Abstract
Antimicrobial resistance (AMR) has become a global public health threat. Experts agree that unless proper actions are taken, the number of deaths due to AMR will increase. Many strategies are being pursued to tackle AMR, one of the most important being the development of efficient vaccines. Similar to other bacterial pathogens, AMR in Helicobacter pylori (Hp) is rising worldwide. Hp infects half of the human population and its prevalence ranges from <10% in developed countries to up to 90% in low-income countries. Currently, there is no vaccine available for Hp. This review provides a brief summary of the use of antibiotic-based treatment for Hp infection and its related AMR problems together with a brief description of the status of vaccine development for Hp. It is mainly dedicated to genetic tools and strategies that can be used to develop an oral recombinant Hp vaccine delivery platform that is (i) completely attenuated, (ii) can survive, synthesize in situ and deliver antigens, DNA vaccines, and adjuvants to antigen-presenting cells at the gastric mucosa, and (iii) possibly activate desired compartments of the gut-associated mucosal immune system. Recombinant Hp vaccine delivery vehicles can be used for therapeutic or prophylactic vaccination for Hp and other microbial pathogens.
1. Introduction
The discovery of penicillin, the first antibiotic, by Sir Alexander Fleming represented a major breakthrough in treating infectious diseases. This initial discovery was followed by many other natural or synthetic molecules, and antibio-therapy has since saved millions of lives worldwide. However, as anticipated by Fleming himself at the time of his discovery, bacteria have developed abilities to resist antibiotics at a rate similar to or higher than the availability of new molecules. Today, AMR has become a global public health threat. Multidrug resistant (MDR) clones of important human pathogens are emerging at an alarming rate [1], and experts agree that if proper actions are not taken, the number of deaths due to resistant bacteria will increase in the future. The increasing trend of AMR was confirmed by a recent review showing the global burden of AMR for 2019 [2]. Not surprisingly, sub-Saharan Africa (sSA) was the world region with the highest death rates directly due to or associated with AMR [2]. To address AMR, many strategies are being conducted both locally and globally. These strategies are based on (i) the production and sharing of quality data on AMR, (ii) capacity building for bacterial culture, antimicrobial susceptibility testing and its interpretation, (iii) surveillance based on a one health approach and (iv) development of alternatives to antibiotic-based treatment. Although challenging, the development of prophylactic and therapeutic vaccines appears to be a powerful means that can be harnessed to tackle the rise of AMR. This strategy is strongly advocated by international organizations at the forefront of the efforts developed to tackle the rise of AMR including the WHO, FAO, OIE. The rapid and efficient development of different vaccines against SARS-CoV-2 during the COVID pandemic has shown that existing scientific tools can serve this purpose.
Similar to other bacterial pathogens, AMR in Helicobacter pylori (Hp) has become an important public health concern, given the increasing prevalence of clones resistant to antibiotics used to treat infection [3]. This is especially true in sSA, where the prevalence of Hp is high and quality data on the resistance of circulating clones are scarce. Alternative methods to manage Hp infection and its associated diseases are most needed in sSA and other low-income countries (LIC). Development of therapeutic and prophylactic vaccines against Hp is, without doubt, the best means to cure and prevent infection respectively, and reduce the burden of diseases associated with this bacterium. While there is no available vaccine against Hp, this short review intends to present pieces of evidence that recombinant Hp can be developed to serve as an oral vaccine delivery platform. After short summaries on AMR and the status of vaccine development for Hp, this review will discuss detailed knowledge of the available tools that can be used to construct recombinant Hp vehicles that are safe, can synthesize and deliver antigens in situ, and activate different compartments of the gut-associated mucosal immune system (G-MIS). Hp delivery platform can be used not only for Hp, but for other microbial pathogens as well.
2. Treatment of Helicobacter pylori Infection and Problems Posed by Eradication Failure
Hp is one of the most successful bacterial colonizers of humans. It has been associated with mankind for over 100,000 years and today, the average prevalence of Hp infection globally is >50% [2]. This prevalence varies greatly, ranging from <10% to >90%, with large differences between countries and between communities in the same country [2]. Several factors contribute to Hp prevalence and its associated diseases, including socioeconomic conditions, host genetic predisposition, age, ethnicity, and environmental factors [2,3]. Poverty is one of the main contributors to the Hp burden and explains the consistent differences between developed and developing countries. Hp infection often lasts for the host’s lifetime if untreated. In most individuals, chronic infection by Hp results in asymptomatic stomach inflammation. In subsets of subjects, Hp carriage leads to different diseases, including peptic ulcers, chronic atrophic gastritis, gastric cancers, and gut lymphoma [4,5].
Treatment of Hp infection is based on the use of a combination of drugs, including one to three antibiotics in addition to a proton pump inhibitor (PPI) and/or bismuth salts [6]. PPIs inhibit gastric acid secretion [7], while bismuth has a direct killing effect against Hp [8]. Both PPIs and bismuth act synergistically with antibiotics against Hp [9,10]. The antibiotics used in the Hp eradication regimen include clarithromycin, metronidazole, ampicillin, fluoroquinolone, and tetracycline [11]. Nowadays, recommendations for first-line Hp treatment depend on local rates of resistance to the above-mentioned antibiotics and may correspond to PPI-based triple therapy (PPI plus two antibiotics), bismuth-based quadruple therapy (bismuth, PPI, and two antibiotics), non-bismuth concomitant quadruple therapy (PPI plus three antibiotics) or other combinations [12]. In case of treatment failure, it is advised to perform Hp isolation and antibiotic susceptibility testing (AST) before using a different eradication regimen [13]. This requires biopsies to be taken and Hp isolated. In LIC, especially in sSA, there is a lack of qualified microbiologists and of laboratories properly equipped to perform Hp culture and AST. Consequently, despite high carriage, reports of Hp prevalence and incidence in the continent greatly vary among regions, with certain countries making available quality data while there are rare to no data for the majority. Therefore, the resistance profile of circulating clones is mostly unknown and treatment is largely empirical. An efficient vaccine is, without doubt, the best means to reduce Hp infection and its associated diseases in sSA.
3. Current Status of Vaccines for Helicobacter pylori
There is currently no vaccine approved for Hp. Despite many candidates developed in preclinical studies (Table 1), Hp vaccines are poorly performed in clinical trials [11]. This is primarily due to a fascinating arsenal of immune escape strategies that Hp has developed during its long co-evolution with humans [14,15]. Hp immune escape targets both innate [16,17,18] and adaptive immune systems [17,18,19,20] leading to unresponsiveness or to activation of inappropriate responses that fail to sterilize the host. Nevertheless, recent advances in understanding Hp pathogenesis and its interaction with the host immune system, in addition to the availability of genetic engineering tools enable a better design of potentially successful vaccines. Important Hp virulence factors including urease, flagellar subunit, catalase, CagA, VacA, NapA, HpaA, immune escape proteins OipA and GGT, adhesion BabA, SabA, and Omp, have been well-studied [19,21]. Innovative approaches, such as immunoinformatics, have been used to identify various epitopes that can be combined in multiepitope vaccines [22] including for oral delivery [23]. These novel approaches can be combined in a recombinant Hp vaccine platform that can immunize for Hp and other pathogenic microbes.

Table 1.
Helicobacter pylori vaccine under the preclinical and clinical phases of development.
