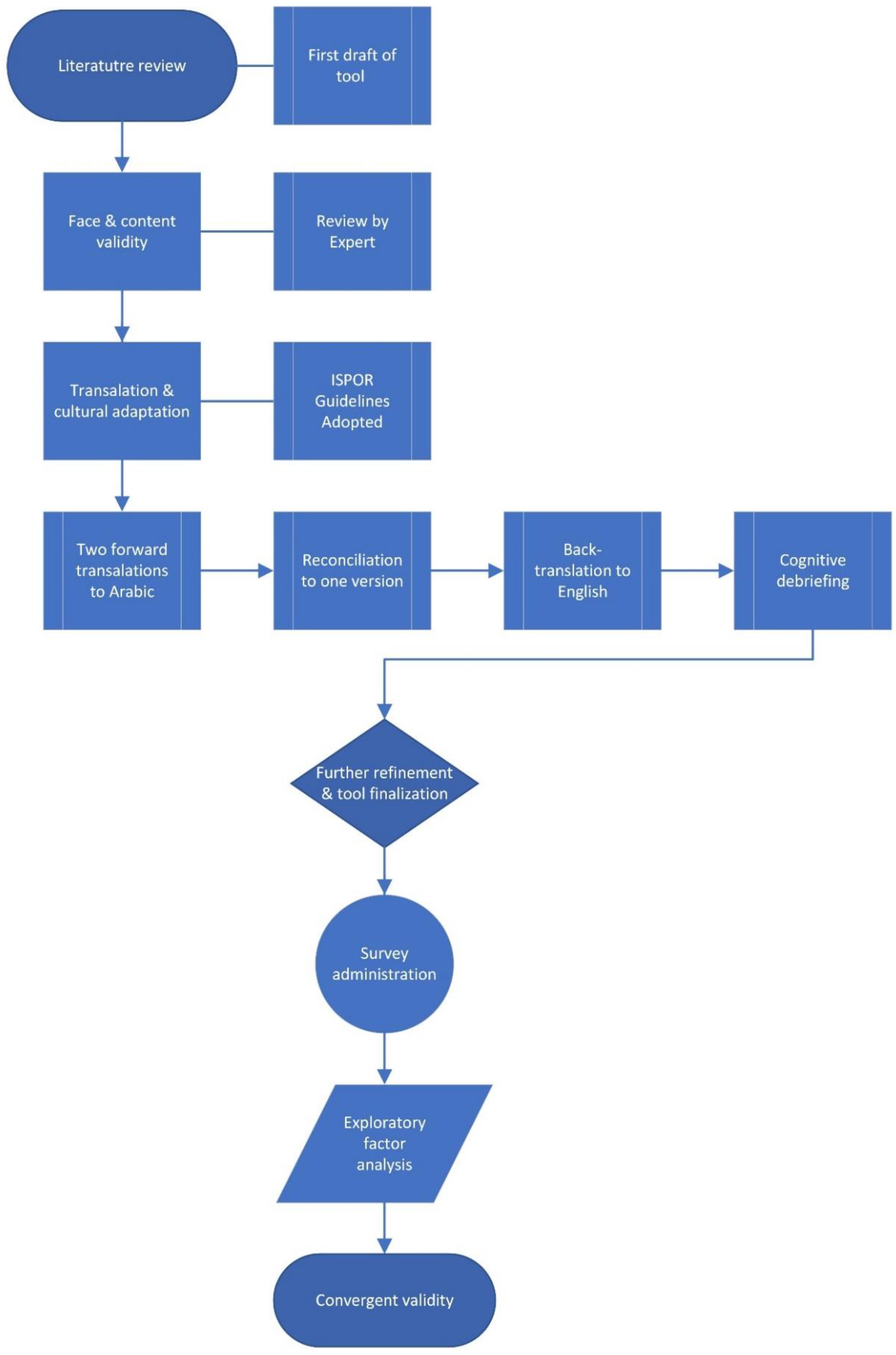
4. Materials and Methods
4.1. Tool Development
4.2. Face and Content Validity
4.3. Tool Translation to Arabic
4.4. Cognitive Debriefings
4.5. Inclusion and Exclusion Criteria
4.6. Sample Size
4.7. Survey Administration
4.8. Convergent Validity
4.9. Data Analysis
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [PubMed]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr.; The Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- McCullough, A.R.; Parekh, S.; Rathbone, J.; Del Mar, C.B.; Hoffmann, T.C. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2015, 71, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, E.A.; Borg, M.A.; Gür, D.; Rasslan, O.; Taher, I.; Ben Redjeb, S.; Elnassar, Z.; Bagatzouni, D.P.; Daoud, Z. Self-medication with antibiotics in the ambulatory care setting within the Euro-Mediterranean region; results from the Armed Project. J. Infect. Public Health 2009, 2, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, L.; Burgerhof, J.G.M.; Degener, J.E.; Deschepper, F.; Lundborg, C.S.; Monnet, D.L.; Scicluna, E.A.; Birkin, J.; Haai-jer-Ruskamp, F.M. Attitudes, beliefs and knowledge concerning antibiotic use and self-medication: A comparative European study. Pharmacoepidemiol. Drug Saf. 2007, 16, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- André, M.; Vernby, Å.; Berg, J.; Lundborg, C.S. A survey of public knowledge and awareness related to antibiotic use and resistance in Sweden. J. Antimicrob. Chemother. 2010, 65, 1292–1296. [Google Scholar] [CrossRef]
- Shehadeh, M.B.; Suaifan, G.A.R.Y.; Hammad, E.A. Active educational intervention as a tool to improve safe and appropriate use of antibiotics. Saudi Pharm. J. 2016, 24, 611–615. [Google Scholar] [CrossRef]
- Kim, S.S.; Moon, S.; Kim, E.J. Public knowledge and attitudes regarding antibiotic use in South Korea. J. Korean Acad. Nurs. 2011, 41, 742. [Google Scholar] [CrossRef]
- Norris, P.; Ng, L.F.; Kershaw, V.; Hanna, F.; Wong, A.; Talekar, M.; Oh, J.; Azer, M.; Cheong, L. Knowledge and reported use of antibiotics amongst immigrant ethnic groups in New Zealand. J. Immigr. Minor. Health 2010, 12, 107–112. [Google Scholar] [CrossRef]
- Vanden, E.J.; Marcus, R.; Hadler, J.L.; Imhoff, B.; Vugia, D.J.; Cieslak, P.R.; Zell, E.; Deneen, V.; McCombs, K.G.; Zansky, S.M.; et al. Consumer attitudes and use of antibiotics. Emerg. Infect. Dis. 2003, 9, 1128–1135. [Google Scholar] [CrossRef]
