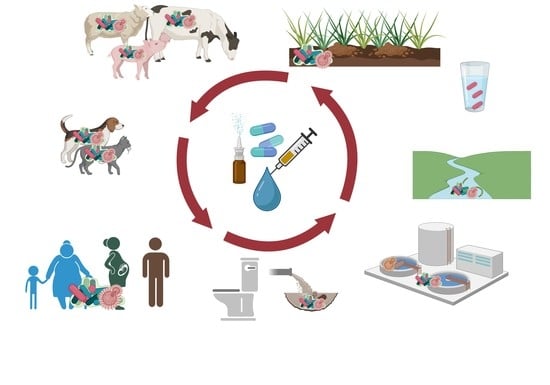
An Overview of the Impact of Pharmaceuticals on Aquatic Microbial Communities
Abstract
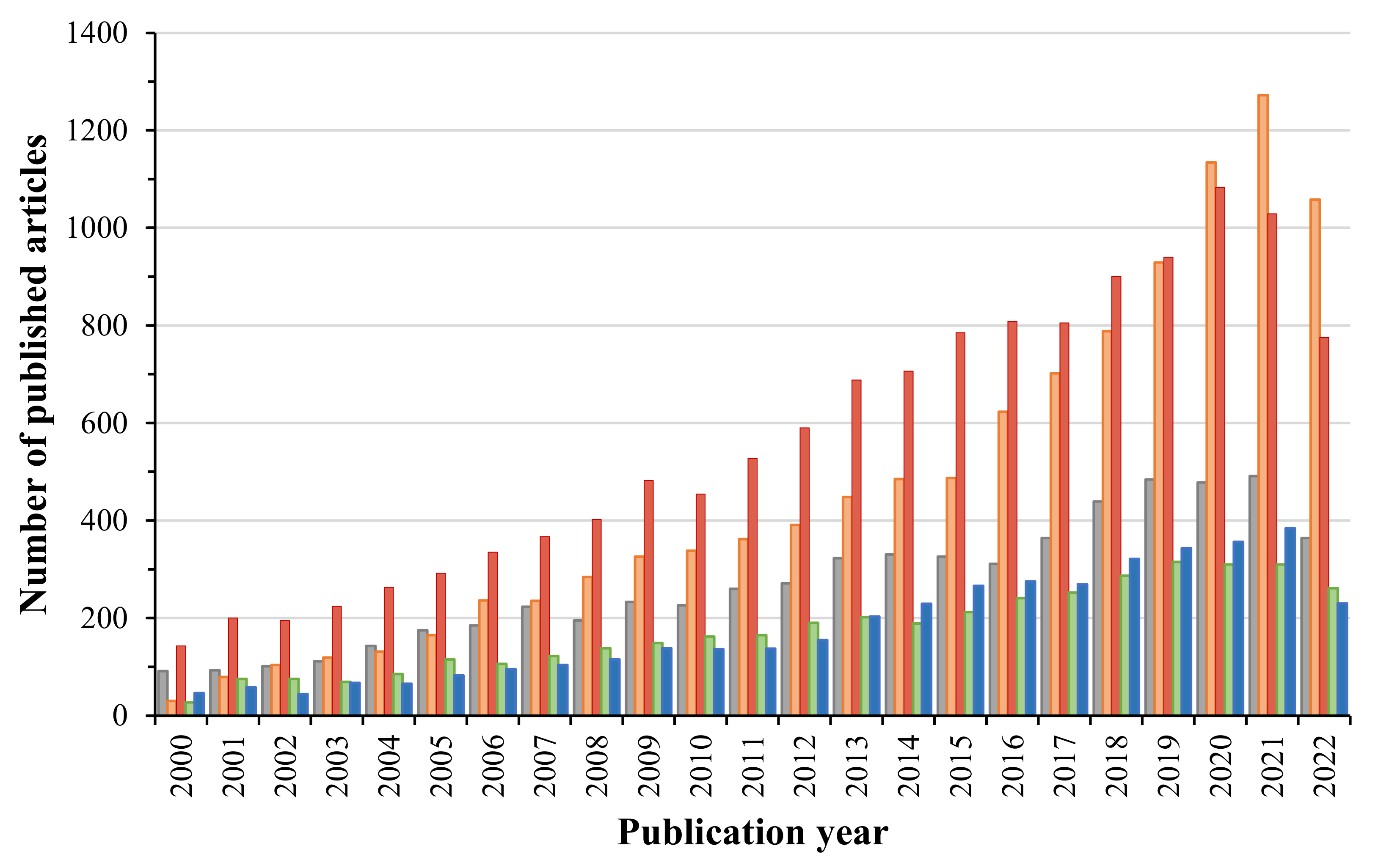
1. Introduction
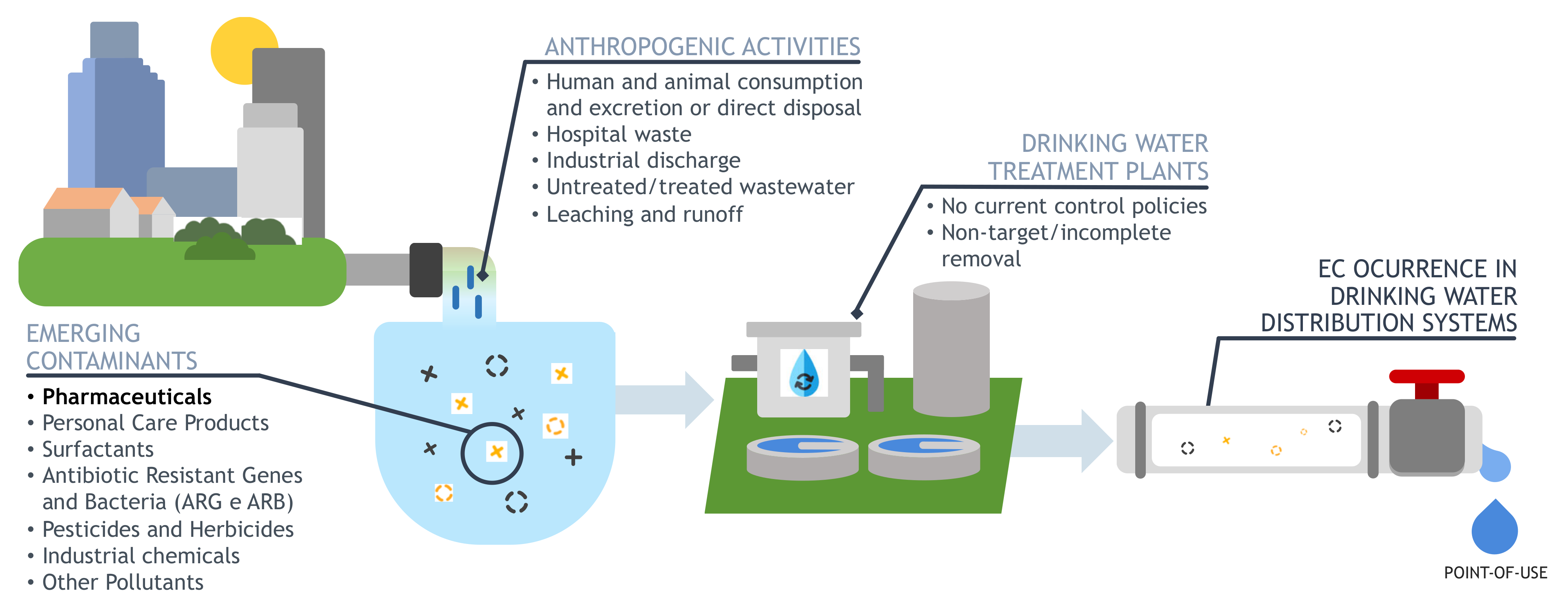
2. Pharmaceuticals Occurrence and Exposure Pathways
3. Environmental Impact of Pharmaceutical Contaminants
3.1. Effects of Pharmaceutical Exposure on Aquatic Biofilms Behaviour
3.2. Effect of Pharmaceuticals on Drinking Water Associated Biofilms
3.3. The Role of Antibiotics and Non-Antibiotics on Acquired Antibiotic Resistance
3.4. Controlling Pharmaceuticals Level in Water Sources and Wastewater
4. Hardships in Assessing Potential Environmental Risks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Water, J. Contaminants of Emerging Concern—An Emerging Risk in Our Waters. 2020. Available online: http://www.waterjpi.eu/implementation/thematic-activities/water-jpi-knowledge-hub-1/water-jpi-knowledge-hub-on-contaminants-of-emerging-concern (accessed on 30 September 2022).
