Pre-Emptive Antimicrobial Locks Decrease Long-Term Catheter-Related Bloodstream Infections in Hemodialysis Patients
Abstract
1. Introduction
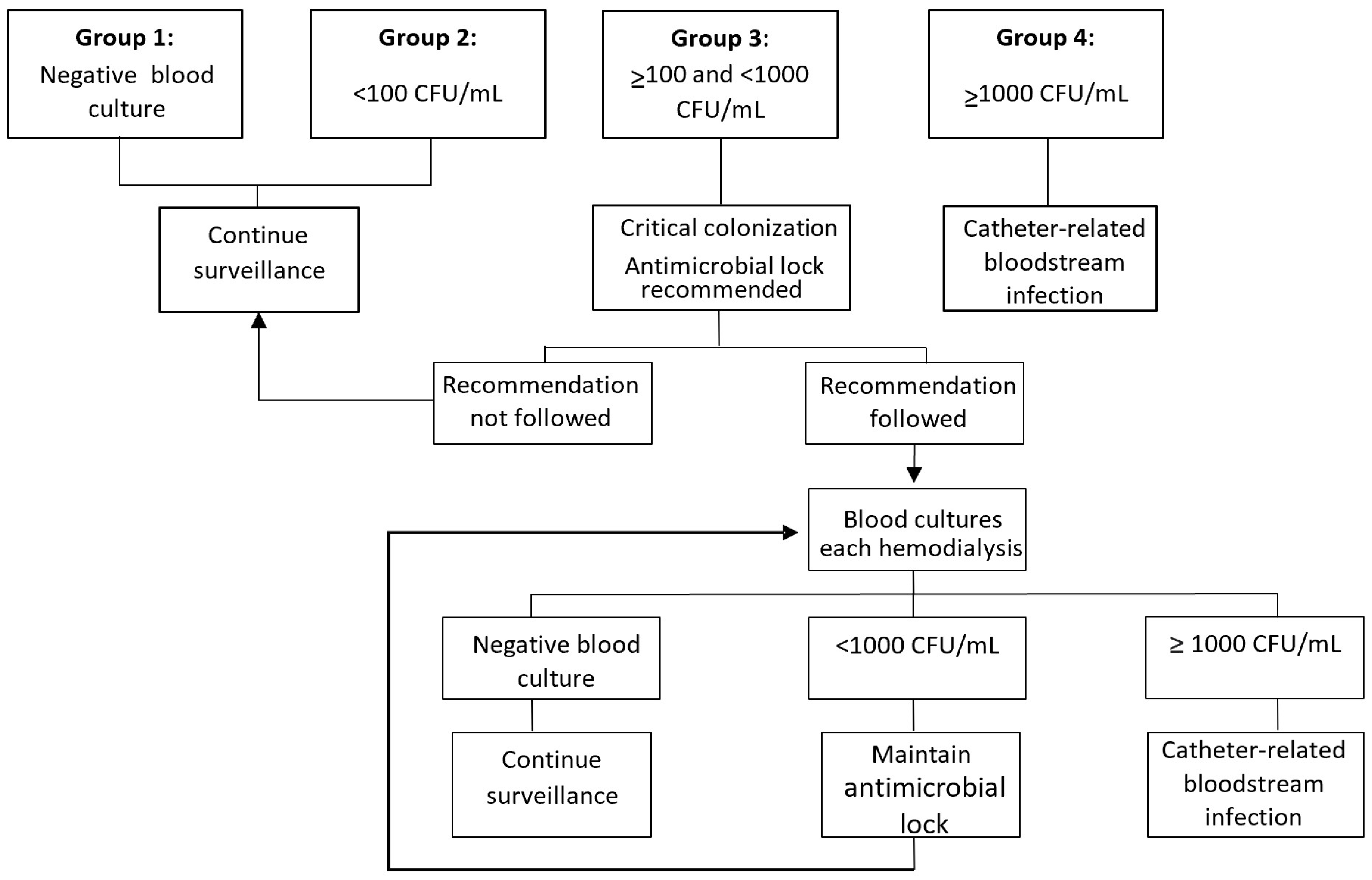
2. Materials and Methods
3. Results
3.1. Demographic
3.2. Isolates
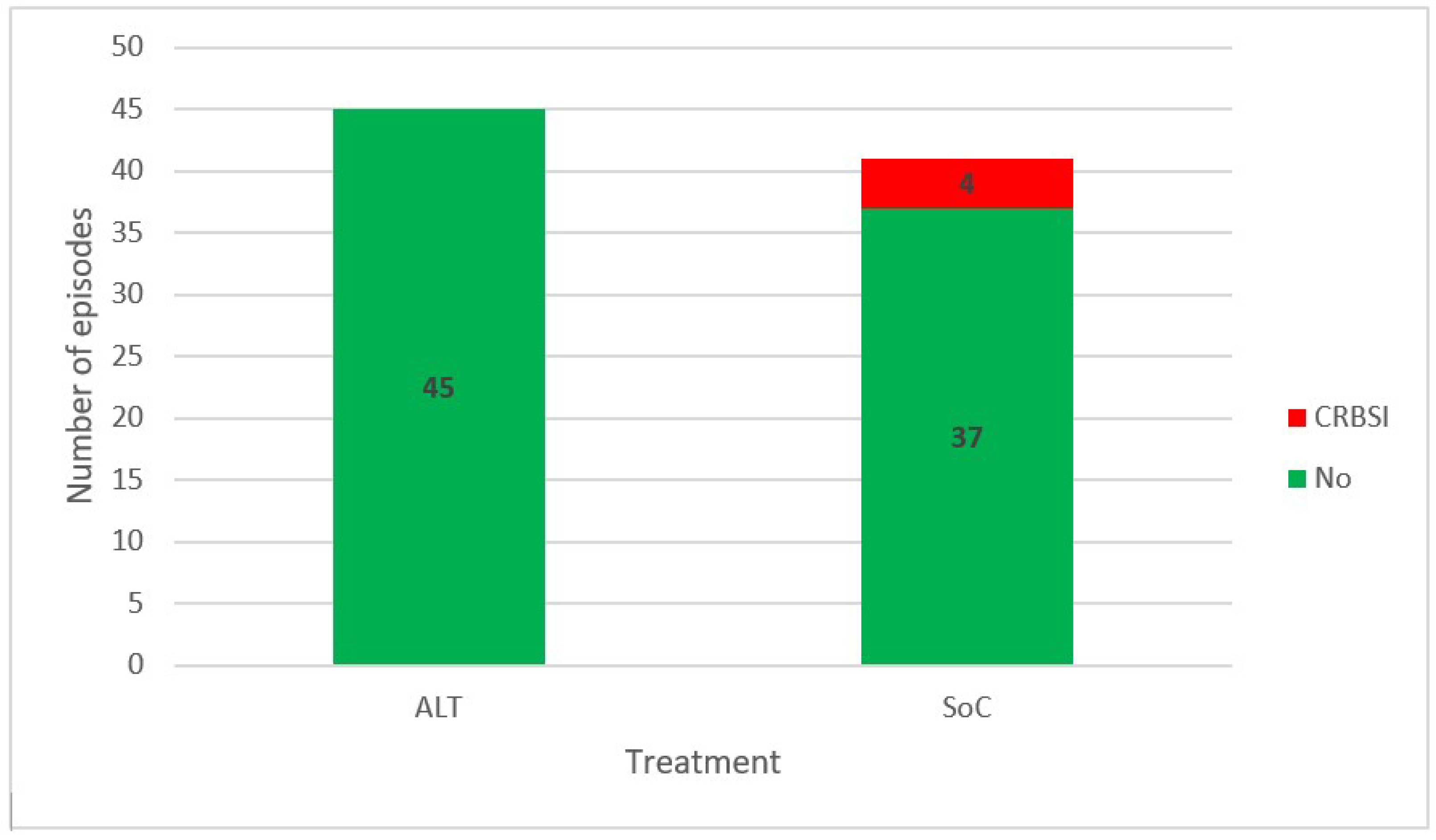
3.3. Progression to Bacteremia
3.4. Eradication Ability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Kurella Tamura, M.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2021, 77 (Suppl. 1), A7–A8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Organització Catalana de Trasplantaments (OCATT). Registre de Malalts Renals de Catalunya, Informe Estadístic 2018; Departament de Salut, Generalitat de Catalunya: Barcelona, Spain, 2018.