4. Helicobacter pylori as a Platform for the Delivery of Oral Vaccines
As one of the most successful bacterial colonizers of humans, Hp can be an ideal vehicle for delivering biologically active molecules, including vaccines and therapeutic drugs, to the digestive tract [35]. Hp presents several advantages for this type of application. Firstly, there are avirulent (type II) strains [36] and the most important virulence factors of Hp have been well characterized and can be easily inactivated. Secondly, Hp efficiently colonizes the human stomach, a site that is less populated by microbial flora than the intestines. Therefore, recombinant Hp cells expressing vaccines will face less competition for the occupation of gastric niches, a factor that impacts the delivery of antigens. Thirdly, Hp can gain access to gastric lymph nodes [37,38] where it encounters antigen-presenting cells (APC) and other immune cells. Additionally, Hp cells can reach Peyer’s patches in the small intestine, yet another important immune induction site [39]. Therefore, Hp can deliver antigens to two primary G-MIS induction sites. Fourthly, substantial knowledge has been accumulated on the physiology and the pathogenicity of Hp. Several aspects of the interaction of the bacterium with the G-MIS are known at the molecular level [40]. This makes it possible to selectively inactivate genes involved in immune escape or to construct recombinant strains that favor the desired type of immune response. Fifthly, it is possible to take advantage of the technologies, approaches, and strategies developed in other models of bacterial vaccine delivery vehicles including attenuated pathogen and commensal/non-pathogenic vectors.
4.1. Genetic Tools and Technologies for Recombinant Helicobacter pylori Vaccine Delivery Vehicle
An intermediary step toward the use of Hp as a vaccine delivery vehicle is the design of genetic and molecular tools that enable modification of the genome, expression, export, and targeting of antigens in different compartments of the bacterium. Hp is a fastidious bacterium that has numerous requirements for growth. Also, there are fewer tools for genetic manipulation available in Hp than in model organisms such as E. coli and Salmonella. Early mutagenesis systems developed in Hp included a few random and targeted mutagenesis strategies using transposon and suicide-plasmid based systems [41,42]. The development of a mutagenesis system based on a counterselectable marker represented a significant advance in Hp mutagenesis [43]. In this system a two-gene DNA cassette specifying an antibiotic resistance marker and a dominant allele of the rpsL ribosomal gene that confers sensitivity to streptomycin is used to replace chromosomal gene. Mutations constructed with this system are easily transferable to other Hp strains by natural transformation using genomic DNA from the mutant. Unmarked deletions can be efficiently obtained after the excision of the mutating cassette from the chromosome. Following this, Xer-Cise targeted mutagenesis that enables one-step construction of unmarked chromosomal deletions has been developed in Hp [44]. Xer-Cise uses the site-specific recombinase XerH, and its cognate target dif sequence that is involved in the resolution of chromosome dimers [45]. In this system, the two-gene counterselectable cassette described above is flanked by dif sequences and used to replace the chromosomal genes (Figure 1). Subsequently, unmarked deletions are counterselected without the need for the introduction of additional DNA material. XerCise system is very similar to the lambda red and Frt-Flip technologies first developed in E. coli [46] and as with that method could possibly be used to insert vaccine constructs in Hp chromosome [47] (Figure 1).
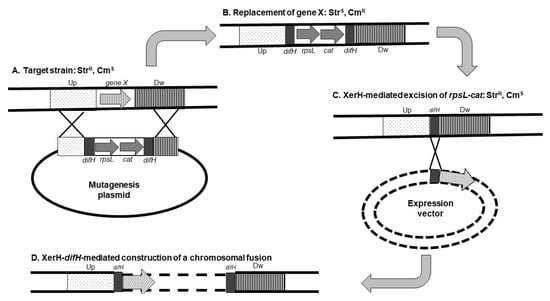
Figure 1.
Construction of unmarked deletion and chromosomal fusion in Helicobacter pylori. A mutagenesis plasmid is introduced in the strain from which a gene (gene X) is to be deleted. This strain harbors a mutation of the rpsL gene that confers resistance to streptomycin (StrR). The mutagenesis plasmid contains the following elements: (i) two copies of difH, a target of the site-specific recombinase XerH flanking (ii) an rpsL allele that restores sensitivity to streptomycin (StrS), and (iii) a cat gene that confers resistance to chloramphenicol (CmR); the difH-rpsL-cat-difH cassette is flanked by (iv) regions corresponding to sequences upstream (Up) and downstream (Dw) of the gene to be deleted. After the introduction of the mutagenesis plasmid (A), the target gene is replaced by the difH-rpsL-cat-difH cassette via homologous recombination between the upstream and downstream sequences from the plasmid and the chromosome; the resulting strain has an StrS-CmR phenotype (B). Growth of this strain in the absence of chloramphenicol and the presence of streptomycin enables the selection of excision of the rpsL-cat cassette via the activity of H. pylori XerH recombinase (C) leaving a single difH copy at the chromosomal location of the deleted gene (StrR-CmS phenotype). This difH copy can be used to insert a genetic construct in the chromosome of the resulting strain (D). Partially adapted from [30].
4.2. Recombinant Protein Expression Systems for Helicobacter pylori
The development of recombinant vaccine delivery vehicles requires expression systems that enable sufficient and stable production of antigens. Both chromosomes and plasmids have been used as locations for antigen constructs. Chromosomal expression presents the advantage of the stability of vaccine constructs. However, it suffers from low levels of protein production. In contrast, plasmids that can exist in multiple copies in a bacterial host often enable high expression levels. Several plasmid vectors have been developed in Hp and used to express foreign genes [48]. However, plasmid expression suffers a few drawbacks for use in Hp delivery vehicles. Firstly, since Hp can be difficult to manipulate, vaccine constructs would typically be performed in easily manipulable hosts such as E. coli before transfer to Hp. Although natural competence and conjugation systems enable the introduction of DNA material into Hp cells, the success rate is often low and varies greatly from strain to strain. This low success is likely due to the strong restriction enzyme barriers that exist in Hp [49]. Secondly, constructs expressed from plasmids are more susceptible to instability and additionally can negatively affect the fitness of the bacterial vehicle during host colonization because of the metabolic burden that results from plasmid maintenance and gene expression. Several alternatives can be proposed to enable stable expression of vaccine constructs in Hp without affecting bacterial fitness in host tissues. The design of constructs with optimized translational signals and their insertion in the chromosome as transcriptional fusions with highly expressed genes may support a sufficient level of antigen production. For example, urease, constituted by UreA and UreB subunits, is highly expressed making up to 10% of total Hp proteins [50]. The regulation of the ureAB operon has been well studied and this enzyme is essential for the colonization of gastric tissues [51]. Transcriptional fusion of vaccine constructs with ureAB operon can be a means to achieve in vivo induction of antigen production. Antigen expression can be further enhanced by optimizing translational signals (ribosome binding site and sequence around the initiation codon) of vaccine constructs. Another alternative could be the use of inducible promoters on the middle to high copy-number plasmids. Unfortunately, only a few inducible systems are available in Hp [52] and their functionality during colonization of the host’s stomach was not evaluated. Interestingly, in vivo-induced promoters have been identified in Hp cells infecting mice [53]. These promoters could be used to design vaccine constructs that are repressed or moderately expressed (from chromosome or plasmid) during growth in vitro and yet highly expressed in the host stomach. Such an approach can relieve the metabolic burden that results from the constitutive expression of heterologous vaccine constructs and improve in situ synthesis and delivery of antigens.