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Singh, B.K.; Nathanail, C.P.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef] [PubMed]
- Kot-Wasik, A.; Jakimska, A.; Śliwka-Kaszyńska, M. Occurrence and seasonal variations of 25 pharmaceutical residues in wastewater and drinking water treatment plants. Environ. Monit. Assess. 2016, 18, 661. [Google Scholar] [CrossRef] [PubMed]
- López-Serna, R.; Jurado, A.; Vázquez-Suñé, E.; Carrera, J.; Petrović, M.; Barceló, D. Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain. Environ. Pollut. 2013, 174, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Maillard, J.Y.; Simões, L.C.; Simões, M. Emerging contaminants affect the microbiome of water systems—Strategies for their mitigation. NPJ Clean Water 2020, 3, 39. [Google Scholar] [CrossRef]
- Rosenfeld, P.E.; Feng, L.G.H. 16—Emerging Contaminants. In Risks of Hazardous Wastes; Rosenfeld, P.E., Feng, L.G.H., Eds.; William Andrew Publishing: Boston, MA, USA, 2011; pp. 215–222. [Google Scholar]
- Da Silva, B.F.; Jelic, A.; López-Serna, R.; Mozeto, A.A.; Petrovic, M.; Barceló, D. Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere 2011, 85, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.L.; Hooda, P.S.; Swinden, J.; Barker, J.; Barton, S. Spatial distribution of organic contaminants in three rivers of Southern England bound to suspended particulate material and dissolved in water. Sci. Total Environ. 2017, 593, 487–497. [Google Scholar] [CrossRef]
- Wang, C.; Ye, D.; Li, X.; Jia, Y.; Zhao, L.; Liu, S.; Xu, J.; Du, J.; Tian, L.; Li, J.; et al. Occurrence of pharmaceuticals and personal care products in bottled water and assessment of the associated risks. Environ. Int. 2021, 155, 106651. [Google Scholar] [CrossRef]
- Chen, H.; Chen, W.; Guo, H.; Lin, H.; Zhang, Y. Pharmaceuticals and personal care products in the seawater around a typical subtropical tourist city of China and associated ecological risk. Environ. Sci. Pollut. Res. 2021, 28, 22716–22728. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging pollutants in the urban water cycle in Latin America: A review of the current literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, E.; Cren-Olivé, C. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ. Pollut. 2011, 159, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; Van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Bhandari, A.; Surampalli, R.Y. Contaminants of Concern Task Committee. III. Environmental and Water Resources; The American Society of Civil Engineers: Reston, VA, USA, 2009. [Google Scholar]
- Sárria, M.P.; Santos, M.M.; Reis-Henriques, M.A.; Vieira, N.M.; Monteiro, N.M. The unpredictable effects of mixtures of androgenic and estrogenic chemicals on fish early life. Environ. Int. 2011, 37, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Gwenzi, W. Emerging Contaminants in the Terrestrial-Aquatic-Atmosphere: Continuum Occurrence, Health Risks and Mitigation; Elsevier: Potsdam, Germany, 2022. [Google Scholar]
- Couto, C.F.; Lange, L.C.; Amaral, M.C. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef] [PubMed]
- Carson, R. Silent Spring III; Houghton Mifflin Company: Boston, MA, USA, 1962; Volume 23. [Google Scholar]
- Cronon, W. Foreword. In DDT, Silent Spring, and the Rise of Environmentalism; Dunlap, T.R., Ed.; University of Washington Press: Washington, WA, USA, 2008; pp. ix–xii. [Google Scholar]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Amiard-Triquet, C.; Amiard, J.-C.; Rainbow, P.S. Ecological Biomarkers: Indicators of Ecotoxicological Effects; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- WHO. Pharmaceuticals in Drinking Water Public Health and Environment, Water, Sanitation, Hygiene and Health; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- FDA. FDA at a Glance: Regulated Products and Facilities. 2021. Available online: https://www.fda.gov/about-fda/fda-basics/fact-sheet-fda-glance (accessed on 1 October 2022).
- Wilkinson, J.L.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: A review of environmental, receptor-mediated, developmental, and epigenetic toxicity with discussion of proposed toxicity to humans. Crit. Rev. Environ. Sci. Technol. 2016, 46, 336–381. [Google Scholar] [CrossRef]
- Afonso-Olivares, C.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Assessment of the Presence of Pharmaceutical Compounds in Seawater Samples from Coastal Area of Gran Canaria Island (Spain). Antibiotics 2013, 2, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar]
- Meffe, R.; De Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef]
- Comber, S.D.W.; Gardner, M.J.; Ellor, B. Seasonal variation of contaminant concentrations in wastewater treatment works effluents and river waters. Environ. Technol. 2020, 41, 2716–2730. [Google Scholar] [CrossRef] [PubMed]