- Kumbar, L.; Yee, J. Current Concepts in Hemodialysis Vascular Access Infections. Adv. Chronic Kidney Dis. 2019, 26, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Arechabala, M.C.; Catoni, M.I.; Claro, J.C.; Rojas, N.P.; Rubio, M.E.; Calvo, M.A.; Letelier, L.M. Antimicrobial Lock Solutions for Preventing Catheter-Related Infections in Haemodialysis. Cochrane Database Syst. Rev. 2018, 2018, CD010597. [Google Scholar] [CrossRef] [PubMed]
- Soi, V.; Kumbar, L.; Yee, J.; Moore, C. Prevention of Catheter-Related Bloodstream Infections in Patients on Hemodialysis: Challenges and Management Strategies. Int. J. Nephrol. Renovasc. Dis. 2016, 9, 95. [Google Scholar] [CrossRef]
- Ibeas, J.; Roca-Tey, R.; Vallespín, J.; Moreno, T.; Moñux, G.; Martí-Monrós, A.; del Pozo, J.L.; Gruss, E.; Ramírez de Arellano, M.; Fontseré, N.; et al. Guía Clínica Española Del Acceso Vascular Para Hemodiálisis. Nefrología 2017, 37, 1–191. [Google Scholar] [CrossRef] [PubMed]
- Hebeisen, U.P.; Atkinson, A.; Marschall, J.; Buetti, N. Catheter-Related Bloodstream Infections with Coagulase-Negative Staphylococci: Are Antibiotics Necessary If the Catheter Is Removed? Antimicrob. Resist. Infect. Control 2019, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Chaves, F.; Garnacho-Montero, J.; del Pozo, J.L.; Bouza, E.; Capdevila, J.A.; de Cueto, M.; Domínguez, M.Á.; Esteban, J.; Fernández-Hidalgo, N.; Fernández Sampedro, M.; et al. Diagnosis and Treatment of Catheter-Related Bloodstream Infection: Clinical Guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology and (SEIMC) and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine A. Med. Intensiva 2018, 42, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID* Guideline for the Diagnosis and Treatment of Biofilm Infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Marschall, J.; Mermel, L.A.; Fakih, M.; Hadaway, L.; Kallen, A.; O’Grady, N.P.; Pettis, A.M.; Rupp, M.E.; Sandora, T.; Maragakis, L.L.; et al. Strategies to Prevent Central Line–Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infect. Control Hosp. Epidemiol. 2014, 35, 753–771. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Aguinaga, A.; Garcia-Fernandez, N.; Hernaez, S.; Serrera, A.; Alonso, M.; Ramos, A.; Guillen-Grima, F.; Leiva, J. Intra-Catheter Leukocyte Culture to Monitor Hemodialysis Catheter Colonization. a Prospective Study to Prevent Catheter-Related Bloodstream Infections. Int. J. Artif. Organs 2008, 31, 820–826. [Google Scholar] [CrossRef]
- Mermel, L.A. What Is the Evidence for Intraluminal Colonization of Hemodialysis Catheters? Kidney Int. 2014, 86, 28–33. [Google Scholar] [CrossRef]
- Sheng, K.X.; Zhang, P.; Li, J.W.; Cheng, J.; He, Y.C.; Böhlke, M.; Chen, J.H. Comparative Efficacy and Safety of Lock Solutions for the Prevention of Catheter-Related Complications Including Infectious and Bleeding Events in Adult Haemodialysis Patients: A Systematic Review and Network Meta-Analysis. Clin. Microbiol. Infect. 2020, 26, 545–552. [Google Scholar] [CrossRef]
- Lutwick, L.; Al-Maani, A.S.; Mehtar, S.; Memish, Z.; Rosenthal, V.D.; Dramowski, A.; Lui, G.; Osman, T.; Bulabula, A.; Bearman, G. Managing and Preventing Vascular Catheter Infections: A Position Paper of the International Society for Infectious Diseases. Int. J. Infect. Dis. 2019, 84, 22–29. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections (2011). Am. J. Infect. Control 2017, 39, S1–S34. [Google Scholar] [CrossRef]
