Therapeutic Options for Chlamydia trachomatis Infection: Present and Future
Abstract
:1. Introduction
2. Clinical Presentation of Chlamydial Infection
3. Current Therapeutic Options
4. Treatment Failure and Novel Approaches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayes, D.; Merrick, R.; Pulford, C.; Buitendam, E.; Mohammed, H.; Saunders, J. What is the role of sexual health services in the delivery of primary prevention of sexually transmitted infections. A narrative review. Sex. Health 2022, 19, 319–328. [Google Scholar] [CrossRef]
- Starnbach, M.N.; Roan, N.R. Conquering sexually transmitted diseases. Nat. Rev. Immunol. 2008, 8, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Sung, S.; Takov, V. Chlamydia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lausen, M.; Christiansen, G.; Bouet Guldbæk Poulsen, T.; Birkelund, S. Immunobiology of monocytes and macrophages during Chlamydia trachomatis infection. Microbes Infect. 2019, 21, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Tietzel, I.; Quayle, A.J.; Carabeo, R.A. Alternatively Activated Macrophages Are Host Cells for Chlamydia trachomatis and Reverse Anti-chlamydial Classically Activated Macrophages. Front. Microbiol. 2019, 10, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkin, S.S.; Minis, E.; Athanasiou, A.; Leizer, J.; Linhares, I.M. Chlamydia trachomatis: The Persistent Pathogen. Clin. Vaccine Immunol. 2017, 24, e00203-17. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.M.; McKay, P.F. Chlamydia trachomatis: Cell biology, immunology and vaccination. Vaccine 2021, 39, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Poston, T.B.; Darville, T. Chlamydia trachomatis: Protective Adaptive Responses and Prospects for a Vaccine. Curr. Top Microbiol. Immunol. 2018, 412, 217–237. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Brunham, R.C.; Byrne, G.I.; Martin, D.H.; Xu, F.; Berman, S.M. Introduction: The natural history and immunobiology of Chlamydia trachomatis genital infection and implications for chlamydia control. J. Infect. Dis. 2010, 201 (Suppl. 2), S85–S87. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Xu, S.; Liu, Z.; Xue, Y.; Xie, W.; Yang, S.; Liu, L.; Bao, X. Inhibitory Activity of Pyrroloisoxazolidine Derivatives against Chlamydia trachomatis. BioMed Res. Int. 2021, 2021, 8889247. [Google Scholar] [CrossRef]
- Rodrigues, R.; Sousa, C.; Vale, N. Chlamydia trachomatis as a Current Health Problem: Challenges and Opportunities. Diagnostics 2022, 12, 1795. [Google Scholar] [CrossRef]
- Fields, K.A.; Hackstadt, T. The Chlamydial Inclusion: Escape from the Endocytic Pathway. Annu. