Antimicrobial Resistance Challenged with Platinum(II) and Palladium(II) Complexes Containing 1,10-Phenanthroline and 5-Amino-1,3,4-Thiadiazole-2(3H)-Thione in Campylobacter jejuni
Abstract
:Highlights
- Two new complexes containing mixed ligands were prepared and characterized;
- NMR data indicated that two thiolate ligands coordinate a metal ion via the sulphur atom after deprotonation.
- The platinum complex was active against three strains of Campylobacter jejuni;
- A synergistic effect was observed when the Pt (II) complex was combined with ciprofloxacin.
Abstract
1. Introduction
2. Results and Discussion
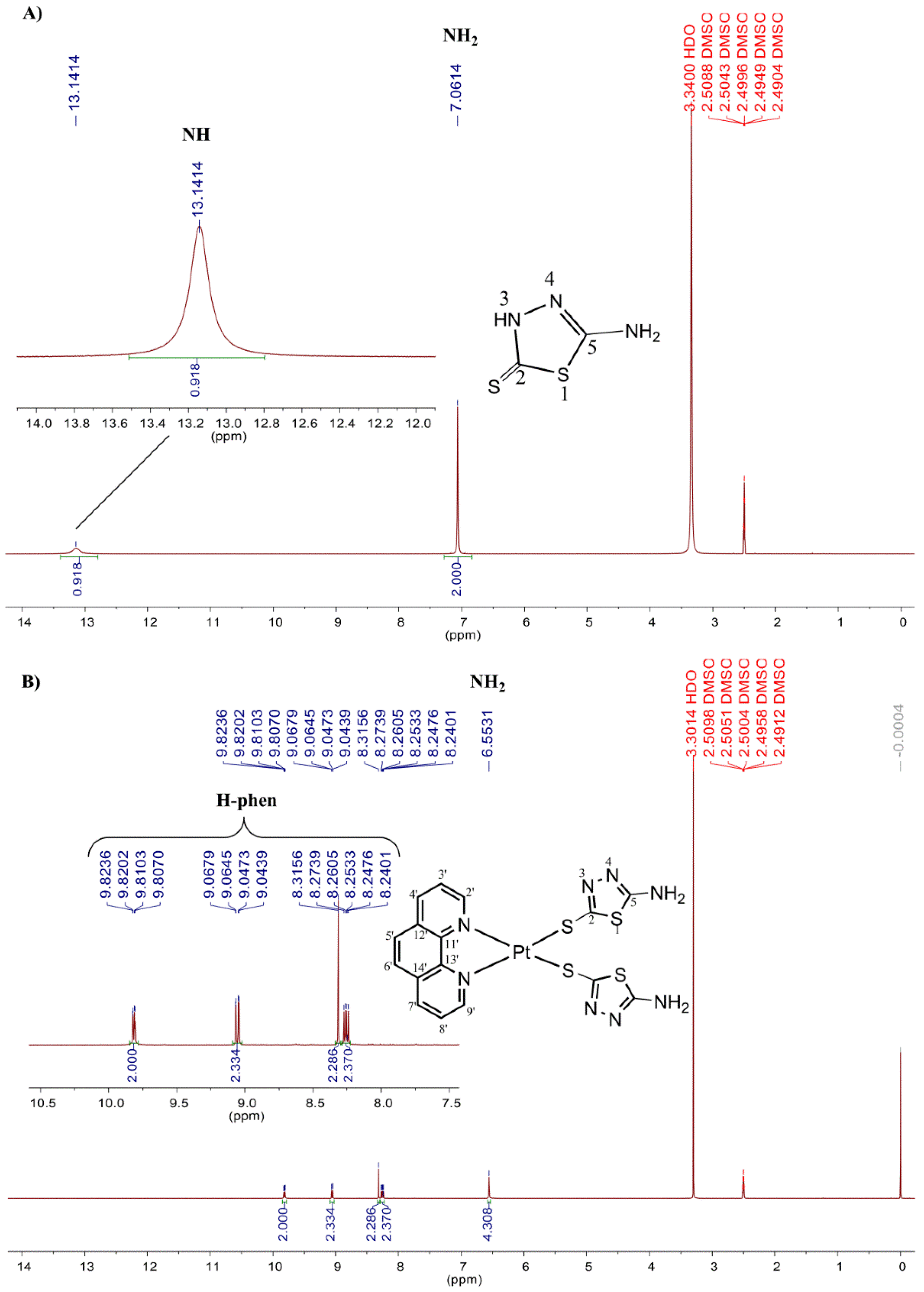
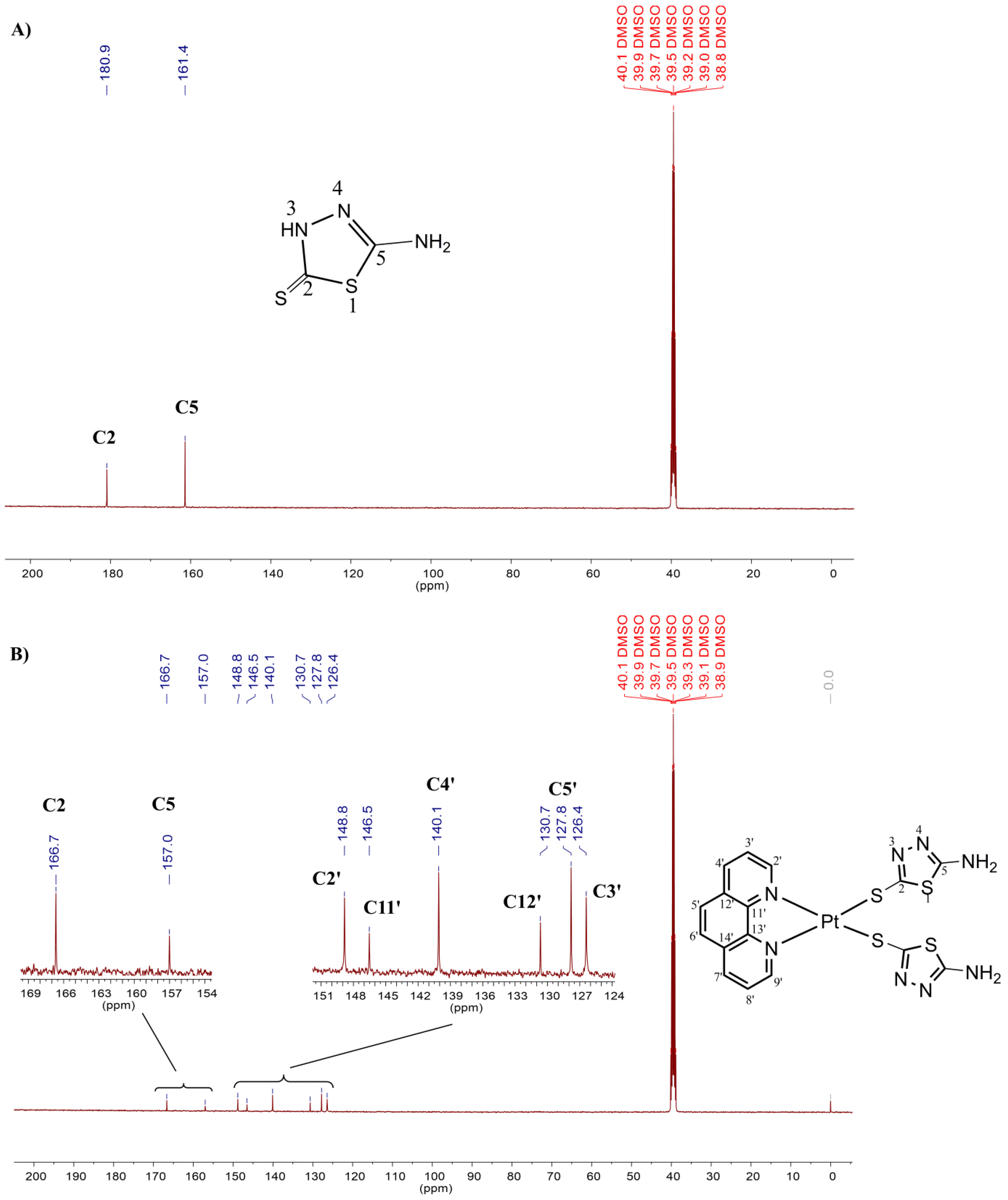
2.1. Chemistry
2.2. Epidemiological and Antimicrobial Resistance Study of the Bank of 235 Strains of C. jejuni
2.3. Effect of Metal Complexes
3. Materials and Methods
3.1. Metal Complexes
3.2. Preparation of Complexes
3.2.1. Complex I-[Pt(L1)2(phen)]
3.2.2. Complex II-[Pd(L1)2(phen)]
3.3. Strains
3.4. Preparations of Strains, Antimicrobial and Metal Complexes
3.5. Minimum Inhibitory Concentration (MIC) and Bactericidal Concentration (MBC) Test
3.6. Isolated and Synergistic Effect of Metal Compounds
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Xu, C.; Kong, L.; Gao, H.; Cheng, X.; Wang, X. A Review of current bacterial resistance to antibiotics in food animals. Front. Microbiol. 2022, 13, 1458. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Sproston, E.L.; Wimalarathna, H.M.L.; Sheppard, S.K. Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom. 2018, 4, e000198. [Google Scholar] [CrossRef]
- Woźniak-Biel, A.; Bugla-Płoskońska, G.; Kielsznia, A.; Korzekwa, K.; Tobiasz, A.; Korzeniowska-Kowal, A.; Wieliczko, A. High prevalence of resistance to fluoroquinolones and tetracycline Campylobacter spp. isolated from poultry in Poland. Microb. Drug Resist. 2018, 24, 314–322. [Google Scholar] [CrossRef]