4.3. Selection of Recombinant Bacteria and Stabilization of Antigen-Encoding Plasmids
The use of a plasmid for antigen expression poses two problems that need to be addressed in a vaccine delivery vehicle. Firstly, commonly used plasmids harbor antibiotic resistance genes that enable the selection of recombinant bacteria. However, the use of antibiotic markers in vaccine carriers is unacceptable for regulatory reasons. Indeed, resistance gene-encoding plasmids can be horizontally transferred to other bacteria that reside in the same niches as the recombinant vehicle during host colonization. The risk of dissemination of drug resistance to other bacteria, including pathogens, poses threats that make necessary the prohibition of antibiotic resistance markers from recombinant bacteria destined to be used in human or animal health. The use of antibiotic markers has been addressed by developing antibiotic-free plasmids that can be used for biotechnology purposes [54,55,56,57]. These antibiotic marker-free plasmids are based on different mechanisms including complementation of auxotrophic mutation [40,58], operator-repressor titration [43,44], plasmid addiction such as the toxin-antitoxin system [45], RNA-based regulation [59,60] and resistance to a non-antibiotic marker [61]. Besides precluding the use of antibiotic selection, the mechanisms mentioned above also contribute to stabilizing recombinant plasmids enabling the continuous expression of vaccine constructs. Indeed, loss of the plasmid results in the death of the bacterial cells. Plasmid instability and loss are frequent in recombinant bacteria during host colonization, especially for high copy number replicons. This is due to the metabolic burden resulting from plasmid maintenance and gene expression. Plasmid loss or instability in the bacterial vaccine carrier compromises the expression and delivery of antigens, and ultimately immune response. Furthermore, inducible expression systems have been incorporated in these vectors in order to provide a controlled expression of vaccine constructs and find an optimal balance between antigen production and bacterial fitness in host organs [62,63,64].
The antibiotic-free vectors have been widely in the two existing categories of bacterial vaccine delivery vehicles including attenuated pathogens and non-pathogenic/commensal bacteria, the most represented species of the two types being Salmonella [64] and Lactococcus lactis [63]. We describe below the balanced-lethal vector-host system developed in Salmonella, a vehicle of the same category as H. pylori, as an example of one of the most attracting systems that serve as both antibiotic-free and stabilization systems and additionally enable within-host inducible expression of vaccine construct. Balanced-lethal system relies on a gene that is (i) essential and (ii) can be complemented by the addition of a metabolite in growth media. Bacterial vaccine carriers lack the essential gene and require supplementation of the metabolite for growth. The essential gene is cloned in an expression plasmid that when introduced in the vaccine carrier provides complementation. Growth media not supplemented with the metabolite are used to select for bacterial cells carrying the complementing plasmid. A balanced-lethal system developed in Salmonella used the asdA gene that encodes aspartate β-semialdehyde dehydrogenase, an enzyme required for the synthesis of diamino pimelic acid (DAP). DAP is a unique constituent of the peptidoglycan layer of bacterial cell wall necessary to maintain cell shape and stability. DAP is only synthesized by bacteria and is unavailable in eukaryotic tissues. The DAP synthesis pathway has been studied in Hp [65] and a requirement for DAP was demonstrated in Hp dapE mutants. Therefore, this system can be implemented in Hp to construct an antigen expression vector (Figure 2). An important improvement of the asd balance-lethal system of Salmonella consisted in adding a plasmid encoding a regulated repressor of the promoter governing the expression of the vaccine construct enabling the induction of antigen production in host organs [64]. This delayed regulated expression improves the fitness of the recombinant delivery vehicle in the host.
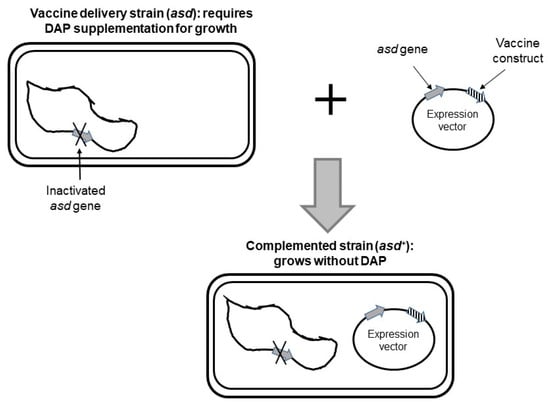
Figure 2.
Balanced-lethal expression system for Helicobacter pylori. The recombinant vaccine carrier strain lacks asd gene and requires supplementation of diaminopimelic acid (DAP) for growth in vitro. Introduction of a plasmid vector harboring asd gene and expressing a vaccine construct complements for inactivated chromosomal asd gene. Adapted from [40].
4.4. Targeting of Antigen Constructs in Helicobacter pylori
The subcellular location of an antigen in a bacterial carrier may impact the immune response [66,67]. It is therefore important to test antigens expressed in different compartments of the Hp delivery vehicle including cytoplasm, cell surface, or release into the surrounding environment (Figure 3). Cytoplasmic expression is straightforward and mainly requires optimal transcription and translation signals. Export of antigens outside Hp cells might be more challenging. Several export systems that enable protein crossing of the inner and outer membrane exist in Gram-negative bacteria [68]. Several of these systems have been characterized in or identified in the genome of Hp. Sec-dependant, twin-arginine translocation, lipoprotein, type IV secretion, and autotransporter pathways are present and studies of the Hp secretome have identified a number of proteins that are released in the supernatant of liquid cultures [69,70,71]. In addition, the Hp genome encodes dozens of outer membrane proteins (OMP) that are targeted to the bacterial surface by different mechanisms [72]. Export and targeting signals from these exported proteins can be used to construct recombinant Hp cells that display antigens at the cell surface or release them into the surrounding environment. Similarly, the Cag (cytotoxin-associated gene) type four secretion system (T4SS) could be engineered to construct Hp cells that directly inject antigens inside eukaryotic cells, a location particularly efficient for induction of a cell-mediated immune response [73,74]. Another means of delivery could be through outer membrane vesicles (OMV). OMVs are naturally produced by Gram-negative bacteria and OMV biogenesis is a controlled and regulated process that fulfills several functions [75]. Hp OMVs have been shown to interact with epithelial cells and to deliver bacterial proteins to host cells [76,77]. Although the mechanism of OMV biogenesis and regulation in Gram-negative bacteria is not fully elucidated, it can be anticipated that fusion to proteins located in Hp OMVs [77,78] could be a means of antigen delivery to eukaryotic cells.
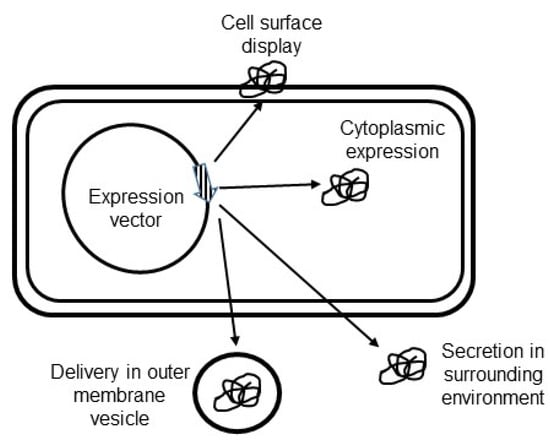
Figure 3.