- Padilla-Orozco, M.; Mendoza-Flores, L.; Herrera-Alonso, A.; Garza González, E.; Gutiérrez Ferman, J.L.; Rodríguez-López, J.M.; Bocanegra-Ibarias, P.; Camacho-Ortiz, A. Generalized and Prolonged Use of Gentamicin-Lock Therapy Reduces Hemodialysis Catheter-Related Infections Due to Gram Negatives. Nephron 2019, 143, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Norris, L.B.; Kablaoui, F.; Brilhart, M.K.; Bookstaver, P.B. Systematic Review of Antimicrobial Lock Therapy for Prevention of Central-Line-Associated Bloodstream Infections in Adult and Pediatric Cancer Patients. Int. J. Antimicrob. Agents 2017, 50, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Mataraci Kara, E.; Ozbek Celik, B. Investigation of the Effects of Various Antibiotics against Klebsiella Pneumoniae Biofilms on in Vitro Catheter Model. J. Chemother. 2018, 30, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, B.; Li, R.; Ge, L.; Chen, K.H.; Tian, J. Does Antimicrobial Lock Solution Reduce Catheter-Related Infections in Hemodialysis Patients with Central Venous Catheters? A Bayesian Network Meta-Analysis. Int. Urol. Nephrol. 2017, 49, 701–716. [Google Scholar] [CrossRef]
- Safdar, N.; Fine, J.P.; Maki, D.G. Meta-Analysis: Methods for Diagnosing Intravascular Device–Related Bloodstream Infection. Ann. Intern. Med. 2005, 142, 451. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease and Prevention. Control National Healthcare Safety Network (NHSN) Overview Patient Safety Component Manual; Center for Disease and Prevention: Atlanta, GA, USA, 2020; p. 305. [Google Scholar]
- Del Pozo, J.L.; Rodil, R.; Aguinaga, A.; Yuste, J.R.; Bustos, C.; Montero, A.; Espinosa, G.; García-Fernández, N. Daptomycin Lock Therapy for Grampositive Long-Term Catheter-Related Bloodstream Infections. Int. J. Clin. Pract. 2012, 66, 305–308. [Google Scholar] [CrossRef]
- Van de Wetering, M.D.; van Woensel, J.B. Prophylactic Antibiotics for Preventing Early Central Venous Catheter Gram Positive Infections in Oncology Patients. In Cochrane Database of Systematic Reviews; van de Wetering, M.D., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2007. [Google Scholar]
- Abdul Salim, S.; Masoud, A.T.; Thongprayoon, C.; Cheungpasitporn, W.; Soliman, K.M.; Garla, V.; Sofy, A.A.; Ahmed, A.S.; Abdelsattar, A.T.; Zsom, L.; et al. Systematic Review and Meta-Analysis of Antibiotic and Antimicrobial Lock Solutions for Prevention of Hemodialysis Catheter-Related Infections. ASAIO J. 2021, 67, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Golestaneh, L.; Allon, M.; Abreo, K.; Mokrzycki, M.H. Prevention of Bloodstream Infections in Patients Undergoing Hemodialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 132–151. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L. Novel Treatment Dynamics for Biofilm-Related Infections. Expert Rev. Anti. Infect. Ther. 2021, 19, 1443–1456. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.P.; Li, H.J.; Wang, R.J.; Wu, Q.; Chen, H.; Ren, J.J.; Tian, J.H. Comparative Efficacy of Various Antimicrobial Lock Solutions for Preventing Catheter-Related Bloodstream Infections: A Network Meta-Analysis of 9099 Patients from 52 Randomized Controlled Trials. Int. J. Infect. Dis. 2019, 87, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.P.; do Nascimento, M.M.; Chula, D.C.; Riella, M.C. Minocycline-EDTA Lock Solution Prevents Catheter-Related Bacteremia in Hemodialysis. J. Am. Soc. Nephrol. 2011, 22, 1939–1945. [Google Scholar] [CrossRef]
- Luther, M.K.; Mermel, L.A.; LaPlante, K.L. Comparison of Linezolid and Vancomycin Lock Solutions with and without Heparin against Biofilm-Producing Bacteria. Am. J. Health Pharm. 2017, 74, e193–e201. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, P.; Wang, Y.; Hao, Y. Mechanisms and Control Measures of Mature Biofilm Resistance to Antimicrobial Agents in the Clinical Context. ACS Omega 2020, 5, 22684–22690. [Google Scholar] [CrossRef]