- Vianez Peregrino, I.; Ferreira Ventura, R.; Borghi, M.; Pinto Schuenck, R.; Devereux, M.; McCann, M.; Souza dos Santos, A.L.; FerreiraNunes, A.P. Antibacterial activity and carbapenem re-sensitizing ability of 1,10-phenanthroline-5,6-dione and its metal complexes against KPC-producing Klebsiella pneumoniae clinical strains. Lett. Appl. Microbiol. 2021, 73, 139–148. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [Green Version]
- Karges, J.; Stokes, R.W.; Cohen, S.M. Metal complexes for therapeutic applications. Trends Chem. 2021, 3, 523–534. [Google Scholar] [CrossRef]
- Monteiro, G.P.; de Melo, R.T.; Guidotti-Takeuchi, M.; Dumont, C.F.; Ribeiro, R.A.C.; Guerra, W.; Ramos, L.M.S.; Paixão, D.A.; dos Santos, F.A.L.; Dos Prazeres Rodrigues, D.; et al. A ternary copper (II) complex with 4-fluorophenoxyacetic acid hydrazide in combination with antibiotics exhibits positive synergistic effect against salmonella typhimurium. Antibiotics 2022, 11, 388. [Google Scholar] [CrossRef]
- Machado, P.H.A.; Paixão, D.A.; Lino, R.C.; de Souza, T.R.; de Souza Bontempo, N.J.; Sousa, L.M.; Van Petten de Vasconcelos Azevedo, F.; Orsolin, P.C.; Lima, P.M.A.P.; Martins, I.C.; et al. A selective CuII complex with 4-fluorophenoxyacetic acid hydrazide and phenanthroline displays DNA-cleaving and pro-apoptotic properties in cancer cells. Sci. Rep. 2021, 11, 24450. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Majeed, A.; Al-Sammarrae, K.; Salih, N.; Salimon, J.; Abdullah, B. Metal complexes of Schiff base: Preparation, characterization and antibacterial activity. Arab. J. Chem. 2017, 10, S1639–S1644. [Google Scholar] [CrossRef] [Green Version]
- da Silva, P.B. Síntese, Caracterização e Investigação das Atividades Biológicas de Complexos de Cobre(II) Contendo Moléculas Bioativas e Ligantes Nitrogenados. Ph.D. Thesis, Universidade Estadual Paulista, São Paulo, Brasil, 2012. [Google Scholar]
- Neto, E.A.B.; Ribeiro, C.; Zucolotto, V. Síntese de nanopartículas de prata para aplicação na sanitização de embalagens. Comun. Técnico Embrapa Instrum. 2008, 99, 1–4. [Google Scholar]
- Salishcheva, O.; Prosekov, A. Antimicrobial activity of mono- and polynuclear platinum and palladium complexes. Foods Raw Mater. 2020, 8, 298–311. [Google Scholar] [CrossRef]