Antigen targeting in Helicobacter pylori vaccine delivery vehicle. Recombinant vaccine constructs can be expressed in the cytoplasm, displayed at the cell surface, secreted in the surrounding environment, or delivered in outer membrane vesicles.
4.5. Delivery of DNA Vaccines by Helicobacter pylori
A potential limitation of antigens delivered by bacteria is the absence of post-translational protein modifications that occur in eukaryotic cells but not in bacteria. These modifications can lead to the formation of epitopes that induce protective immunity against viruses and eukaryotic microbes. Delivery of antigen-encoding DNA to host cells represents a means to overcome such a limitation. Besides this, DNA vaccines present other advantages. Firstly, DNA eliminates the need for antigen synthesis that imposes a metabolic burden on the bacterial vaccine carrier and negatively affects its fitness in host tissues. Secondly, antigens synthesized in the cytosol of host cells are efficient in inducing cell-mediated immunity, are properly processed by host chaperones, and preserve both linear and conformational epitopes. Thirdly, DNA molecules can be engineered to harbor innate immune activators such as CpG motifs that are recognized by TLR (toll-like receptor) 9 [79]. Several attenuated bacterial pathogens have been used to deliver DNA vaccines including Salmonella, Shigella, and Vibrio [80]. In these systems, antigen-encoding DNAs are harbored by plasmids that additionally contain signals for efficient antigen synthesis by eukaryotic cells including nuclear transport, eukaryotic transcriptional, and translational signals. Hp presents a potential for the delivery of antigen-encoding DNA to eukaryotic cells. DNA transfer between Hp cells during growth in vitro and in host tissues is established [81,82,83]. Plasmid transfer by Hp can occur through at least three pathways including natural transformation, conjugation via T4SS, and a recently identified yet not fully characterized mechanism [84]. Interestingly, Cag-T4SS can deliver chromosomal Hp DNA into host cells [85,86]. To be effective in inducing an immune response, a DNA vaccine should be delivered to APC. Hp interacts, is internalized, and survives in gastric APC including dendritic cells [87] and macrophages [88]. These findings indicate that recombinant Hp could possibly be engineered to deliver DNA vaccine constructs into gastric APC.
4.6. Overcoming Oral Tolerance and Immune Suppression by Helicobacter pylori
The design of a Hp oral vaccine delivery system needs to take into account oral tolerance, a state of immune unresponsiveness to an antigen delivered by the oral route [89] on the one hand, and the diverse immune escape strategies used by Hp on the other. These two issues can be properly addressed by using as a vaccine delivery vehicle, strains with a mutation in one or more immune escape strategies and that additionally express one or several adjuvants. Mucosal tolerance is an essential regulatory mechanism of the G-MIS that prevents inappropriate and detrimental activation against non-threatening compounds in the mucosa. Mucosal vaccine formulation typically includes mucosal adjuvants to overcome immune tolerance [90]. An adjuvant is an immune stimulant that, when co-administered with an antigen, enhances the potency and the quality of the immune response induced. The most efficient mucosal adjuvants comprise bacterial enterotoxins, TLR ligands, and cytokines. Bacterial enterotoxins, in particular ADP-ribosylating toxins such as cholera toxin (CT) and E. coli heat-labile toxin (LT), represent a class of mucosal adjuvants that have been well characterized [91]. These toxins are oligomeric proteins composed of one enzymatically active subunit and a homopentameric receptor binding subunit [92]. The toxin binds ganglioside GM1 [93] present at the surface of most eukaryotic cells including enterocytes and APC. The toxin’s binding to its receptor and its subsequent internalization activate signaling pathways that result in pleiotropic effects with activation of both innate and adaptive mucosal immune systems [94,95,96]. Concerns regarding the toxicity of the toxins have been solved by constructing mutants of CT and LT that are devoid of toxicity while retaining full adjuvant properties [95,97,98,99,100]. Additionally, it has been shown that the receptor-binding pentamer without the catalytic subunit still displays immunostimulatory activities [95,101,102,103]. For a Hp vaccine carrier, it is important to stress that CT and LT are bacterial proteins, and therefore can be efficiently synthesized and properly folded in a bacterial host. Another class of adjuvant used in mucosal immunization groups a number of TLR agonists [104]. TLR activation stimulates signaling pathways that result in the production of pro-inflammatory cytokines, recruitment of immune cells including APC, helper, and effector cells, and initiation of an adaptive immune response [104]. For a Hp vaccine delivery platform, the use of TLR signaling may primarily consist in restoring TLR4 and TLR5 signaling that are compromised by wild-type Hp cells [105]. In addition to these, recombinant Hp may be genetically engineered to express ligands of other TLRs. Cytokines represent a third class of adjuvant that can be considered for the Hp vaccine carrier. Co-delivery of antigen with cytokines has been tested with other bacterial vaccine carriers including attenuated pathogens [106] and commensal bacteria [63,66] resulting in enhanced immune responses against the antigen. The interest in co-delivery of cytokine with antigen lies in that this class of adjuvant represents an efficient means of directing immune activation toward a desired type of response. However, due to their pleiotropic effects cytokine expression by vaccine carriers should be carefully controlled to avoid adverse reactions in the host.
4.7. Bio-Containment of Recombinant Helicobacter pylori Vaccine Carrier
Bio-containment is an important concern when using genetically modified microorganisms [107]. Recombinant bacterial delivery vehicles should (i) be safe, (ii) lack or have limited abilities to uptake from or transmit genetic material to other microorganisms and (iii) be unable to disseminate once excreted outside their hosts. An ideal approach should combine not only one, but several bio-containment methods, providing redundancy such that failure of all the containment mechanisms becomes highly unlikely. Safety concern for a Hp vaccine carrier has been discussed above. Regarding the transmission of genetic materials, Hp is one of the most genetically diverse bacterial species likely because of efficient DNA import and export abilities along with a strong recombination system [108]. Hp strains can encode up to four types four secretion systems (TFSS) three of which are involved in DNA exchange including ComB-TFSS that mediate import from the environment, and TFS3 and TFS4 that are involved in conjugative transfer of chromosomal and plasmid DNA [108]. Additionally, a conjugation-independent mechanism not well characterized yet has been reported in Hp [84]. These mechanisms favor intra-species DNA exchange. Besides, the transmission of genetic material by Hp to other species can occur [109] although this is not well-documented. Acquisition of foreign DNA by recombinant Hp vehicle should be avoided in order to preserve its vaccine attributes. This can be accomplished by inactivating genes involved in DNA import. Interestingly, mutation of the comB gene prevented DNA uptake by natural transformation or by conjugation [84]. This gene is therefore a good candidate to be mutated to reduce foreign DNA uptake by Hp delivery vehicles. DNA export can be reduced by mutating relaxase genes of Hp tfs3 and tfs4 and by using expression plasmids devoid of any signal for mobilization in their sequences. Additionally, a plasmid addiction system can be used to design recombinant plasmids with reduced transferability. For example, a Hp vaccine carrier that harbors a chromosomal gene encoding an antitoxin can be used as a host of a plasmid expressing the cognate toxin. Transmission of the plasmid to other bacteria will result in the death of recipient microorganisms that normally do not encode antitoxin. Dissemination of recombinant Hp excreted by its host in the environment will presumably be limited since this bacterium mostly converts to coccoid cells that are a viable but non-culturable form outside the human stomach and its natural ecological niche [110]. However, additional strategies developed in other bacterial delivery vehicles including attenuated pathogens [111] and non-pathogenic vectors [107,111,112] can be imported to Hp. An example relevant to Hp is an elegant regulated delayed lysis in vivo developed in Salmonella in which the addition of arabinose during culture in vitro of the recombinant delivery vehicle induces the synthesis of the cell wall. After administration to the host, cell wall integrity is maintained in vivo during a few generations when the vaccine constructed is expressed and delivered after which the bacterial cells undergo lysis due to the absence of arabinose [111]. Hp harbors homologs of the asdA and murA genes involved in cell wall synthesis in Salmonella opening the possibility of using this system in this bacterium.
5. Conclusions
Vaccination is a strategy of choice to address the growing threat of AMR globally. The development of vaccines against the most critical human and animal bacterial pathogens will result in a decrease in antimicrobial consumption and of resistance to the antibiotics used for the treatment of infections. Active researches aiming to develop prophylactic and therapeutic vaccines, including orally delivered formulations are ongoing. Hp by its long association with humans, its ability to colonize stomach mucosa, and the health benefits it could confer could be an ideal oral vaccine delivery system. Little work has been dedicated to this topic so far. The extensive knowledge accumulated on the pathogenesis of Hp, especially on its immune escape mechanisms, and the available genetic engineering tools enable the construction of recombinant strains that are completely attenuated while retaining the ability to colonize and multiply in the gastric mucosa. These strains can be vectored to carry multiple antigens or DNA vaccines that are synthesized in situ and delivered with immune stimuli and immune modulation molecules enabling an efficient activation of all the desired compartments of the immune system including the innate, humoral, cell-mediated, and memory branches. Furthermore, recombinant Hp delivery vectors can be endowed with biocontainment attributes precluding their dissemination outside their host. These oral vaccines will represent an important breakthrough that will contribute to addressing AMR in Hp. Besides Hp, they can serve as a delivery vehicle for oral vaccines against other microbial pathogens most relevant for AMR. Oral Hp vaccine will be especially suited for LIC such as in sSA where the threat of AMR is most pressing.
Author Contributions
Literature review, Y.D., C.M.N., F.T., A.A.M.D., C.F.; writing—original draft preparation, Y.D.; writing—review and editing, C.M.N., F.T., A.A.M.D., C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moodley, Y.; Linz, B.; Bond, R.P.; Nieuwoudt, M.; Soodyall, H.; Schlebusch, C.M.; Bernhoft, S.; Hale, J.; Suerbaum, S.; Mugisha, L.; et al. Age of the association between Helicobacter pylori and man. PLoS Pathog. 2012, 8, e1002693. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.M.; Sharaf, R.R.; Aziz, R.K. Helicobacter pylori: A poor man’s gut pathogen? Gut. Pathog. 2010, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Ricci, V.; Romano, M.; Bouquet, P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J. Gastroenterol. 2011, 17, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef]
- Wang, A.Y.; Peura, D.A. The prevalence and incidence of Helicobacter pylori-associated peptic ulcer disease and upper gastrointestinal bleeding throughout the world. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.I.; Ajayi, A.; Jolaiya, T.; Onyekwere, C.; Setshedi, M.; Schulz, C.; Otegbayo, J.A.; Ndip, R.; Dieye, Y.; Alboraie, M.; et al. Helicobacter pylori Infection in Africa: Update of the Current Situation and Challenges. Dig. Dis. 2022, 40, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.R.; Sachs, G.; Marcus, E.A. The role of acid inhibition in Helicobacter pylori eradication. F1000Research 2016, 5, 1747. [Google Scholar] [CrossRef]
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkim, C. Role of Bismuth in the Eradication of Helicobacter pylori. Am. J. Ther. 2017, 24, e751–e757. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.T. High-dose dual therapy versus bismuth-containing quadruple therapy for the treatment of Helicobacter pylori infection—A review of the strengths, weaknesses, and proposed solutions. Tzu-Chi Med. J. 2022, 34, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Malizia, T.; Tejada, M.; Marchetti, F.; Favini, P.; Pizzarelli, G.; Campa, M.; Senesi, S. Synergic interactions of macrolides and proton-pump inhibitors against Helicobacter pylori: A comparative in-vitro study. J. Antimicrob. Chemother. 1998, 41, 29–35. [Google Scholar] [CrossRef][Green Version]
- Dos Santos Viana, I.; Cordeiro Santos, M.L.; Santos Marques, H.; Lima de Souza Goncalves, V.; Bittencourt de Brito, B.; Franca da Silva, F.A.; Oliveira, E.S.N.; Dantas Pinheiro, F.; Fernandes Teixeira, A.; Tanajura Costa, D.; et al. Vaccine development against Helicobacter pylori: From ideal antigens to the current landscape. Expert Rev. Vaccines 2021, 20, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P. “Rescue” regimens after Helicobacter pylori treatment failure. World J. Gastroenterol. 2008, 14, 5385–5402. [Google Scholar] [CrossRef]
- Chen, X.G.; Correa, P.; Offerhaus, J.; Rodriguez, E.; Janney, F.; Hoffmann, E.; Fox, J.; Hunter, F.; Diavolitsis, S. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am. J. Clin. Pathol. 1986, 86, 575–582. [Google Scholar] [CrossRef]
- Ruggiero, P. Helicobacter pylori and inflammation. Curr. Pharm. Des. 2010, 16, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Mentucci, F.; Voli, A.; Petrella, A.; Porta, A.; Tosco, A. Helicobacter pylori Pathogen-Associated Molecular Patterns: Friends or Foes? Int. J. Mol. Sci. 2022, 23, 3531. [Google Scholar] [CrossRef] [PubMed]
- Karkhah, A.; Ebrahimpour, S.; Rostamtabar, M.; Koppolu, V.; Darvish, S.; Vasigala, V.K.R.; Validi, M.; Nouri, H.R. Helicobacter pylori evasion strategies of the host innate and adaptive immune responses to survive and develop gastrointestinal diseases. Microbiol. Res. 2019, 218, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mejias-Luque, R.; Gerhard, M. Immune Evasion Strategies and Persistence of Helicobacter pylori. Curr. Top. Microbiol. Immunol. 2017, 400, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.T.B. Strategies used by Helicobacter pylori to establish persistent infection. World J. Gastroenterol. 2017, 23, 2870–2882. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Leone, I.; Suraci, E.; Imeneo, M.; Luzza, F. Helicobacter pylori and T Helper Cells: Mechanisms of Immune Escape and Tolerance. J. Immunol. Res. 2015, 2015, 981328. [Google Scholar] [CrossRef] [PubMed]