- Corcoll, N.; Acuña, V.; Barceló, D.; Casellas, M.; Guasch, H.; Huerta, B.; Petrovic, M.; Ponsatí, L.; Rodríguez-Mozaz, S.; Sabater, S. Pollution-induced community tolerance to non-steroidal anti-inflammatory drugs (NSAIDs) in fluvial biofilm communities affected by WWTP effluents. Chemosphere 2014, 112, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.-S.; Yang, J.-J.; Metcalfe, C.D. Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant. Environ. Sci. Technol. 2005, 39, 7469–7475. [Google Scholar] [CrossRef]
- Rzymski, P.; Drewek, A.; Klimaszyk, P. Pharmaceutical pollution of aquatic environment: An emerging and enormous challenge. Limnol. Rev. 2017, 17, 97–107. [Google Scholar] [CrossRef]
- Stefanakis, A.; Becker, J. A Review of Emerging Contaminants in Water: Classification, Sources, and Potential Risks. In Impact of Water Pollution on Human Health and Environmental Sustainability; McKeown, A.E., Bugyi, G., Eds.; IGI Global Publisher: Hershey, PA, USA, 2015; pp. 57–82. [Google Scholar]
- Houeto, P.; Carton, A.; Guerbet, M.; Mauclaire, A.C.; Gatignol, C.; Lechat, P.; Masset, D. Assessment of the health risks related to the presence of drug residues in water for human consumption: Application to carbamazepine. Regul. Toxicol. Pharmacol. 2012, 62, 41–48. [Google Scholar] [CrossRef]
- Gallagher, M.T.; Reisinger, A.J. Effects of ciprofloxacin on metabolic activity and algal biomass of urban stream biofilms. Sci. Total Environ. 2020, 706, 135728. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, B. Emerging contaminants in Stormwater. In Proceedings of the National Conference on Energy and Environment, Coimbatore, India, 16–17 September 2016. [Google Scholar]
- Yamamoto, H.; Nakamura, Y.; Moriguchi, S.; Nakamura, Y.; Honda, Y.; Tamura, I.; Hirata, Y.; Hayashi, A.; Sekizawa, J. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: Laboratory photolysis, biodegradation, and sorption experiments. Water Res. 2009, 43, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Shi, X.; Yu, G.; Huang, J.; Wang, B. Assessing the persistence of pharmaceuticals in the aquatic environment: Challenges and needs. Emerg. Contam. 2016, 2, 145–147. [Google Scholar] [CrossRef]
- Leusch, F.D.; Neale, P.A.; Busetti, F.; Card, M.; Humpage, A.; Orbell, J.D.; Ridgway, H.F.; Stewart, M.B.; van de Merwe, J.P.; Escher, B.I. Transformation of endocrine disrupting chemicals, pharmaceutical and personal care products during drinking water disinfection. Sci. Total Environ. 2019, 657, 1480–1490. [Google Scholar] [CrossRef]
- Lynch, J.M.; Wiseman, A.; De Leij, F.A.A.M. Ecotoxicology. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2001; pp. 118–126. [Google Scholar]
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manag. 2014, 133, 378–387. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abdallah, M.A.E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Saaristo, M.; Brodin, T.; Balshine, S.; Bertram, M.G.; Brooks, B.W.; Ehlman, S.M.; McCallum, E.S.; Sih, A.; Sundin, J.; Wong, B.B.M.; et al. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. Biol. Sci. 2018, 285, 20181297. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, L.; Petitjean, Q.; Côte, J.; Laffaille, P.; Jean, S. Effects of Pollution on Fish Behavior, Personality, and Cognition: Some Research Perspectives. Front. Ecol. Evol. 2020, 8, 86. [Google Scholar] [CrossRef]
- Noman, M.A.; Feng, W.; Zhu, G.; Hossain, M.B.; Chen, Y.; Zhang, H.; Sun, J. Bioaccumulation and potential human health risks of metals in commercially important fishes and shellfishes from Hangzhou Bay, China. Sci. Rep. 2022, 12, 4634. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, D.; Grabic, R.; Grabicová, K.; Randák, T.; Larsson, D.J.; Johnson, A.C.; Jürgens, M.D.; Tysklind, M.; Lindberg, R.H.; Fick, J. Neuroactive drugs and other pharmaceuticals found in blood plasma of wild European fish. Environ. Int. 2021, 146, 106188. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Yeh, A.; Gallagher, E.P. Adverse metabolic effects in fish exposed to contaminants of emerging concern in the field and laboratory. Environ. Pollut. 2018, 236, 850–861. [Google Scholar] [CrossRef]
- Grabicova, K.; Grabic, R.; Blaha, M.; Kumar, V.; Cerveny, D.; Fedorova, G.; Randak, T. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015, 72, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of psychoactive pharmaceuticals in fish in an effluent dominated stream. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef]