- Riswan Ahamed, M.A.; Azarudeen, R.S.; Kani, N.M. Antimicrobial applications of transition metal complexes of benzothiazole based terpolymer: Synthesis, characterization, and effect on bacterial and fungal strains. Bioinorg. Chem. Appl. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, M.Y.; McBain, A.J.; Butler, J.A.; Banks, C.E.; Whitehead, K.A. Antimicrobial efficacy and synergy of metal ions against Enterococcus faecium, Klebsiella pneumoniae and Acinetobacter baumannii in planktonic and biofilm phenotypes. Sci. Rep. 2017, 7, 5911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanelli, M.; Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M.; Paoli, P. New trends in platinum and palladium complexes as antineoplastic agents. Coord. Chem. Rev. 2016, 310, 41–79. [Google Scholar] [CrossRef]
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef]
- Zou, L.; Wang, J.; Gao, Y.; Ren, X.; Rottenberg, M.E.; Lu, J.; Holmgren, A. Synergistic antibacterial activity of silver with antibiotics correlating with the upregulation of the ROS production. Sci. Rep. 2018, 8, 11131. [Google Scholar] [CrossRef] [Green Version]
- Din, M.I.; Ali, F.; Intisar, A. Metal based drugs and chelating agents as therapeutic agents and their antimicrobial activity. Rev. Roum. Chim. 2019, 64, 5–17. [Google Scholar] [CrossRef]
- European Food Safety Authority. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 286. [Google Scholar] [CrossRef]
- CDC. Campylobacter (Campylobacteriosis)—Information for Health Profissionals; CDC: Atlanta, GA, USA, 2019.
- Brasil Manual Integrado de Prevenção e Controle de Doenças Transmitidas por Alimentos; Ministério da Saúde. Secretaria de Vigilância Sanitária: Brasília, Brazil, 2013; pp. 1–136.
- Oliveira, J.J.; Rezende, C.; Feistel, J.; Moreira, N.; Oliveira, A. Campylobacter spp. e sua ocorrência em abatedouro de aves. Enciclopédia Biosf. 2013, 9, 13. [Google Scholar]