- Mandolfo, S.; Acconcia, P.; Bucci, R.; Corradi, B.; Farina, M.; Rizzo, M.A.; Stucchi, A. Hemodialysis Tunneled Central Venous Catheters: Five-Year Outcome Analysis. J. Vasc. Access 2014, 15, 461–465. [Google Scholar] [CrossRef]
- Rijnders, B.; DiSciullo, G.J.; Csiky, B.; Rutkowski, B.; Appelt, K.; Cheronis, J.; Aitchison, R.; Gordon, G.; Jadoul, M.; Fluck, R. Locking Hemodialysis Catheters With Trimethoprim-Ethanol-Ca-EDTA to Prevent Bloodstream Infections: A Randomized, Evaluator-Blinded Clinical Trial. Clin. Infect. Dis. 2019, 69, 130–136. [Google Scholar] [CrossRef]
| Antimicrobial | Concentration (mg/mL) | Sodium Heparin Concentration (UI) |
|---|---|---|
| Teicoplanin | 10 | 500 |
| Daptomycin | 5 | 500 |
| Vancomycin | 10 | 100 |
| Standard of care | - | 5000 |
| Variable | All Patients (n = 149) | At Least One CRBSI Patients (n = 22) | CCC Episode Patients (n = 13) | Never CCC or CRBSI Patients (n = 114) | p |
|---|---|---|---|---|---|
| Age in years, median (IQR) | 68 (54–74.5) | 69.5 (60.5–73.5) | 72 (50–79.5) | 67 (52.75–74) | 0.437 |
| Gender (male), n. (%) | 85 (57) | 8 (36.4) | 5 (38.5) | 72 (63.2) | 0.025 |
| Charlson score, median (IQR) | 6 (5–8) | 6.5 (5–8) | 7 (5–8) | 6 (5–8) | 0.892 |
| Time from TCVC insertion to beginning the study in months, median (IQR) | 1.02 (0.5–1.88) | 1.64 (1.26–16.04) | 1.36 (0.78–3.19) | 0.89 (0.46–1.52) | <0.001 |
| Time from TCVC insertion to first CRBSI/CCC in months, median (IQR) | - | 12.19 (5.22–27.68) | 13.52 (4.44–62.83) | - | 0.375 |
| Lifespan of TCVC from insertion to removal in months, median (IQR) | 7.72 (3.2–18.78) | 31.02 (12.36–60.58) | 9.89 (4.99–87.2) | 5.98 (2.61–14.49) | <0.001 |
| Follow-up in months, median (IQR) | 8.80 (2.82–26.72) | 50.41 (12.82–82.79) | 38.28 (26.17–99) | 4.94 (1.93–16.59) | <0.001 |
| Deaths during follow-up, n. (%) | 71 (47.7) | 18 (81.8) | 7 (53.8) | 46 (40.4) | 0.001 |
| Time from CRBSI/CCC to death in months, median (IQR) | - | 29.31 (11.06–61.93) | 12.62 (2.76–42.12) | - | 0.216 |
| Ratio TCVC-patients | 1.47 | 2.59 | 2 | 1.19 | 0.368 |
| Cause of ESRD, n. (%) | 0.533 | ||||
| -Glomerulonephritis | 32 (21.47) | 5 (22.72) | 2 (15.4) | 25 (21.9) | |
| -Diabetic kidney disease | 20 (13.42) | 3 (13.64) | 4 (30.7) | 13 (11.4) | |
| -Nephroangiosclerosis | 19 (12.75) | 2 (9.1) | 1 (7.7) | 16 (14) | |
| -Polycystic nephropathy | 15 (10.07) | 5 (22.72) | 3 (23.1) | 7 (6.1) | |
| -Others or unknown | 63 (42.28) | 7 (31.82) | 3 (23.1) | 53 (46.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Di Matteo, A.; Garcia-Fernandez, N.; Aguinaga Pérez, A.; Carmona-Torre, F.; Oteiza, A.C.; Leiva, J.; Del Pozo, J.L. Pre-Emptive Antimicrobial Locks Decrease Long-Term Catheter-Related Bloodstream Infections in Hemodialysis Patients. Antibiotics 2022, 11, 1692. https://doi.org/10.3390/antibiotics11121692
Blanco-Di Matteo A, Garcia-Fernandez N, Aguinaga Pérez A, Carmona-Torre F, Oteiza AC, Leiva J, Del Pozo JL. Pre-Emptive Antimicrobial Locks Decrease Long-Term Catheter-Related Bloodstream Infections in Hemodialysis Patients. Antibiotics. 2022; 11(12):1692. https://doi.org/10.3390/antibiotics11121692
Chicago/Turabian StyleBlanco-Di Matteo, Andres, Nuria Garcia-Fernandez, Aitziber Aguinaga Pérez, Francisco Carmona-Torre, Amaya C. Oteiza, Jose Leiva, and Jose Luis Del Pozo. 2022. "Pre-Emptive Antimicrobial Locks Decrease Long-Term Catheter-Related Bloodstream Infections in Hemodialysis Patients" Antibiotics 11, no. 12: 1692. https://doi.org/10.3390/antibiotics11121692
APA StyleBlanco-Di Matteo, A., Garcia-Fernandez, N., Aguinaga Pérez, A., Carmona-Torre, F., Oteiza, A. C., Leiva, J., & Del Pozo, J. L. (2022). Pre-Emptive Antimicrobial Locks Decrease Long-Term Catheter-Related Bloodstream Infections in Hemodialysis Patients. Antibiotics, 11(12), 1692. https://doi.org/10.3390/antibiotics11121692