- Kovacs, E.D.; Silaghi-Dumitrescu, L.; Roman, C.; Tian, D. Structural and Metabolic Profiling of Lycopersicon esculentum Rhizosphere Microbiota Artificially Exposed at Commonly Used Non-Steroidal Anti-Inflammatory Drugs. Microorganisms 2022, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Stedtfeld, R.D.; Guo, X.; Bhalsod, G.D.; Jeon, S.; Tiedje, J.M.; Li, H.; Zhang, W. Pharmaceutical exposure changed antibiotic resistance genes and bacterial communities in soil-surface- and overhead-irrigated greenhouse lettuce. Environ. Int. 2019, 131, 105031. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in treated wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Microplastics and associated emerging contaminants in the environment: Analysis, sorption mechanisms and effects of co-exposure. Trends Environ. Anal. Chem. 2022, 35, e00170. [Google Scholar] [CrossRef]
- Besha, A.T.; Liu, Y.; Fang, C.; Bekele, D.N.; Naidu, R. Assessing the interactions between micropollutants and nanoparticles in engineered and natural aquatic environments. Crit. Rev. Environ. Sci. Technol. 2020, 50, 135–215. [Google Scholar] [CrossRef]
- Besemer, K. Biodiversity, community structure and function of biofilms in stream ecosystems. Res. Microbiol. 2015, 166, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Aubertheau, E.; Stalder, T.; Mondamert, L.; Ploy, M.C.; Dagot, C.; Labanowski, J. Impact of wastewater treatment plant discharge on the contamination of river biofilms by pharmaceuticals and antibiotic resistance. Sci. Total Environ. 2017, 579, 1387–1398. [Google Scholar] [CrossRef]
- Sandhya, M.; Huang, Y.; Li, J.; Wu, X.; Zhou, Z.; Lei, Q.; Bhatt, P.; Chen, S. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 2022, 294, 133609. [Google Scholar]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Fact. 2016, 15, 165. [Google Scholar] [CrossRef]
- Huq, A.; Whitehouse, C.A.; Grim, C.J.; Alam, M.; Colwell, R.R. Biofilms in water, its role and impact in human disease transmission. Curr. Opin. Biotechnol. 2008, 19, 244–247. [Google Scholar] [CrossRef]
- Cortés, M.E.; Bonilla, J.C.; Sinisterra, R.D. Biofilm formation, control and novel strategies for eradication. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 2, 896–905. [Google Scholar]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A Review of the Role of Extracellular Polymeric Substances (EPS) in Wastewater Treatment Systems. Int. J. Env. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef]
- Romero, F.; Acuña, V.; Sabater, S. Multiple Stressors Determine Community Structure and Estimated Function of River Biofilm Bacteria. Appl. Environ. Microbiol. 2020, 86, e00291-20. [Google Scholar] [CrossRef] [PubMed]
- Corcoll, N.; Casellas, M.; Huerta, B.; Guasch, H.; Acuña, V.; Rodríguez-Mozaz, S.; Serra-Compte, A.; Barceló, D.; Sabater, S. Effects of flow intermittency and pharmaceutical exposure on the structure and metabolism of stream biofilms. Sci. Total Environ. 2015, 503, 159–170. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, L.C.A.; de Oliveira, M.B.M. Effect of heavy metals on the biofilm formed by microorganisms from impacted aquatic environments. In Bacterial Biofilms; IntechOpen Limited: London, UK, 2020. [Google Scholar]
- Wang, H.; Hu, C.; Shen, Y.; Shi, B.; Zhao, D.; Xing, X. Response of microorganisms in biofilm to sulfadiazine and ciprofloxacin in drinking water distribution systems. Chemosphere 2019, 218, 197–204. [Google Scholar] [CrossRef]
- Proia, L.; Lupini, G.; Osorio, V.; Pérez, S.; Barceló, D.; Schwartz, T.; Amalfitano, S.; Fazi, S.; Romaní, A.M.; Sabater, S. Response of biofilm bacterial communities to antibiotic pollutants in a Mediterranean river. Chemosphere 2013, 92, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Rosi-Marshall, E.J.; Kincaid, D.W.; Bechtold, H.A.; Royer, T.V.; Rojas, M.; Kelly, J.J. Pharmaceuticals suppress algal growth and microbial respiration and alter bacterial communities in stream biofilms. Ecol. Appl. 2013, 23, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Subirats, J.; Timoner, X.; Sànchez-Melsió, A.; Balcázar, J.L.; Acuña, V.; Sabater, S.; Borrego, C.M. Emerging contaminants and nutrients synergistically affect the spread of class 1 integron-integrase (intI1) and sul1 genes within stable streambed bacterial communities. Water Res. 2018, 138, 77–85. [Google Scholar] [CrossRef]
- Aristi, I.; Casellas, M.; Elosegi, A.; Insa, S.; Petrovic, M.; Sabater, S.; Acuña, V. Nutrients versus emerging contaminants–Or a dynamic match between subsidy and stress effects on stream biofilms. Environ. Pollut. 2016, 212, 208–215. [Google Scholar] [CrossRef]