- Ministério da Saúde. Secretaria de Vigilância em Saúde. Manual técnico de diagnóstico laboratorial de Campylobacter: Gênero Campylobacter: Diagnóstico laboratorial clássico e molecular/Ministério da Saúde; Secretaria de Vigilância em Saúde, Fundação Oswaldo Cruz, Laboratório de Referência Nacional de Enteroinfecções Bacterianas, Instituto Adolfo Lutz, Ministério da Saúde: Brasília, Brasil, 2011; p. 40.
- Alves, J.; De Oliveira, T.C.R.M. Presença de Campylobacter spp. em cortes refrigerados de frango. Semin. Cienc. Agrar. 2013, 34, 2829–2836. [Google Scholar] [CrossRef]
- Souza, W.A.; de Almeida, A.M.; Pivatto, M.; de Almeida, M.V.; Guedes, G.P.; Resende, J.A.L.C.; Guerra, W. Crystal structure and spectroscopy properties of new PtII complexes containing 5-alkyl-1,3,4-oxadiazol-2-thione derivatives. J. Mol. Struct. 2021, 1226, 129250. [Google Scholar] [CrossRef]
- Nath, M.; Sulaxna; Song, X.; Eng, G. Synthesis, spectral and thermal studies of some organotin(IV) derivatives of 5-amino-3H-1,3,4-thiadiazole-2-thione. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Keefer, C.E.; Bereman, R.D.; Purrington, S.T.; Knight, B.W.; Boyle, P.D. The 195 Pt NMR of L 2 Pt(1,2-dithiolene) Complexes. Inorg. Chem. 1999, 38, 2294–2302. [Google Scholar] [CrossRef]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef] [PubMed]
- Suman Kumar, M.; Ramees, T.P.; Dhanze, H.; Gupta, S.; Dubal, Z.B.; Kumar, A. Occurrence and antimicrobial resistance of Campylobacter isolates from broiler chicken and slaughter house environment in India. Anim. Biotechnol. 2021, 9, 8692. [Google Scholar] [CrossRef]
- Poudel, S.; Li, T.; Chen, S.; Zhang, X.; Cheng, W.-H.; Sukumaran, A.T.; Kiess, A.S.; Zhang, L. Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter isolated from broilers and broiler meat raised without antibiotics. Microbiol. Spectr. 2022, 10, e00251-22. [Google Scholar] [CrossRef]
- Melo, R.T.; Grazziotin, A.L.; Júnior, E.C.V.; Prado, R.R.; Mendonça, E.P.; Monteiro, G.P.; Peres, P.A.B.M.; Rossi, D.A. Evolution of Campylobacter jejuni of poultry origin in Brazil. Food Microbiol. 2019, 82, 489–496. [Google Scholar] [CrossRef]
- Mapa, B. INSTRUÇÃO NORMATIVA No 14, DE 17 DE MAIO DE 2012; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brasil, 2012.