- Stivers, T.; Mangione-Smith, R.; Elliott, M.N.; McDonald, L.; Heritage, J. Why do physicians think parents expect antibiotics? What parents report vs. what physicians believe. J. Fam. Pract. 2003, 52, 140–148. [Google Scholar] [PubMed]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Ranji, S.R.; Steinman, M.A.; Shojania, K.G.; Gonzales, R. Interventions to reduce unnecessary antibiotic prescribing: A systematic review and quantitative analysis. Med. Care 2008, 46, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Finch, R.G.; Metlay, J.P.; Davey, P.G.; Baker, L.J. Educational interventions to improve antibiotic use in the community: Report from the International Forum on antibiotic resistance (IFAR) colloquium, 2002. Lancet Infect. Dis. 2004, 4, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Deschepper, R.; Grigoryan, L.; Lundborg, C.S.; Hofstede, G.; Cohen, J.; Kelen, G.V.D.; Deliens, L.; Haaijer-Ruskamp, F.M. Are cultural dimensions relevant for explaining cross-national differences in antibiotic use in Europe? BMC Health Serv. Res. 2008, 8, 123. [Google Scholar] [CrossRef]
- Widayati, A.; Suryawati, S.; de Crespigny, C.; Hiller, J.E. Knowledge and beliefs about antibiotics among people in Yogyakarta City Indonesia: A cross sectional population-based survey. Antimicrob. Resist. Infect. Control 2012, 1, 38. [Google Scholar] [CrossRef]
- Shehadeh, M.; Suaifan, G.; Darwish, R.M.; Wazaify, M.; Zaru, L.; Alja’fari, S. Knowledge, attitudes and behavior regarding antibiotics use and misuse among adults in the community of Jordan. A pilot study. Saudi Pharm. J. 2012, 20, 125–133. [Google Scholar] [CrossRef]
- Pavydė, E.; Veikutis, V.; Mačiulienė, A.; Mačiulis, V.; Petrikonis, K.; Stankevičius, E. Public knowledge, beliefs and behavior on antibiotic use and self-medication in Lithuania. Int. J. Environ. Res. Public Health 2015, 12, 7002–7016. [Google Scholar] [CrossRef]
- Mouhieddine, T.; Olleik, Z.; Itani, M.M.; Kawtharani, S.; Nassar, H.; Hassoun, R.; Houmani, Z.; El Zein, Z.; Fakih, R.; Mortada, I.K.; et al. Assessing the Lebanese population for their knowledge, attitudes and practices of antibiotic usage. J. Infect. Public Health 2015, 8, 20–31. [Google Scholar] [CrossRef]
- Lim, K.K.; Teh, C.C. A Cross Sectional Study of Public Knowledge and Attitude towards Antibiotics in Putrajaya, Malaysia. South. Med. Rev. 2012, 5, 26–33. [Google Scholar] [PubMed]
- Cheaito, L.; Azizi, S.; Saleh, N.; Salameh, P. Assessment of self-medication in population buying antibiotics in pharmacies: A pilot study from Beirut and its suburbs. Int. J. Public Health 2013, 59, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Darwish, D.A.; Abdelmalek, S.; Abu Dayyih, W.; Hamadi, S. Awareness of antibiotic use and antimicrobial resistance in the Iraqi community in Jordan. J. Infect. Dev. Ctries. 2014, 8, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Farghadani, G.; Khalid AlHaideri, S.; Abdelraouf Fathy, M. Pharmacist opportunities to improve public self-medicating practices in the UAE. Pharmacol. Pharm. 2016, 7, 459–471. [Google Scholar] [CrossRef]
- Mohanna, M. Self-medication with antibiotic in children in Sana’a city, Yemen. Oman Med. J. 2010, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Ghaieth, M.F.; Elhag, S.R.; Hussien, M.E.; Konozy, E.H. Antibiotics self-medication among medical and nonmedical students at two prominent universities in Benghazi City, Libya. J. Pharm. Bioallied Sci. 2015, 7, 109. [Google Scholar]
