Contribution of RND-Type Efflux Pumps in Reduced Susceptibility to Biocides in Acinetobacter baumannii
Abstract
:1. Introduction
2. Results
2.1. Susceptibility Testing
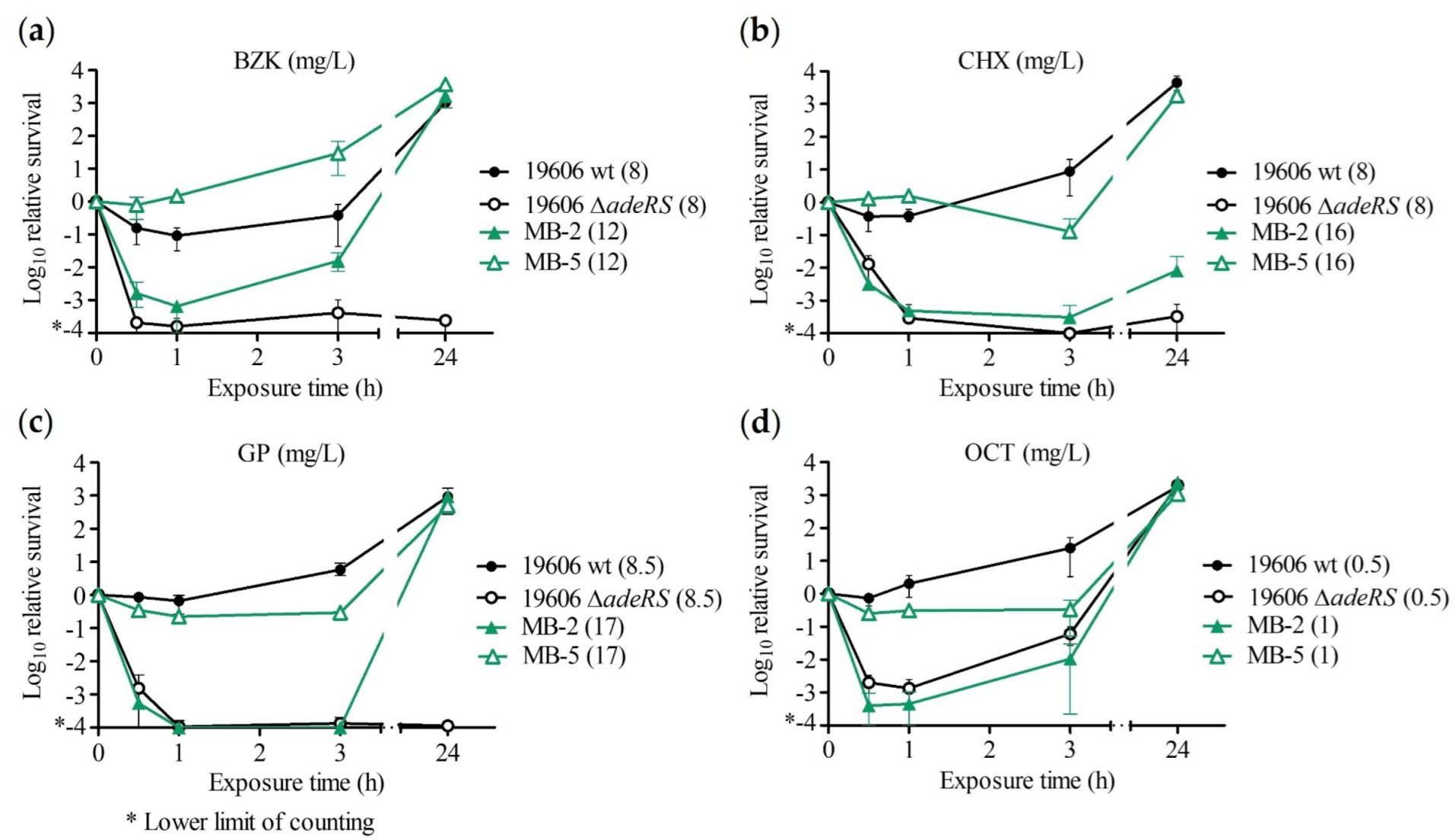
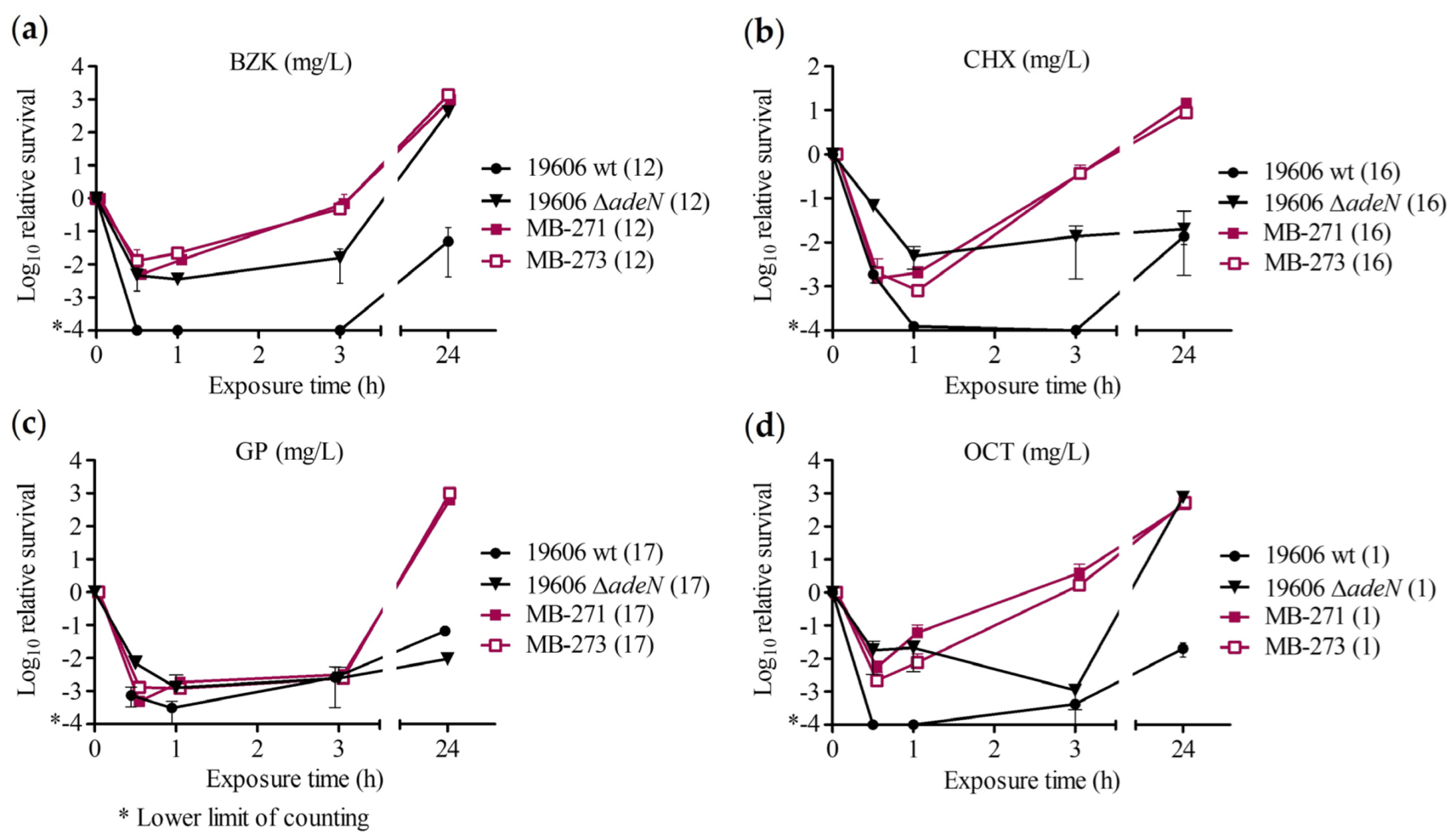
2.2. Time–Kill Assay
2.3. Dose–Response Assay
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Biocides
4.3. Susceptibility Testing
4.4. Kill Kinetics
4.5. Dose–Response Assay with Triclosan
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartal, C.; Rolston, K.V.I.; Nesher, L. Carbapenem-resistant Acinetobacter baumannii: Colonization, infection and current treatment options. Infect. Dis. Ther. 2022, 11, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Maillard, J.Y. Resistance of bacteria to biocides. Microbiol. Spectr. 2018, 6, 2. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Schmitt, R.; Wohrmann, A.; Stefanik, D.; Seifert, H. Resistance to disinfectants in epidemiologically defined clinical isolates of Acinetobacter baumannii. J. Hosp. Infect. 2007, 66, 174–181. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [Green Version]
- Hübner, N.O.; Siebert, J.; Kramer, A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin Pharmacol. Physiol. 2010, 23, 244–258. [Google Scholar] [CrossRef]
- Widmer, A.E.; Frei, R. Antimicrobial activity of glucoprotamin: A clinical study of a new disinfectant for instruments. Infect. Control Hosp. Epidemiol. 2003, 24, 762–764. [Google Scholar] [CrossRef] [Green Version]
- Manian, F.A.; Griesenauer, S.; Senkel, D.; Setzer, J.M.; Doll, S.A.; Perry, A.M.; Wiechens, M. Isolation of Acinetobacter baumannii complex and methicillin-resistant Staphylococcus aureus from hospital rooms following terminal cleaning and disinfection: Can we do better? Infect. Control Hosp. Epidemiol. 2011, 32, 667–672. [Google Scholar] [CrossRef]
- Kawamura-Sato, K.; Wachino, J.; Kondo, T.; Ito, H.; Arakawa, Y. Reduction of disinfectant bactericidal activities in clinically isolated Acinetobacter species in the presence of organic material. J. Antimicrob. Chemother. 2008, 61, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikaido, H.; Pagès, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef] [Green Version]
- Grkovic, S.; Brown, M.H.; Skurray, R.A. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 2002, 66, 671–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damier-Piolle, L.; Magnet, S.; Brémont, S.; Lambert, T.; Courvalin, P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnet, S.; Courvalin, P.; Lambert, T. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 2001, 45, 3375–3380. [Google Scholar] [CrossRef] [Green Version]