- Proia, L.; Osorio, V.; Soley, S.; Köck-Schulmeyer, M.; Pérez, S.; Barceló, D.; Romaní, A.M.; Sabater, S. Effects of pesticides and pharmaceuticals on biofilms in a highly impacted river. Environ. Pollut. 2013, 178, 220–228. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Swerhone, G.D.; Wassenaar, L.I.; Neu, T.R. Effects of selected pharmaceuticals on riverine biofilm communities. Can. J. Microbiol. 2005, 51, 655–669. [Google Scholar] [CrossRef]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.K.; Kemal, S.; Henrik, N.R.; Alexander, E.; Natalia, C.; Henrik, J.C.; Thomas, B.; Hans, B.; Erik, K. Triclosan changes community composition and selects for specific bacterial taxa in marine periphyton biofilms in low nanomolar concentrations. Ecotoxicology 2020, 29, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Phung, C.; Grace, M. Pharmaceuticals and personal care products alter growth and function in lentic biofilms. Environ. Chem. 2015, 12, 301–306. [Google Scholar] [CrossRef]
- Potgieter, S.; Pinto, A.; Sigudu, M.; Du Preez, H.; Ncube, E.; Venter, S. Long-term spatial and temporal microbial community dynamics in a large-scale drinking water distribution system with multiple disinfectant regimes. Water Res. 2018, 139, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Percival, S.; Walker, J. Contamination potential of biofilms in water distribution systems. Water Sci. Technol. Water Supply 2002, 2, 271–280. [Google Scholar] [CrossRef]
- Johnson, L.R. Microcolony and biofilm formation as a survival strategy for bacteria. J. Theor. Biol. 2008, 251, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yu, Z.; Chen, X.; Liu, R.; Zhang, H. Molecular characterization of natural biofilms from household taps with different materials: PVC, stainless steel, and cast iron in drinking water distribution system. Appl. Microbiol. Biotechnol. 2013, 97, 8393–8401. [Google Scholar] [CrossRef] [PubMed]
- Simões, L.C.; Simões, M. Biofilms in drinking water: Problems and solutions. RSC Adv. 2013, 3, 2520–2533. [Google Scholar] [CrossRef]
- Martinez, E.R. Assessing Biofilm Development in Drinking Water Distribution Systems by Machine Learning Methods; Universitat Politècnica de València: Valencia, Spain, 2016. [Google Scholar]
- Aggarwal, S.; Gomez-Smith, C.K. Overview of Drinking Water Distribution System Microbiome and Water Quality. In Encyclopedia of Water: Science, Technology, and Society; Wiley & Sons: New York, NY, USA, 2019; pp. 1–17. [Google Scholar]
- Hull, N.M.; Ling, F.; Pinto, A.J.; Albertsen, M.; Jang, H.G.; Hong, P.Y.; Konstantinidis, K.T.; LeChevallier, M.; Colwell, R.R.; Liu, W.T. Drinking Water Microbiome Project: Is it Time? Trends Microbiol. 2019, 27, 670–677. [Google Scholar] [CrossRef]
- Gomes, I.; Madureira, D.; Simões, L.C.; Simões, M. The effects of pharmaceutical and personal care products on the behavior of Burkholderia cepacia isolated from drinking water. Int. Biodeterior. Biodegrad. 2019, 141, 87–93. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, L.C.; Simões, M. The effects of emerging environmental contaminants on Stenotrophomonas maltophilia isolated from drinking water in planktonic and sessile states. Sci. Total Environ. 2018, 643, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Querido, M.M.; Teixeira, J.P.; Pereira, C.C.; Simões, L.C.; Simões, M. Prolonged exposure of Stenotrophomonas maltophilia biofilms to trace levels of clofibric acid alters antimicrobial tolerance and virulence. Chemosphere 2019, 235, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Liu, L.; Zhou, L.; Zhao, Z. Impacts of antibiotics on biofilm bacterial community and disinfection performance on simulated drinking water supply pipe wall. Environ. Pollut. 2021, 288, 117736. [Google Scholar] [CrossRef]
- Arruda, V.; Simões, M.; Gomes, I.B. The impact of synthetic musk compounds in biofilms from drinking water bacteria. J. Hazard. Mater. 2022, 436, 129185. [Google Scholar] [CrossRef]
- Tsvetanova, Z. Quantification of the bacterial community of drinking water-associated biofilms under different flow velocities and changing chlorination regimes. Appl. Water Sci. 2020, 10, 3. [Google Scholar] [CrossRef]
- Khan, S.; Beattie, T.K.; Knapp, C.W. Relationship between antibiotic-and disinfectant-resistance profiles in bacteria harvested from tap water. Chemosphere 2016, 152, 132–141. [Google Scholar] [CrossRef]
- Bertelli, C.; Courtois, S.; Rosikiewicz, M.; Piriou, P.; Aeby, S.; Robert, S.; Loret, J.-F.; Greub, G. Reduced chlorine in drinking water distribution systems impacts bacterial biodiversity in biofilms. Front. Microbiol. 2018, 9, 2520. [Google Scholar] [CrossRef] [PubMed]
- Brennan, G.; Collins, S. Growth responses of a green alga to multiple environmental drivers. Nat. Clim. Chang. 2015, 5, 892–897. [Google Scholar] [CrossRef]
- Malik, A.; Batool, S.; Farooqi, A. Chapter 7—Advances in biodegradation and bioremediation of emerging contaminants in the environment. In Biological Approaches to Controlling Pollutants; Kumar, S., Hashmi, M.Z., Eds.; Woodhead Publishing: Thorston, UK, 2022; pp. 121–138. [Google Scholar]