- Conner, M.; Norman, P. Predicting Health Behaviour: Research and Practice with Social Cognition Models, 2nd ed.; Open University Press: Maidenhead, UK, 2005. [Google Scholar]
- Kehoe, J. Basic Item Analysis for Multiple-Choice Tests. Pract. Assess. Res. Eval. 1994, 4, 10. [Google Scholar]
- Li, D.; Yang, H.; Gong, Y.; Zhao, Y.; Qiu, L.; Sun, N.; Yin, X. Development and nationwide application of an antibiotic knowledge scale. Prev. Med. 2020, 141, 106262. [Google Scholar] [CrossRef]
- Belongia, E.A.; Naimi, T.S.; Gale, C.M.; Besser, R.E. Antibiotic use and upper respiratory infections: A survey of knowledge, attitudes, and experience in Wisconsin and Minnesota. Prev. Med. 2002, 34, 346–352. [Google Scholar] [CrossRef]
- Ling Oh, A.; Hassali, M.A.; Al-Haddad, M.S.; Syed Sulaiman, S.A.; Shafie, A.A.; Awaisu, A. Public knowledge and attitudes towards antibiotic usage: A cross-sectional study among the general public in the State of Penang, Malaysia. J. Infect. Dev. Ctries. 2011, 5, 338–347. [Google Scholar] [CrossRef]
- Radyowijati, A.; Haak, H. Determinants of Antimicrobial Use in the Developing World; Child Health Research ProjectSpecial Report; Johns Hopkins University, Office of Design and Publications: Baltimore, MD, USA, 2002; Volume 4, p. 37. [Google Scholar]
- Maciulaitis, R.; Janusonis, T.; Petrikaite, V.; Aukstakalniene, A. Assessment of antibiotic use and comparison with recommendations for their rational use. Medicina 2006, 42, 999–1005. [Google Scholar] [PubMed]
- Väänänen, M.H.; Pietilä, K.; Airaksinen, M. Self-medication with antibiotics—Does it really happen in Europe? Health Policy 2006, 77, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Skliros, E.; Merkouris, P.; Papazafiropoulou, A.; Gikas, A.; Matzouranis, G.; Papafragos, C.; Tsakanikas, I.; Zarbala, I.; Vasibosis, A.; Stamataki, P.; et al. Self-medication with antibiotics in rural population in Greece: A cross-sectional Multicenter Study. BMC Fam. Pract. 2010, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Widayati, A.; Suryawati, S.; de Crespigny, C.; Hiller, J.E. Self medication with antibiotics in Yogyakarta City Indonesia: A cross sectional population-based survey. BMC Res. Notes 2011, 4, 491. [Google Scholar] [CrossRef]
- Muras, M.; Krajewski, J.; Nocun, M.; Godycki-Cwirko, M. A survey of patient behaviours and beliefs regarding antibiotic self-medication for respiratory tract infections in Poland. Arch. Med. Sci. 2013, 5, 854–857. [Google Scholar] [CrossRef]
- Grigoryan, L.; Germanos, G.; Zoorob, R.; Juneja, S.; Raphael, J.L.; Paasche-Orlow, M.K.; Trautner, B.W. Use of antibiotics without a prescription in the U.S. population. Ann. Intern. Med. 2019, 171, 257. [Google Scholar] [CrossRef]