- Ministério da Agricultura. P. e A.–M. INSTRUÇÃO NORMATIVA No 20, DE 21 DE OUTUBRO DE 2016; Diário Oficial da União; Ministério da Agricultura: Brasília, Brasil, 2016; p. 13.
- Mapa, B. INSTRUÇÃO NORMATIVA No 41, DE 23 DE OUTUBRO DE 2017; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brasil, 2017.
- Whelan, M.V.X.; Ardill, L.; Koide, K.; Nakajima, C.; Suzuki, Y.; Simpson, J.C.; Ó Cróinín, T. Acquisition of fluoroquinolone resistance leads to increased biofilm formation and pathogenicity in Campylobacter jejuni. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Elhadidy, M.; Ali, M.M.; El-Shibiny, A.; Miller, W.G.; Elkhatib, W.F.; Botteldoorn, N.; Dierick, K. Antimicrobial resistance patterns and molecular resistance markers of Campylobacter jejuni isolates from human diarrheal cases. PLoS ONE 2020, 15, e0227833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payot, S.; Bolla, J.-M.; Corcoran, D.; Fanning, S.; Mégraud, F.; Zhang, Q. Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microbes Infect. 2006, 8, 1967–1971. [Google Scholar] [CrossRef]
- Salles, T.S.; de Almeida Figueira, A.; Costa, G.A.; Costa da Cunha, N.; Rossi, D.A.; Torres de Melo, R.; Léo de Almeida Pereira, V.; Cosendey de Aquino, M.H. SVR-flaA alleles and molecular characterization of resistance to ciprofloxacin and erythromycin in Campylobacter jejuni isolated from broiler carcasses in southern Brazil. Microbiology 2022, in press. [Google Scholar]
- Nascimento, R.J.; Frasão, B.S.; Dias, T.S.; Nascimento, E.R.; Tavares, L.S.B.; Almeida, V.L.; Aquino, M.H.C. Detection of efflux pump CmeABC in enrofloxacin resistant Campylobacter spp. strains isolated from broiler chickens (Gallus gallus domesticus) in the state of Rio de Janeiro, Brazil. Pesqui. Veterinária Bras. 2019, 39, 728–733. [Google Scholar] [CrossRef]
- de Moura, H.M. Isolamento e Análise de Resistência a Antimicrobianos de Cepas de Campylobacter jejuni em Amostras de Carne de Aves Resfriadas Comercializadas no Distrito Federal. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 2010. [Google Scholar]
- de Oliveira, J.J. Isolamento e Caracterização de Campylobacter spp. em Carcaças de frango e ovos Comerciais. Master’s Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2014. [Google Scholar]
- Djennad, A.; Lo Iacono, G.; Sarran, C.; Lane, C.; Elson, R.; Höser, C.; Lake, I.R.; Colón-González, F.J.; Kovats, S.; Semenza, J.C.; et al. Seasonality and the effects of weather on Campylobacter infections. BMC Infect. Dis. 2019, 19, 255. [Google Scholar] [CrossRef]
- Friedrich, A.; Marshall, J.C.; Biggs, P.J.; Midwinter, A.C.; French, N.P. Seasonality of Campylobacter jejuni isolates associated with human campylobacteriosis in the Manawatu region, New Zealand. Epidemiol. Infect. 2016, 144, 820–828. [Google Scholar] [CrossRef]
- Baali, M.; Lounis, M.; Al Amir, H.L.; Ayachi, A.; Hakem, A.; Kassah-Laouar, A. Prevalence, seasonality, and antimicrobial resistance of thermotolerant Campylobacter isolated from broiler farms and slaughterhouses in East Algeria. Vet. World 2020, 13, 1221–1228. [Google Scholar] [CrossRef]