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef]
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef] [PubMed]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems—A review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef] [PubMed]
- Alderton, I.; Palmer, B.R.; Heinemann, J.A.; Pattis, I.; Weaver, L.; Gutiérrez-Ginés, M.J.; Horswell, J.; Tremblay, L.A. The role of emerging organic contaminants in the development of antimicrobial resistance. Emerg. Contam. 2021, 7, 160–171. [Google Scholar] [CrossRef]
- Suzuki, S.; Pruden, A.; Virta, M.; Zhang, T. Editorial: Antibiotic Resistance in Aquatic Systems. Front. Microbiol. 2017, 8, 14. [Google Scholar] [CrossRef]
- Jang, J.; Park, J.; Hwang, C.Y.; Choi, J.; Shin, J.; Kim, Y.M.; Cho, K.H.; Kim, J.H.; Lee, Y.M.; Lee, B.Y. Abundance and diversity of antibiotic resistance genes and bacterial communities in the western Pacific and Southern Oceans. Sci. Total Environ. 2022, 822, 153360. [Google Scholar] [CrossRef] [PubMed]
- Sanganyado, E.; Gwenzi, W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks. Sci. Total Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B. Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infect. Ecol. Epidemiol. 2015, 5, 28564. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Sun, J.; Fang, M.; Luo, S.; Tian, Y.; Dong, P.; Xu, B.; Zheng, C. Occurrence of antibiotics in the main rivers of Shenzhen, China: Association with antibiotic resistance genes and microbial community. Sci. Total Environ. 2019, 653, 334–341. [Google Scholar] [CrossRef]
- Bergeron, S.; Boopathy, R.; Nathaniel, R.; Corbin, A.; LaFleur, G. Presence of antibiotic resistant bacteria and antibiotic resistance genes in raw source water and treated drinking water. Int. Biodeterior. Biodegrad. 2015, 102, 370–374. [Google Scholar] [CrossRef]
- Singh, A.K.; Kaur, R.; Verma, S.; Singh, S. Antimicrobials and Antibiotic Resistance Genes in Water Bodies: Pollution, Risk, and Control. Front. Environ. Sci. 2022, 10, 830861. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Zhang, J.; Qi, W.; Li, Y.; Chen, S.; Zhou, W. Prevalence of antibiotic resistance genes in drinking water and biofilms: The correlation with the microbial community and opportunistic pathogens. Chemosphere 2020, 259, 127483. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.B.; Li, H.B.; Chen, Z.S.; Shi, D.Y.; Chen, T.J.; Yang, D.; Yin, J.; Zhou, S.Q.; Cheng, C.Y.; Shao, Y.F.; et al. Spatial behavior and source tracking of extracellular antibiotic resistance genes in a chlorinated drinking water distribution system. J. Hazard. Mater. 2022, 425, 127942. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, A.Z.; He, M.; Li, D.; Chen, J. Subinhibitory Concentrations of Disinfectants Promote the Horizontal Transfer of Multidrug Resistance Genes within and across Genera. Env. Sci. Technol. 2017, 51, 570–580. [Google Scholar] [CrossRef]
- Ching, C.; Orubu, E.S.; Wirtz, V.J.; Zaman, M.H. Bacterial antibiotic resistance development and mutagenesis following exposure to subinhibitory concentrations of fluoroquinolones in vitro: A systematic review of the literature. JAC-Antimicrob. Resist. 2020, 2, e030747. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front. Microbiol 2015, 6, 1216. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Mao, L.; Li, J.; Yuan, Z.; Bond, P.L.; Guo, J. Antiepileptic drug carbamazepine promotes horizontal transfer of plasmid-borne multi-antibiotic resistance genes within and across bacterial genera. ISME J. 2019, 13, 509–522. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Engelstädter, J.; Zhang, S.; Ding, P.; Mao, L.; Yuan, Z.; Bond, P.L.; Guo, J. Non-antibiotic pharmaceuticals enhance the transmission of exogenous antibiotic resistance genes through bacterial transformation. ISME J. 2020, 14, 2179–2196. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Chen, J.; Lu, H.; Liu, G.; Zhou, J.; Guan, X. PAHs accelerate the propagation of antibiotic resistance genes in coastal water microbial community. Environ. Pollut. 2017, 231, 1145–1152. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, A.Z.; Cen, T.; Li, X.; He, M.; Li, D.; Chen, J. Sub-inhibitory concentrations of heavy metals facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes in water environment. Environ. Pollut. 2018, 237, 74–82. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Li, J.; Mao, L.; Nguyen, S.H.; Duarte, T.; Coin, L.; Bond, P.; Yuan, Z.; Guo, J. Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera. Environ. Int. 2018, 121, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Lu, J.; Chen, Z.; Nguyen, S.H.; Mao, L.; Li, J.; Yuan, Z.; Guo, J. Antidepressant fluoxetine induces multiple antibiotics resistance in Escherichia coli via ROS-mediated mutagenesis. Environ. Int. 2018, 120, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Bhaskarla, C.; Sadhir, I.; Sreedharan, S.; Nandi, D. Non-steroidal anti-inflammatory drugs, acetaminophen and ibuprofen, induce phenotypic antibiotic resistance in Escherichia coli: Roles of marA and acrB. FEMS Microbiol. Lett. 2018, 365, fny251. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Update on Progress and Implementation: European Union Strategic Approach to Pharmaceuticals in the Environment; European Union: Luxembourg, 2020. [Google Scholar]