- Stivers, T. Managing patient pressure to prescribe antibiotics in the clinic. Paediatr. Drugs 2021, 23, 437–443. [Google Scholar] [CrossRef]
- Silverman, J.; Kurtz, S.; Draper, J. Skills for Communicating with Patients; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Colliers, A.; Bombeke, K.; Philips, H.; Remmen, R.; Coenen, S.; Anthierens, S. Antibiotic prescribing and doctor-patient communication during consultations for respiratory tract infections: A video observation study in out-of-hours primary care. Front. Med. 2021, 8, 735276. [Google Scholar] [CrossRef]
- Hasan, S.; Gamal, M.; Samorinha, C.; Hassan, N.A.G. Antibiotic use numeracy: Developing the infectious numeracy test (INT). Res. Soc. Adm. Pharm. 2022, 18, 3580–3587. [Google Scholar] [CrossRef]
- Yee, L.M.; Simon, M.A. The role of Health Literacy and numeracy in contraceptive decision-making for Urban Chicago Women. J. Community Health 2014, 39, 394–399. [Google Scholar] [CrossRef]
- Hasan, S.; Halabi, M.I. Development and validation of the New Asthma Numeracy Test. Value Health Reg. Issues 2021, 25, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Mahameed, S. Assessing patient knowledge of asthma using a newly validated tool. Value Health Reg. Issues 2020, 22, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Vallin, M.; Polyzoi, M.; Marrone, G.; Rosales-Klintz, S.; Tegmark Wisell, K.; Stålsby Lundborg, C. Knowledge and attitudes towards antibiotic use and resistance—A latent class analysis of a Swedish population-based sample. PLoS ONE 2016, 11, e0152160. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P.; ISPOR Task Force for Translation and Cultural Adaptation. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: Report of the ISPOR task force for translation and cultural adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef] [PubMed]
- De Vellis, R.F. Scale Development: Theory and Applications, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2003. [Google Scholar]
| Sociodemographic Information | N (%) |
| Gender: Male Female | 123 (31.9) 263 (68.1) |
| Age: 18–30 31–40 41–50 51 | 250 (65.0) 64 (16.5) 42 (10.8) 30 (7.7) |
| Nationality: UAE National Other Arab Non-Arab | 214 (55.5) 138 (35.7) 34 (8.8) |
| Education: Tertiary school or less Postgraduate Higher degree | 178 (46.1) 175 (45.3) 33 (8.6) |
| Monthly Income (AED): <10,000 10,000–20,000 20,000–30,000 >30,000 | 272 (70.5) 57 (14.8) 33 (8.5) 24 (6.2) |
| Do you have any persistent or long-lasting illness? Yes No | 38 (9.8) 348 (90.2) |
| Have you had any medical/health related education? Yes No | 25 (6.5) 361 (93.5) |
| Familiarity and consumption of antibiotics | N (%) |
| Please name below some of the antibiotics you have heard of: Correct Incorrect | 108 (28.0) 278 (72.0) |