- do Nascimento Veras, H. Diagnóstico Molecular e Detecção de Genes de Virulência de Campylobacter jejuni em Crianças com Diarreia Moderada a Severa na Cidade de Fortaleza–CE, Brasil. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2016. [Google Scholar]
- Rodrigues, C.S.; Armendaris, P.M.; de Sá, C.V.G.C.; Haddad, J.P.A.; de Melo, C.B. Prevalence of Campylobacter spp. in chicken carcasses in slaughterhouses from south of Brazil. Curr. Microbiol. 2021, 78, 2242–2250. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Mendiola-Garza, G.; de Melo, E.M.; Dugmore, T.I.J.; Matharu, A.S.; Morones-Ramirez, J.R. Antimicrobial activity of a silver-microfibrillated cellulose biocomposite against susceptible and resistant bacteria. Sci. Rep. 2020, 10, 7281. [Google Scholar] [CrossRef]
- de Oliveira, L.P.; Carneiro, Z.A.; Ribeiro, C.M.; Lima, M.F.; Paixão, D.A.; Pivatto, M.; de Souza, M.V.N.; Teixeira, L.R.; Lopes, C.D.; de Albuquerque, S.; et al. Three new platinum complexes containing fluoroquinolones and DMSO: Cytotoxicity and evaluation against drug-resistant tuberculosis. J. Inorg. Biochem. 2018, 183, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Eğlence-Bakır, S.; Şahin, M.; Salt, B.Z.; Tüzün, E.; Kara, E.M.; Atun, G.; Çavuş, S.; Kızılcıklı, İ. Palladium (II) complexes with thione and thioalkylated thiosemicarbazones: Electrochemical, antimicrobial and thermogravimetric investigations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237, 118358. [Google Scholar] [CrossRef] [PubMed]
- İnci, D.; Aydın, R. Structures, hydrolysis, stabilities of palladium(II) complexes containing biologically active ligands and species distribution in aqueous solution. J. Mol. Struct. 2019, 1187, 23–37. [Google Scholar] [CrossRef]
- Kantoury, M.; Eslami Moghadam, M.; Tarlani, A.A.; Divsalar, A. Structure effect of some new anticancer Pt(II) complexes of amino acid derivatives with small branched or linear hydrocarbon chains on their DNA interaction. Chem. Biol. Drug Des. 2016, 88, 76–87. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.M.; de Oliveira, B.A.; de Castro, P.P.; de Mendonça, C.C.; Furtado, R.A.; Nicolella, H.D.; da Silva, V.L.; Diniz, C.G.; Tavares, D.C.; Silva, H.; et al. Lipophilic gold(I) complexes with 1,3,4-oxadiazol-2-thione or 1,3-thiazolidine-2-thione moieties: Synthesis and their cytotoxic and antimicrobial activities. BioMetals 2017, 30, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Associação Brasileira de Proteína Animal. Relatório Anual 2021 ABPA; Associação Brasileira de Proteína Animal: São Paulo, Brazil, 2021. [Google Scholar]
- ABPA. Associação Brasileira de Proteína Animal 2022 Relatório Anual; ABPA: São Paulo, Brazil, 2022; pp. 44–49. [Google Scholar]
- ISO/DIS 10272-1:2015; Microbiology of the Food Chain—Horizontal Method for Detection and Enumeration of Campylobacter—Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2015.