- Denic, M.; Touati, E.; De Reuse, H. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2020, 25, e12736. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.; Ali, A.; Akbar, H.; Sayaf, A.M.; Khan, A.; Wei, D.Q. Immunoinformatics approaches to explore Helicobacter Pylori proteome (Virulence Factors) to design B and T cell multi-epitope subunit vaccine. Sci. Rep. 2019, 9, 13321. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Baca, V.H.; Gomez-Flores, R.; De La Garza-Ramos, M.A.; Tamez-Guerra, P.; Lucio-Sauceda, D.G.; Rodriguez-Padilla, M.C. Immunoinformatics Approach to Design a Novel Epitope-Based Oral Vaccine Against Helicobacter pylori. J. Comput. Biol. 2019, 26, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Mao, X.H.; Li, J.X.; Tong, W.D.; Wang, B.; Zhang, Y.J.; Guo, G.; Zhao, Z.J.; Li, L.; Wu, D.L.; et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Formichella, L.; Romberg, L.; Bolz, C.; Vieth, M.; Geppert, M.; Gottner, G.; Nolting, C.; Walter, D.; Schepp, W.; Schneider, A.; et al. A novel line immunoassay based on recombinant virulence factors enables highly specific and sensitive serologic diagnosis of Helicobacter pylori infection. Clin. Vaccine Immunol. 2013, 20, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.F.; Moise, L.; Lee, D.S.; Kim, W.; Zhang, S.; Lee, J.; Rogers, A.B.; Martin, W.; De Groot, A.S. HelicoVax: Epitope-based therapeutic Helicobacter pylori vaccination in a mouse model. Vaccine 2011, 29, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dai, L.X.; Pan, X.; Wang, H.; Li, B.; Zhu, J.; Li, M.Y.; Shi, X.L.; Wang, B.N. Protection against Helicobacter pylori infection in BALB/c mice by oral administration of multi-epitope vaccine of CTB-UreI-UreB. Pathog. Dis. 2015, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, C.; Cheng, W.; Duan, G.; Shi, Q.; Chen, S.; Fan, Q. Delivery of Helicobacter pylori HpaA to gastrointestinal mucosal immune sites using Lactococcus lactis and its immune efficacy in mice. Biotechnol. Lett. 2018, 40, 585–590. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Ye, J.; Ning, L.; Luo, J.; Zhang, L.; Jiang, Y.; Xi, Y.; Ning, Y. Antibody Production and Th1-biased Response Induced by an Epitope Vaccine Composed of Cholera Toxin B Unit and Helicobacter pylori Lpp20 Epitopes. Helicobacter 2016, 21, 234–248. [Google Scholar] [CrossRef]
- Sutton, P.; Boag, J.M. Status of vaccine research and development for Helicobacter pylori. Vaccine 2019, 37, 7295–7299. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Selgrad, M.; Wex, T.; Romi, B.; Borgogni, E.; Spensieri, F.; Zedda, L.; Ruggiero, P.; Pancotto, L.; Censini, S.; et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: A randomised, placebo-controlled phase 1/2 study. Lancet Gastroenterol. Hepatol. 2018, 3, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Ren, B.; Zhou, X.; Cheng, L. Helicobacter pylori in the Oral Cavity: Current Evidence and Potential Survival Strategies. Int. J. Mol. Sci. 2022, 23, 13646. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, T.; Laforsch, S.; Hurwitz, R.; Brombacher, F.; Meyer, T.F. Immunity against Helicobacter pylori: Significance of interleukin-4 receptor alpha chain status and gender of infected mice. Infect. Immun. 2001, 69, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, H.; Tang, F.; Yin, R.; Liu, H.; Gong, X.; Wei, J.; Zhang, Y.; Xu, G.; Liu, K. Oral Immunization with a Multivalent Epitope-Based Vaccine, Based on NAP, Urease, HSP60, and HpaA, Provides Therapeutic Effect on H. pylori Infection in Mongolian gerbils. Front. Cell Infect. Microbiol. 2017, 7, 349. [Google Scholar] [CrossRef]
- Marshall, B.; Schoep, T. Helicobacter pylori as a vaccine delivery system. Helicobacter 2007, 12, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Axon, A.T. Are all helicobacters equal? Mechanisms of gastroduodenal pathology and their clinical implications. Gut 1999, 45, I1–I4. [Google Scholar] [CrossRef][Green Version]
- Andersen, L.P.; Holck, S. Possible evidence of invasiveness of Helicobacter (Campylobacter) pylori. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Necchi, V.; Candusso, M.E.; Tava, F.; Luinetti, O.; Ventura, U.; Fiocca, R.; Ricci, V.; Solcia, E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology 2007, 132, 1009–1023. [Google Scholar] [CrossRef]
- Nagai, S.; Mimuro, H.; Yamada, T.; Baba, Y.; Moro, K.; Nochi, T.; Kiyono, H.; Suzuki, T.; Sasakawa, C.; Koyasu, S. Role of Peyer’s patches in the induction of Helicobacter pylori-induced gastritis. Proc. Natl. Acad. Sci. USA 2007, 104, 8971–8976. [Google Scholar] [CrossRef]
- Robinson, K.; Lehours, P. Review - Helicobacter, inflammation, immunology and vaccines. Helicobacter 2020, 25, e12737. [Google Scholar] [CrossRef]
- Bijlsma, J.J.; Vandenbroucke-Grauls, C.M.; Phadnis, S.H.; Kusters, J.G. Identification of virulence genes of Helicobacter pylori by random insertion mutagenesis. Infect. Immun. 1999, 67, 2433–2440. [Google Scholar] [CrossRef]
- Salama, N.R.; Shepherd, B.; Falkow, S. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J. Bacteriol. 2004, 186, 7926–7935. [Google Scholar] [CrossRef] [PubMed]
- Dailidiene, D.; Dailide, G.; Kersulyte, D.; Berg, D.E. Contraselectable streptomycin susceptibility determinant for genetic manipulation and analysis of Helicobacter pylori. Appl. Environ. Microbiol. 2006, 72, 5908–5914. [Google Scholar] [CrossRef][Green Version]
- Debowski, A.W.; Gauntlett, J.C.; Li, H.; Liao, T.; Sehnal, M.; Nilsson, H.O.; Marshall, B.J.; Benghezal, M. Xer-cise in Helicobacter pylori: One-step transformation for the construction of markerless gene deletions. Helicobacter 2012, 17, 435–443. [Google Scholar] [CrossRef]
- Debowski, A.W.; Carnoy, C.; Verbrugghe, P.; Nilsson, H.O.; Gauntlett, J.C.; Fulurija, A.; Camilleri, T.; Berg, D.E.; Marshall, B.J.; Benghezal, M. Xer recombinase and genome integrity in Helicobacter pylori, a pathogen without topoisomerase IV. PLoS ONE 2012, 7, e33310. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Ellermeier, C.D.; Janakiraman, A.; Slauch, J.M. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 2002, 290, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Heuermann, D.; Haas, R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 1998, 257, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Humbert, O.; Salama, N.R. The Helicobacter pylori HpyAXII restriction-modification system limits exogenous DNA uptake by targeting GTAC sites but shows asymmetric conservation of the DNA methyltransferase and restriction endonuclease components. Nucleic Acids Res. 2008, 36, 6893–6906. [Google Scholar] [CrossRef] [PubMed]