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses; OECD Studies on Water: Paris, France, 2019. [Google Scholar]
- Rodríguez-Serin, H.; Gamez-Jara, A.; De La Cruz-Noriega, M.; Rojas-Flores, S.; Rodriguez-Yupanqui, M.; Gallozzo Cardenas, M.; Cruz-Monzon, J. Literature Review: Evaluation of Drug Removal Techniques in Municipal and Hospital Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 13105. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, Z.; Liu, H.; Dong, S.; Nghiem, L.; Gao, L.; Chaves, A.V.; Zamyadi, A.; Li, X.; Wang, Q. A review on microalgae-mediated biotechnology for removing pharmaceutical contaminants in aqueous environments: Occurrence, fate, and removal mechanism. J. Hazard. Mater. 2023, 443, 130213. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Sun, X.; Ren, H.; Huang, H. Biological filtration for wastewater treatment in the 21st century: A data-driven analysis of hotspots, challenges and prospects. Sci. Total Environ. 2023, 855, 158951. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.C.; Zheng, Y.; Dzakpasu, M. Removal of pharmaceutical active compounds in wastewater by constructed wetlands: Performance and mechanisms. J. Environ. Manag. 2022, 325, 116478. [Google Scholar] [CrossRef] [PubMed]
- Abd Rahman, N.; Choong, C.E.; Pichiah, S.; Nah, I.W.; Kim, J.R.; Oh, S.E.; Yoon, Y.; Choi, E.H.; Jang, M. Recent advances in the TiO2 based photoreactors for removing contaminants of emerging concern in water. Sep. Purif. Technol. 2023, 304, 122294. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewaters by membrane technology: Limitations, successes, and future improvements. Sci. Total Env. 2022, 838, 156010. [Google Scholar] [CrossRef]
- İlyasoglu, G.; Kose-Mutlu, B.; Mutlu-Salmanli, O.; Koyuncu, I. Removal of organic micropollutans by adsorptive membrane. Chemosphere 2022, 302, 134775. [Google Scholar] [CrossRef] [PubMed]
- Marson, E.O.; Paniagua, C.E.; Júnior, O.G.; Gonçalves, B.R.; Silva, V.M.; Ricardo, I.A.; Starling, M.C.V.M.; Amorim, C.C.; Trovó, A.G. A review toward contaminants of emerging concern in Brazil: Occurrence, impact and their degradation by advanced oxidation process in aquatic matrices. Sci. Total Environ. 2022, 836, 155605. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.; Smalling, K.L.; Ahrens, L.; Gros, M.; Miglioranza, K.S.; Picó, Y.; Schoenfuss, H.L. Critical review: Grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Env. Toxicol. Chem. 2019, 38, 46–60. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881. [Google Scholar] [CrossRef]
- Gomes, I.; Simões, M.; Simões, L.C. An overview on the reactors to study drinking water biofilms. Water Res. 2014, 62, 63–87. [Google Scholar] [CrossRef]
- Kitajima, M.; Cruz, M.C.; Williams, R.B.; Wuertz, S.; Whittle, A.J. Microbial abundance and community composition in biofilms on in-pipe sensors in a drinking water distribution system. Sci. Total Environ. 2021, 766, 142314. [Google Scholar] [CrossRef]
- Douterelo, I.; Jackson, M.; Solomon, C.; Boxall, J. Microbial analysis of in situ biofilm formation in drinking water distribution systems: Implications for monitoring and control of drinking water quality. Appl. Microbiol. Biotechnol. 2016, 100, 3301–3311. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, I.; Simões, M.; Gomes, I.B. An Overview of the Impact of Pharmaceuticals on Aquatic Microbial Communities. Antibiotics 2022, 11, 1700. https://doi.org/10.3390/antibiotics11121700
Pinto I, Simões M, Gomes IB. An Overview of the Impact of Pharmaceuticals on Aquatic Microbial Communities. Antibiotics. 2022; 11(12):1700. https://doi.org/10.3390/antibiotics11121700
Chicago/Turabian StylePinto, Isabel, Manuel Simões, and Inês B. Gomes. 2022. "An Overview of the Impact of Pharmaceuticals on Aquatic Microbial Communities" Antibiotics 11, no. 12: 1700. https://doi.org/10.3390/antibiotics11121700
APA StylePinto, I., Simões, M., & Gomes, I. B. (2022). An Overview of the Impact of Pharmaceuticals on Aquatic Microbial Communities. Antibiotics, 11(12), 1700. https://doi.org/10.3390/antibiotics11121700