| Have you ever used antibiotics without a doctor’s prescription? Yes No I don’t know | 111 (28.8) 161 (41.7) 114 (29.5) |
| How many times have you consumed antibiotics during the past 12 months? Never Once only 2–5 times More than 5 | 35 (9.1) 141 (36.5) 134 (34.7) 76 (19.7) |
| Do you consume antibiotics when your body temperature is ________? More than 37 °C More than 37.5 °C More than 38 °C More than 38.5 °C I don’t use antibiotics unless prescribed by my physician | 191 (49.5) 74 (19.2) 32 (8.3) 18 (4.6) 71 (18.4) |
| Which of the following six medications is an antibiotic? (Correct response = 3) | MeanSt.D 1.47 0.77 |
| Item | Correct/ Approp. N (%) | Incorrect/ In-Approp. N (%) | I Don’t Know N (%) |
|---|---|---|---|
| Antibiotics can be used to treat bacterial infections | 261 (67.62) | 53 (13.73) | 72 (18.62) |
| Antibiotics can be used to treat viral infections | 134 (34.72) | 183 (47.41) | 69 (17.88) |
| The body can fight mild infections on its own without antibiotics | 277 (71.76) | 62 (16.06) | 47 (12.18) |
| You can use antibiotics when you have a common cold | 109 (28.24) | 188 (48.70) | 89 (23.06) |
| You can use antibiotics when you have pneumonia (lung infection) | 160 (41.45) | 72 (18.65) | 154 (39.90) |
| You should always use antibiotics if one’s mucous becomes colored when having a cold | 108 (27.98) | 81 (20.98) | 197 (51.04) |
| You should always use antibiotics when you have sore throat | 144 (37.13) | 132 (34.20) | 110 (28.50) |
| An ear infection in a 3–6 year old child has to be treated with antibiotics | 102 (26.42) | 105 (27.20) | 179 (46.37) |
| If one feels better, he/she should stop using the antibiotic as soon as feeling better | 153 (39.64) | 166 (43.01) | 67 (17.36) |
| Antibiotics are used to kill all bacteria in the body | 137 (35.49) | 122 (31.61) | 127 (32.90) |
| Antibiotics make one recover faster when having a cold | 122 (31.61) | 140 (36.27) | 124 (32.12) |
| I usually obtain antibiotics from a pharmacy without a doctor’s visit | 177 (45.85) | 163 (42.23) | 46 (11.92) |
| In my home, leftover antibiotics can be saved for personal future use or given to someone else | 183 (47.41) | 166 (43.01) | 37 (9.59) |
| Sometimes I am able to acquire antibiotics from relatives or acquaintances, without having to be examined by a doctor | 280 (72.54) | 75 (19.43) | 31 (8.03) |
| Sometimes I buy antibiotics online, without having to see a doctor. | 256 (66.32) | 108 (27.98) | 22 (5.07) |
| If I get an infection, I often wait and see. (i.e., rest and take it easy), and see if the infection goes away on its own | 291 (56.74) | 75 (19.43) | 20 (5.18) |
| Antibiotics often cause side effects such as diarrhea | 146 (37.82) | 73 (18.91) | 167 (43.26) |
| Antibiotics can cause negative effects on the body’s own bacteria | 144 (37.31) | 89 (23.06) | 153 (39.64) |
| Bacteria can become resistant to antibiotics | 168 (43.52) | 43 (11.14) | 175 (45.34) |