- Gerson, S.; Nowak, J.; Zander, E.; Ertel, J.; Wen, Y.; Krut, O.; Seifert, H.; Higgins, P.G. Diversity of mutations in regulatory genes of resistance-nodulation-cell division efflux pumps in association with tigecycline resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2018, 73, 1501–1508. [Google Scholar] [CrossRef]
- Rajamohan, G.; Srinivasan, V.B.; Gebreyes, W.A. Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. J. Antimicrob. Chemother. 2010, 65, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Zhang, Y.; Xu, W.; Zhang, X.; Xu, Y.; Sun, Y.; Zhou, T.; Cao, J. Hyper-expression of the efflux pump gene adeB was found in Acinetobacter baumannii with decreased triclosan susceptibility. J. Glob. Antimicrob. Resist. 2020, 22, 367–373. [Google Scholar] [CrossRef]
- Marchand, I.; Damier-Piolle, L.; Courvalin, P.; Lambert, T. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 2004, 48, 3298–3304. [Google Scholar] [CrossRef]
- Rosenfeld, N.; Bouchier, C.; Courvalin, P.; Périchon, B. Expression of the resistance-nodulation-cell division pump AdeIJK in Acinetobacter baumannii is regulated by AdeN, a TetR-type regulator. Antimicrob. Agents Chemother. 2012, 56, 2504–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, J.; Schneiders, T.; Seifert, H.; Higgins, P.G. The Asp20-to-Asn substitution in the response regulator AdeR leads to enhanced efflux activity of AdeB in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 1085–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucaßen, K.; Müller, C.; Wille, J.; Xanthopoulou, K.; Hackel, M.; Seifert, H.; Higgins, P.G. Prevalence of RND efflux pump regulator variants associated with tigecycline resistance in carbapenem-resistant Acinetobacter baumannii from a worldwide survey. J. Antimicrob. Chemother. 2021, 76, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.J.; Wand, M.E.; Sutton, J.M. Varying activity of chlorhexidine-based disinfectants against Klebsiella pneumoniae clinical isolates and adapted strains. J. Hosp. Infect. 2016, 93, 42–48. [Google Scholar] [CrossRef]
- Wand, M.E. Bacterial resistance to hospital disinfection. In Modeling the Transmission and Prevention of Infectious Disease; Hurst, C.J., Ed.; Advances in Environmental Microbiology; Springer: Cham, Switzerland, 2017; Volume 4, pp. 32–37. ISBN 978-3-319-60616-3. [Google Scholar]