| Concentration (mg/L) | CIP n (%) | ERY n (%) |
|---|---|---|
| 0.25 | 39 (16.59%) | 14 (5.95%) |
| 0.5 | 18 (7.65%) | 8 (3.40%) |
| 1 | 57 (24.25%) | 30 (12.76%) |
| 2 | 60 (25.53%) | 42 (17.87%) |
| 4 | 33 (14.04%) | 40 (17.02%) |
| 8 | 20 (8.51%) | 18 (7.65%) |
| 16 | 4 (1.70%) | 5 (2.12%) |
| 32 | 4 (1.70%) | 78 (33.18%) |
| R (%) | 121 a (51.48%) | 83 b (35.31%) |
| MIC50 | 2 | 4 |
| MIC90 | 8 | 32 |
| Epidemiological Factor | Antimicrobial Resistance Profiles-n (%) | ||||
|---|---|---|---|---|---|
| Profile 1: CIP/ERY | Profile 2: CIP | Profile 3: ERY | Profile 4: Susceptibility | Total | |
| Federal State | |||||
| A | 29 (31.18%) | 21 (22.58%) | 24 (25.80%) | 19 (20.43%) | 93 |
| B | 15 (18.29%) | 33 (40.24%) | 6 (7.31%) | 28 (34.14%) | 82 |
| C | 9 (15%) | 14 (23.33%) | 0 (0.00%) | 37 (61.66%) | 60 |
| Season | |||||
| Spring | 30 (22.72%) | 43 (32.57%) | 12 (9.09%) | 47 (35.60%) | 132 |
| Summer | 3 (7.31%) | 12 (29.26%) | 6 (14.63%) | 20 (48.78%) | 41 |
| Autumn | 18 (32.14) | 12 (21.42%) | 9 (16.07%) | 17 (30.35%) | 56 |
| Winter | 2 (33.33%) | 1 (16.66%) | 3 (50%) | 0 | 6 |
| Total | 53 (22.55%) a | 68 (28.93%) a | 30 (12.76%) b | 84 (35.74%) c | 235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacerda, M.L.d.; Rossi, D.A.; Lourenzatto, E.C.A.; Takeuchi, M.G.; Souza, W.A.; Silva, R.T.C.; Julio, L.G.; Guerra, W.; Melo, R.T.d. Antimicrobial Resistance Challenged with Platinum(II) and Palladium(II) Complexes Containing 1,10-Phenanthroline and 5-Amino-1,3,4-Thiadiazole-2(3H)-Thione in Campylobacter jejuni. Antibiotics 2022, 11, 1645. https://doi.org/10.3390/antibiotics11111645
Lacerda MLd, Rossi DA, Lourenzatto ECA, Takeuchi MG, Souza WA, Silva RTC, Julio LG, Guerra W, Melo RTd. Antimicrobial Resistance Challenged with Platinum(II) and Palladium(II) Complexes Containing 1,10-Phenanthroline and 5-Amino-1,3,4-Thiadiazole-2(3H)-Thione in Campylobacter jejuni. Antibiotics. 2022; 11(11):1645. https://doi.org/10.3390/antibiotics11111645
Chicago/Turabian StyleLacerda, Meiry Leandra de, Daise Aparecida Rossi, Eduarda Cristina Alves Lourenzatto, Micaela Guidotti Takeuchi, Wesley Almeida Souza, Raphael Tristão Cruvinel Silva, Luma Gonçalves Julio, Wendell Guerra, and Roberta Torres de Melo. 2022. "Antimicrobial Resistance Challenged with Platinum(II) and Palladium(II) Complexes Containing 1,10-Phenanthroline and 5-Amino-1,3,4-Thiadiazole-2(3H)-Thione in Campylobacter jejuni" Antibiotics 11, no. 11: 1645. https://doi.org/10.3390/antibiotics11111645
APA StyleLacerda, M. L. d., Rossi, D. A., Lourenzatto, E. C. A., Takeuchi, M. G., Souza, W. A., Silva, R. T. C., Julio, L. G., Guerra, W., & Melo, R. T. d. (2022). Antimicrobial Resistance Challenged with Platinum(II) and Palladium(II) Complexes Containing 1,10-Phenanthroline and 5-Amino-1,3,4-Thiadiazole-2(3H)-Thione in Campylobacter jejuni. Antibiotics, 11(11), 1645. https://doi.org/10.3390/antibiotics11111645