| Resistant bacteria can spread from one patient to another | 138 (35.75) | 93 (24.09) | 155 (40.16) |
| The more antibiotics we used in society, the higher the risk that resistance develops | 95 (24.61) | 50 (12.95) | 241 (62.44) |
| Antibiotic use in animals can have an effect on antibiotic treatment for humans | 106 (27.46) | 61 (15.80) | 219 (56.46) |
| Resistance to antibiotics could spread from one country to another when people travel back and forth between countries | 100 (25.91) | 47 (12.18) | 239 (61.92) |
| Item | Factor | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | Antibiotics can be used to treat bacterial infections | * | * | * | * | 0.643 |
| 2 | Antibiotics can be used to treat viral infections | * | * | * | * | 0.788 |
| 3 | The body can fight mild infections on its own without antibiotics | * | * | * | * | 0.479 |
| 4 | You can use antibiotics when you have a common cold | * | * | * | −0.433 | * |
| 5 | You can use antibiotics when you have pneumonia (lung infection) | * | * | * | −0.392 | * |
| 6 | You should always use antibiotics if mucous becomes colored when having a cold | * | * | * | −0.707 | * |
| 7 | You should always use antibiotics when you have sore throat | * | * | * | −0.641 | * |
| 8 | An ear infection in a 3–6 year old child has to be treated with antibiotics | * | * | * | −0.690 | * |
| 9 | If one feels better, he/she should stop using the antibiotic as soon as feeling better | * | * | 0.654 | * | * |
| 10 | Antibiotics are used to kill all bacteria in the body | * | * | 0.627 | * | * |
| 11 | Antibiotics make one recover faster when having a cold | * | * | 0.535 | * | * |
| 12 | I usually obtain antibiotics from a pharmacy without a doctor’s visit | * | 0.702 | * | * | * |
| 13 | In my home, leftover antibiotics can be saved for personal future use or given to someone else | * | 0.726 | * | * | * |
| 14 | Sometimes I am able to acquire antibiotics from relatives or acquaintances, without having to be examined by a doctor | * | 0.792 | * | * | * |
| 15 | Sometimes I buy antibiotics online, without having to see a doctor. | * | 0.795 | * | * | * |
| 16 | If I get an infection, I often wait and see. (i.e., rest and take it easy), and see if the infection goes away on its own | * | 0.637 | * | * | * |
| 17 | Antibiotics often cause side effects such as diarrhea | 0.657 | * | * | * | * |
| 18 | Antibiotics can cause negative effects on the body’s own bacteria | 0.697 | * | * | * | * |
| 19 | Bacteria can become resistant to antibiotics | 0.616 | * | * | * | * |
| 20 | Resistant bacteria can spread from one patient to another | 0.658 | * | * | * | * |
| 21 | The more antibiotics we used in society, the higher the risk that resistance develops | 0.781 | * | * | * | * |
| 22 | Antibiotic use in animals can have an effect on antibiotic treatment for humans | 0.791 | * | * | * | * |