- Slipski, C.J.; Jamieson, T.R.; Lam, A.; Shing, V.L.; Bell, K.; Zhanel, G.G.; Bay, D.C. Plasmid transmitted small multidrug resistant (SMR) efflux pumps differ in gene regulation and enhance tolerance to quaternary ammonium compounds (QAC) when grown as biofilms. BioRxiv 2019, 768630. [Google Scholar] [CrossRef]
- Yoon, E.J.; Courvalin, P.; Grillot-Courvalin, C. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: Major role for AdeABC overexpression and AdeRS mutations. Antimicrob. Agents Chemother. 2013, 57, 2989–2995. [Google Scholar] [CrossRef] [Green Version]
- Lucaßen, K.; Gerson, S.; Xanthopoulou, K.; Wille, J.; Wille, T.; Seifert, H.; Higgins, P.G. Comparison of the Acinetobacter baumannii reference strains ATCC 17978 and ATCC 19606 in antimicrobial resistance mediated by the AdeABC efflux pump. Antimicrob. Agents Chemother. 2021, 65, e0057021. [Google Scholar] [CrossRef]
- Gerson, S. Diversity of Regulatory Systems and Their Contribution to Colistin and Tigecycline Resistance in Acinetobacter baumannii. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, 2021. Bonndoc. Available online: https://hdl.handle.net/20.500.11811/9448 (accessed on 1 October 2022).
- Higgins, P.G.; Schneiders, T.; Hamprecht, A.; Seifert, H. In vivo selection of a missense mutation in adeR and conversion of the novel blaOXA-164 gene into blaOXA-58 in carbapenem-resistant Acinetobacter baumannii isolates from a hospitalized patient. Antimicrob. Agents Chemother. 2010, 54, 5021–5027. [Google Scholar] [CrossRef] [Green Version]
- Migliaccio, A.; Esposito, E.P.; Bagattini, M.; Berisio, R.; Triassi, M.; De Gregorio, E.; Zarrilli, R. Inhibition of AdeB, AceI, and AmvA efflux pumps restores chlorhexidine and benzalkonium susceptibility in Acinetobacter baumannii ATCC 19606. Front. Microbiol. 2022, 12, 790263. [Google Scholar] [CrossRef]
- Rajamohan, G.; Srinivasan, V.B.; Gebreyes, W.A. Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 1919–1925. [Google Scholar] [CrossRef]
- Hassan, K.A.; Jackson, S.M.; Penesyan, A.; Patching, S.G.; Tetu, S.G.; Eijkelkamp, B.A.; Brown, M.H.; Henderson, P.J.; Paulsen, I.T. Transcriptomic and biochemical analyses identify a family of chlorhexidine efflux proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 20254–20259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraud, S.; Campigotto, A.J.; Chen, Z.; Poole, K. MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa: Involvement in chlorhexidine resistance and induction by membrane-damaging agents dependent upon the AlgU stress response sigma factor. Antimicrob. Agents Chemother. 