- Bauerfeind, P.; Garner, R.; Dunn, B.E.; Mobley, H.L. Synthesis and activity of Helicobacter pylori urease and catalase at low pH. Gut 1997, 40, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.; De Reuse, H. Staying alive overdosed: How does Helicobacter pylori control urease activity? Int. J. Med. Microbiol. 2005, 295, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.M.; McDaniel, T.K.; Whitmire, J.M.; Gancz, H.; Guidotti, S.; Censini, S.; Merrell, D.S. Expanding the Helicobacter pylori genetic toolbox: Modification of an endogenous plasmid for use as a transcriptional reporter and complementation vector. Appl. Environ. Microbiol. 2007, 73, 7506–7514. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.R.; Woodruff, A.J.; Connolly, L.E.; Sause, W.E.; Ottemann, K.M. Recombination-based in vivo expression technology identifies Helicobacter pylori genes important for host colonization. Infect. Immun. 2008, 76, 5632–5644. [Google Scholar] [CrossRef] [PubMed]
- Vandermeulen, G.; Marie, C.; Scherman, D.; Preat, V. New generation of plasmid backbones devoid of antibiotic resistance marker for gene therapy trials. Mol. Ther. 2011, 19, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.H.; Mairhofer, J. Marker-free plasmids for biotechnological applications - implications and perspectives. Trends Biotechnol. 2013, 31, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Mignon, C.; Sodoyer, R.; Werle, B. Antibiotic-free selection in biotherapeutics: Now and forever. Pathogens 2015, 4, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Seyed, N.; Zahedifard, F.; Habibzadeh, S.; Yousefi, R.; Lajevardi, M.S.; Gholami, E.; Rafati, S. Antibiotic-Free Nanoplasmids as Promising Alternatives for Conventional DNA Vectors. Vaccines 2022, 10, 1710. [Google Scholar] [CrossRef]
- Galan, J.E.; Nakayama, K.; Curtiss, R., 3rd. Cloning and characterization of the asd gene of Salmonella typhimurium: Use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene 1990, 94, 29–35. [Google Scholar] [CrossRef]
- Mairhofer, J.; Pfaffenzeller, I.; Merz, D.; Grabherr, R. A novel antibiotic free plasmid selection system: Advances in safe and efficient DNA therapy. Biotechnol. J. 2008, 3, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.; Carnes, A.E.; Hodgson, C.P.; Williams, J.A. Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine 2009, 27, 6454–6459. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Good, L. Plasmid selection in Escherichia coli using an endogenous essential gene marker. BMC Biotechnol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- de Castro, C.P.; Drumond, M.M.; Batista, V.L.; Nunes, A.; Mancha-Agresti, P.; Azevedo, V. Vector Development Timeline for Mucosal Vaccination and Treatment of Disease Using Lactococcus lactis and Design Approaches of Next Generation Food Grade Plasmids. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.; Cortes-Perez, N.G.; de Moreno de Leblanc, A.; Loiseau, J.; Aucouturier, A.; Langella, P.; LeBlanc, J.G.; Bermudez-Humaran, L.G. Use of genetically modified lactic acid bacteria and bifidobacteria as live delivery vectors for human and animal health. Gut Microbes 2022, 14, 2110821. [Google Scholar] [CrossRef] [PubMed]
- Clark-Curtiss, J.E.; Curtiss, R., 3rd. Salmonella Vaccines: Conduits for Protective Antigens. J. Immunol. 2018, 200, 39–48. [Google Scholar] [CrossRef]
- Karita, M.; Etterbeek, M.L.; Forsyth, M.H.; Tummuru, M.K.; Blaser, M.J. Characterization of Helicobacter pylori dapE and construction of a conditionally lethal dapE mutant. Infect. Immun. 1997, 65, 4158–4164. [Google Scholar] [CrossRef]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Dieye, Y.; Hoekman, A.J.; Clier, F.; Juillard, V.; Boot, H.J.; Piard, J.C. Ability of Lactococcus lactis to export viral capsid antigens: A crucial step for development of live vaccines. Appl. Environ. Microbiol. 2003, 69, 7281–7288. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. Protein secretion and membrane insertion systems in gram-negative bacteria. J. Membr. Biol. 2006, 214, 75–90. [Google Scholar] [CrossRef]
- Bumann, D.; Aksu, S.; Wendland, M.; Janek, K.; Zimny-Arndt, U.; Sabarth, N.; Meyer, T.F.; Jungblut, P.R. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 2002, 70, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Weeks, D.L.; Shin, J.M.; Scott, D.R.; Young, M.K.; Sachs, G. Proteins released by Helicobacter pylori in vitro. J. Bacteriol. 2002, 184, 6155–6162. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Lim, J.M.; Weinberg, M.V.; Wells, L.; Hoover, T.R. Direct analysis of the extracellular proteome from two strains of Helicobacter pylori. Proteomics 2007, 7, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Alm, R.A.; Bina, J.; Andrews, B.M.; Doig, P.; Hancock, R.E.; Trust, T.J. Comparative genomics of Helicobacter pylori: Analysis of the outer membrane protein families. Infect. Immun. 2000, 68, 4155–4168. [Google Scholar] [CrossRef]
- Panthel, K.; Meinel, K.M.; Sevil Domenech, V.E.; Trulzsch, K.; Russmann, H. Salmonella type III-mediated heterologous antigen delivery: A versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int. J. Med. Microbiol. 2008, 298, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Russman, H. Inverted pathogenicity: The use of pathogen-specific molecular mechanisms for prevention or therapy of disease. Int. J. Med. Microbiol. 2004, 293, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef]
- Parker, H.; Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect. Immun. 2010, 78, 5054–5061. [Google Scholar] [CrossRef]
- Wei, S.; Li, X.; Wang, J.; Wang, Y.; Zhang, C.; Dai, S.; Wang, X.; Deng, X.; Zhao, L.; Shan, B. Outer Membrane Vesicles Secreted by Helicobacter pylori Transmitting Gastric Pathogenic Virulence Factors. ACS Omega 2022, 7, 240–258. [Google Scholar] [CrossRef]
- Mullaney, E.; Brown, P.A.; Smith, S.M.; Botting, C.H.; Yamaoka, Y.Y.; Terres, A.M.; Kelleher, D.P.; Windle, H.J. Proteomic and functional characterization of the outer membrane vesicles from the gastric pathogen Helicobacter pylori. Proteomics Clin. Appl. 2009, 3, 785–796. [Google Scholar] [CrossRef]
- Blaas, S.H.; Stieber-Gunckel, M.; Falk, W.; Obermeier, F.; Rogler, G. CpG-oligodeoxynucleotides stimulate immunoglobulin A secretion in intestinal mucosal B cells. Clin. Exp. Immunol. 2009, 155, 534–540. [Google Scholar] [CrossRef]
- Becker, P.D.; Noerder, M.; Guzman, C.A. Genetic immunization: Bacteria as DNA vaccine delivery vehicles. Hum. Vaccin. 2008, 4, 189–202. [Google Scholar] [CrossRef]
- Kennemann, L.; Didelot, X.; Aebischer, T.; Kuhn, S.; Drescher, B.; Droege, M.; Reinhardt, R.; Correa, P.; Meyer, T.F.; Josenhans, C.; et al. Helicobacter pylori genome evolution during human infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5033–5038. [Google Scholar] [CrossRef] [PubMed]
- Kulick, S.; Moccia, C.; Didelot, X.; Falush, D.; Kraft, C.; Suerbaum, S. Mosaic DNA imports with interspersions of recipient sequence after natural transformation of Helicobacter pylori. PLoS ONE 2008, 3, e3797. [Google Scholar] [CrossRef] [PubMed]