| 23 | Resistance to antibiotics could spread from one country to another when people travel back and forth between countries | 0.797 | * | * | * | * |
| Cronbach’s Alpha | 0.856 | 0.792 | 0.592 | 0.631 | 0.504 | |
| Cronbach’s Alpha for total knowledge scale | 0.848 | |||||
| Variable | N |
Factor 1 (7 Items) St.D P 2.32 ± 2.00 |
Factor 2 (5 Items) St.D P 3.08 ± 1.52 |
Factor 3 (3 Items) St.D P 1.07 ± 0.98 |
Factor 4 (5 Items) St.D P 1.61 ± 1.29 |
Factor 5 (3 Items) St.D P 1.74 ± 0.92 |
|---|---|---|---|---|---|---|
| Gender Male Female | 123 263 | 2.392.21 0.66 2.291.90 | 2.931.65 0.21 3.141.46 | 0.950.97 0.13 1.110.98 | 1.611.38 0.96 1.611.25 | 1.740.99 0.92 1.740.89 |
| Age 18–30 31–40 41–50 51 | 250 64 42 30 | 2.271.95 0.29 2.151.84 2.402.20 2.972.37 | 3.181.47 0.20 2.851.65 2.741.78 3.101.24 | 0.980.96 0.12 1.280.92 1.191.06 1.131.04 | 1.631.24 0.016 1.351.34 1.431.40 2.231.33 | 1.710.89 0.53 1.721.03 1.780.97 1.960.89 |
| Education Tertiary school or less Postgraduate Higher degree | 178 175 33 | 2.031.91 0.001 2.412.02 3.392.03 | 3.001.57 0.70 3.121.50 3.181.44 | 2.031.91 0.046 2.412.02 3.392.03 | 1.511.33 0.30 1.671.24 1.851.35 | 1.680.89 0.000 1.670.93 2.420.75 |
| Monthly Income <10,000 10,000–20,000 20,000–30,000 >3000 | 272 57 33 24 | 2.171.98 0.008 2.241.83 2.362.23 3.671.97 | 2.981.58 0.38 3.261.40 3.241.37 3.371.31 | 1.020.95 0.14 1.291.00 0.911.01 1.251.07 | 1.491.28 0.043 1.881.40 1.901.28 1.961.08 | 1.680.92 0.024 2.030.90 1.570.93 1.950.80 |
| AKCT Mean St.D = 9.82 ± 3.85 | 386 | 2.32 ± 2.00 | 3.08 ± 1.52 | 1.07 ± 0.98 | 1.61 ± 1.29 | 1.74 ± 0.92 |
| INT (Total Score) | |
|---|---|
| Antibiotic knowledge (total score) | 0.205 ** |
| Factor 1—Side-effects and resistance | 0.162 * |
| Factor 2—Access to antibiotics | −0.060 |
| Factor 3—Recovery after use | 0.125 |
| Factor 4—Antibiotics use indications | −0.025 |
| Factor 5—Body response | −0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, S.; Sulieman, H.; Babi, H.; Bloukh, S. Development and Psychometric Evaluation of the Antibiotic Knowledge and Consumption Tool (AKCT). Antibiotics 2022, 11, 1744. https://doi.org/10.3390/antibiotics11121744
Hasan S, Sulieman H, Babi H, Bloukh S. Development and Psychometric Evaluation of the Antibiotic Knowledge and Consumption Tool (AKCT). Antibiotics. 2022; 11(12):1744. https://doi.org/10.3390/antibiotics11121744
Chicago/Turabian StyleHasan, Sanah, Hana Sulieman, Husam Babi, and Samir Bloukh. 2022. "Development and Psychometric Evaluation of the Antibiotic Knowledge and Consumption Tool (AKCT)" Antibiotics 11, no. 12: 1744. https://doi.org/10.3390/antibiotics11121744
APA StyleHasan, S., Sulieman, H., Babi, H., & Bloukh, S. (2022). Development and Psychometric Evaluation of the Antibiotic Knowledge and Consumption Tool (AKCT). Antibiotics, 11(12), 1744. https://doi.org/10.3390/antibiotics11121744