2008, 52, 4478–4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mc Cay, P.H.; Ocampo-Sosa, A.A.; Fleming, G.T.A. Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiology 2010, 156, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, S.B. Active efflux, a common mechanism for biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92, 65s–71s. [Google Scholar] [CrossRef]
- Chen, J.; Kuroda, T.; Huda, M.N.; Mizushima, T.; Tsuchiya, T. An RND-type multidrug efflux pump SdeXY from Serratia marcescens. J. Antimicrob. Chemother. 2003, 52, 176–179. [Google Scholar] [CrossRef] [Green Version]
- Garratt, I.; Aranega-Bou, P.; Sutton, J.M.; Moore, G.; Wand, M.E. Long-term exposure to octenidine in a simulated sink trap environment results in selection of Pseudomonas aeruginosa, Citrobacter, and Enterobacter isolates with mutations in efflux pump regulators. Appl. Environ. Microbiol. 2021, 87, e00210–e00221. [Google Scholar] [CrossRef]
- Wand, M.E.; Jamshidi, S.; Bock, L.J.; Rahman, K.M.; Sutton, J.M. SmvA is an important efflux pump for cationic biocides in Klebsiella pneumoniae and other Enterobacteriaceae. Sci. Rep. 2019, 9, 1344. [Google Scholar] [CrossRef] [Green Version]
- Prieto Martin Gil, S.; Tajuelo, A.; López-Siles, M.; McConnell, M.J. Subinhibitory concentrations of clinically-relevant antimicrobials affect resistance-nodulation-division family promoter activity in Acinetobacter baumannii. Front. Microbiol. 2021, 12, 780201. [Google Scholar] [CrossRef]
- Chuanchuen, R.; Beinlich, K.; Hoang, T.T.; Becher, A.; Karkhoff-Schweizer, R.R.; Schweizer, H.P. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: Exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob. Agents Chemother. 2001, 45, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.; Ruiz, F.M.; Romero, A.; Martínez, J.L. The binding of triclosan to SmeT, the repressor of the multidrug efflux pump SmeDEF, induces antibiotic resistance in Stenotrophomonas maltophilia. PLoS Pathog. 2011, 7, e1002103. [Google Scholar] [CrossRef]
- Fernando, D.M.; Xu, W.; Loewen, P.C.; Zhanel, G.G.; Kumar, A. Triclosan can select for an AdeIJK-overexpressing mutant of Acinetobacter baumannii ATCC 17978 that displays reduced susceptibility to multiple antibiotics. Antimicrob. Agents Chemother. 2014, 58, 6424–6431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.Z.; Chen, J.; Mizushima, T.; Kuroda, T.; Tsuchiya, T. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob. Agents Chemother. 2005, 49, 4362–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuanchuen, R.; Karkhoff-Schweizer, R.R.; Schweizer, H.P. High-level triclosan resistance in Pseudomonas aeruginosa is solely a result of efflux. Am. J. Infect. Control 2003, 31, 124–127. [Google Scholar] [CrossRef]