- Yahara, K.; Kawai, M.; Furuta, Y.; Takahashi, N.; Handa, N.; Tsuru, T.; Oshima, K.; Yoshida, M.; Azuma, T.; Hattori, M.; et al. Genome-wide survey of mutual homologous recombination in a highly sexual bacterial species. Genome Biol. Evol. 2012, 4, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, S.; Holsten, L.; Weiss, E.; Benghezal, M.; Fischer, W.; Haas, R. Multiple pathways of plasmid DNA transfer in Helicobacter pylori. PLoS ONE 2012, 7, e45623. [Google Scholar] [CrossRef]
- Varga, M.G.; Shaffer, C.L.; Sierra, J.C.; Suarez, G.; Piazuelo, M.B.; Whitaker, M.E.; Romero-Gallo, J.; Krishna, U.S.; Delgado, A.; Gomez, M.A.; et al. Pathogenic Helicobacter pylori strains translocate DNA and activate TLR9 via the cancer-associated cag type IV secretion system. Oncogene 2016, 35, 6262–6269. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Linz, B.; Yamaoka, Y.; Backert, S. Unique TLR9 Activation by Helicobacter pylori Depends on the cag T4SS, But Not on VirD2 Relaxases or VirD4 Coupling Proteins. Curr. Microbiol. 2022, 79, 121. [Google Scholar] [CrossRef] [PubMed]
- Neuper, T.; Frauenlob, T.; Sarajlic, M.; Posselt, G.; Wessler, S.; Horejs-Hoeck, J. TLR2, TLR4 and TLR10 Shape the Cytokine and Chemokine Release of H. pylori-Infected Human DCs. Int. J. Mol. Sci. 2020, 21, 3897. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Huang, J.C.; Cheng, H.H.; Wu, M.C.; Huang, M.Z.; Hsu, H.Y.; Chen, Y.A.; Hsu, C.Y.; Pan, Y.J.; Chu, Y.T.; et al. Helicobacter pylori cholesterol glucosylation modulates autophagy for increasing intracellular survival in macrophages. Cell Microbiol. 2018, 20, e12947. [Google Scholar] [CrossRef]
- Rezende, R.M.; Weiner, H.L. Oral tolerance: An updated review. Immunol. Lett. 2022, 245, 29–37. [Google Scholar] [CrossRef]
- Lawson, L.B.; Norton, E.B.; Clements, J.D. Defending the mucosa: Adjuvant and carrier formulations for mucosal immunity. Curr. Opin. Immunol. 2011, 23, 414–420. [Google Scholar] [CrossRef]
- Beddoe, T.; Paton, A.W.; Le Nours, J.; Rossjohn, J.; Paton, J.C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 2010, 35, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; O’Neal, C.J.; Mitchell, D.D.; Robien, M.A.; Zhang, Z.; Pickens, J.C.; Tan, X.J.; Korotkov, K.; Roach, C.; Krumm, B.; et al. Structural biology and structure-based inhibitor design of cholera toxin and heat-labile enterotoxin. Int. J. Med. Microbiol. 2004, 294, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, S.; Magnani, J.L.; Twiddy, E.M.; Holmes, R.K.; Ginsburg, V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 1988, 56, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Anosova, N.G.; Chabot, S.; Shreedhar, V.; Borawski, J.A.; Dickinson, B.L.; Neutra, M.R. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer’s patches. Mucosal Immunol. 2008, 1, 59–67. [Google Scholar] [CrossRef]
- Connell, T.D. Cholera toxin, LT-I, LT-IIa and LT-IIb: The critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev. Vaccines 2007, 6, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Nawar, H.; Tapping, R.I.; Russell, M.W.; Connell, T.D. The Type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect. Immun. 2004, 72, 6351–6358. [Google Scholar] [CrossRef]
- Nawar, H.F.; Arce, S.; Russell, M.W.; Connell, T.D. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect. Immun. 2007, 75, 621–633. [Google Scholar] [CrossRef]
- Norton, E.B.; Lawson, L.B.; Freytag, L.C.; Clements, J.D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kiyono, H.; Yamamoto, M.; Imaoka, K.; Fujihashi, K.; Van Ginkel, F.W.; Noda, M.; Takeda, Y.; McGhee, J.R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 1997, 94, 5267–5272. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Takeda, Y.; Yamamoto, M.; Kurazono, H.; Imaoka, K.; Yamamoto, M.; Fujihashi, K.; Noda, M.; Kiyono, H.; McGhee, J.R. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 1997, 185, 1203–1210. [Google Scholar] [CrossRef]
- Lee, C.H.; Masso-Welch, P.; Hajishengallis, G.; Connell, T.D. TLR2-dependent modulation of dendritic cells by LT-IIa-B5, a novel mucosal adjuvant derived from a type II heat-labile enterotoxin. J. Leukoc. Biol. 2011, 90, 911–921. [Google Scholar] [CrossRef]
- Nashar, T.O.; Webb, H.M.; Eaglestone, S.; Williams, N.A.; Hirst, T.R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: Receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc. Natl. Acad. Sci. USA 1996, 93, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Plant, A.; Williams, R.; Jackson, M.E.; Williams, N.A. The B subunit of Escherichia coli heat labile enterotoxin abrogates oral tolerance, promoting predominantly Th2-type immune responses. Eur. J. Immunol. 2003, 33, 3186–3195. [Google Scholar] [CrossRef]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Melit, L.E.; Marginean, C.O.; Marginean, C.D.; Marginean, M.O. The Relationship between Toll-like Receptors and Helicobacter pylori-Related Gastropathies: Still a Controversial Topic. J. Immunol. Res. 2019, 2019, 8197048. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, Z.S.; Du, Y.Q.; Gong, Y.F.; Yang, H.; Sun, B.; Jin, J. Construction of recombinant attenuated Salmonella typhimurium DNA vaccine expressing H pylori ureB and IL-2. World J. Gastroenterol. 2007, 13, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Smit, E.; Klein, M.R. Evidence-based biosafety: A review of the principles and effectiveness of microbiological containment measures. Clin. Microbiol. Rev. 2008, 21, 403–425. [Google Scholar] [CrossRef]
- Fischer, W.; Tegtmeyer, N.; Stingl, K.; Backert, S. Four Chromosomal Type IV Secretion Systems in Helicobacter pylori: Composition, Structure and Function. Front. Microbiol. 2020, 11, 1592. [Google Scholar] [CrossRef]
- Oyarzabal, O.A.; Rad, R.; Backert, S. Conjugative transfer of chromosomally encoded antibiotic resistance from Helicobacter pylori to Campylobacter jejuni. J. Clin. Microbiol. 2007, 45, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Losurdo, G.; Mileti, A.; Paolillo, R.; Giorgio, F.; Principi, M.; Di Leo, A. The Puzzle of Coccoid Forms of Helicobacter pylori: Beyond Basic Science. Antibiotics 2020, 9, 293. [Google Scholar] [CrossRef]
- Curtiss, R., III; Xin, W.; Li, Y.; Kong, W.; Wanda, S.Y.; Gunn, B.; Wang, S. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit. Rev. Immunol. 2010, 30, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, P. Biocontainment strategies for live lactic acid bacteria vaccine vectors. Bioeng. Bugs 2010, 1, 75–77. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).