- Bonez, P.C.; dos Santos Alves, C.F.; Dalmolin, T.V.; Agertt, V.A.; Mizdal, C.R.; da Costa Flores, V.; Marques, J.B.; Santos, R.C.V.; de Campos, M.M.A. Chlorhexidine activity against bacterial biofilms. Am. J. Infect. Control 2013, 41, e119–e122. [Google Scholar] [CrossRef]
- Fernández-Cuenca, F.; Tomás, M.; Caballero-Moyano, F.J.; Bou, G.; Martínez-Martínez, L.; Vila, J.; Pachón, J.; Cisneros, J.M.; Rodríguez-Baño, J.; Pascual, Á. Reduced susceptibility to biocides in Acinetobacter baumannii: Association with resistance to antimicrobials, epidemiological behaviour, biological cost and effect on the expression of genes encoding porins and efflux pumps. J. Antimicrob. Chemother. 2015, 70, 3222–3229. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pi, B.; Zhou, H.; Yu, Y.; Li, L. Triclosan resistance in clinical isolates of Acinetobacter baumannii. J. Med. Microbiol. 2009, 58, 1086–1091. [Google Scholar] [CrossRef] [Green Version]
- Maillard, J.Y. Antimicrobial biocides in the healthcare environment: Efficacy, usage, policies, and perceived problems. Ther. Clin. Risk Manag. 2005, 1, 307–320. [Google Scholar]
- Merchel Piovesan Pereira, B.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef] [Green Version]
- Stahl, J.; Bergmann, H.; Göttig, S.; Ebersberger, I.; Averhoff, B. Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PLoS ONE 2015, 10, e0138360. [Google Scholar] [CrossRef]
- Bouvet, P.J.; Grimont, P.A. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int. J. Syst. Evol. Microbiol. 1986, 36, 228–240. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; Approved Standard M07-A10; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Boyce, J.M.; Pittet, D. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm. Rep. 2002, 51, 1–45. [Google Scholar] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline M26-A; NCCLS: Wayne, PA, USA, 1999. [Google Scholar]
| A. baumannii Strain | Biocide MIC (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| BZK | CHX | ETH | GP | OCT | TRI | ||
| ATCC 19606 wt | 16 | 16 | 197,500 * | 17 | 4 | 1 | |
| ATCC 19606 ΔadeRS | 16 | 4 | 197,500 | 8.5 | 2 | 1 | |
| ATCC 19606 ΔadeN | 16 | 16 | 197,500 | 17 | 4 | 1 | |
| Isolate pair 1 | MB-2 | 16 | 16 | 197,500 | 17 | 4 | 0.5 |
| MB-5 (ISAba1 disrupts adeS) | 16 | 16 | 197,500 | 34 | 4 | 1 | |
| Isolate pair 2 | MB-7 | 16 | 16 | n.d. | 17 | 4 | 4 |
| MB-43 (ISAba1 disrupts adeS) | 16 | 32 | n.d. | 17 | 4 | 4 | |
| Isolate pair 3 | MB-271 | 16 | 16 | 197,500 | 34 | 4 | 4 |
| MB-273 (ISAba1 disrupts adeN) | 16 | 16 | 197,500 | 34 | 4 | 4 | |
| Isolate pair 4 | MB-131 | 32 | 16 | n.d. | 17 | 4 | 4 |
| MB-1044 (ISAba125 disrupts adeN) | 32 | 16 | n.d. | 17 | 4 | 4 | |
| Isolate pair 5 | Isolate F | 16 | 16 | n.d. | 17 | 4 | 4 |
| Isolate G (mutation in adeR) | 16 | 16 | n.d. | 17 | 4 | 4 | |
| A. baumannii Strain | Relevant Characteristics | Reference | ||
|---|---|---|---|---|
| Laboratory strains | ATCC 19606 | Reference strain | [53] | |
| ATCC 19606 ΔadeRS | adeABC not expressed | [28] | ||
| ATCC 19606 ΔadeN | 2.5-fold increase in adeIJK expression | [29] | ||
| Clinical strains | Isolate pair 1 | MB-2 | Wildtype | [17] |
| MB-5 | ISAba1 insertion in adeS, 45-fold increase in adeABC expression | [17] | ||
| Isolate pair 2 | MB-7 | Wildtype | [17] | |
| MB-43 | ISAba1 insertion in adeS, 35-fold increase in adeABC expression | [17] | ||
| Isolate pair 3 | MB-271 | Wildtype | [17] | |
| MB-273 | ISAba1 insertion in adeN, 6-fold increase in adeIJK expression | [17] | ||
| Isolate pair 4 | MB-131 | Wildtype | [17] | |
| MB-1044 | ISAba125 insertion in adeN, 2-fold increase in adeIJK expression | [17] | ||
| Isolate pair 5 | Isolate F | Wildtype | [30] | |
| Isolate G | Missense mutation in adeR, 7-fold increase in adeABC expression | [30] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, C.; Lucaβen, K.; Gerson, S.; Xanthopoulou, K.; Wille, T.; Seifert, H.; Higgins, P.G. Contribution of RND-Type Efflux Pumps in Reduced Susceptibility to Biocides in Acinetobacter baumannii. Antibiotics 2022, 11, 1635. https://doi.org/10.3390/antibiotics11111635
Meyer C, Lucaβen K, Gerson S, Xanthopoulou K, Wille T, Seifert H, Higgins PG. Contribution of RND-Type Efflux Pumps in Reduced Susceptibility to Biocides in Acinetobacter baumannii. Antibiotics. 2022; 11(11):1635. https://doi.org/10.3390/antibiotics11111635
Chicago/Turabian StyleMeyer, Christina, Kai Lucaβen, Stefanie Gerson, Kyriaki Xanthopoulou, Thorsten Wille, Harald Seifert, and Paul G. Higgins. 2022. "Contribution of RND-Type Efflux Pumps in Reduced Susceptibility to Biocides in Acinetobacter baumannii" Antibiotics 11, no. 11: 1635. https://doi.org/10.3390/antibiotics11111635





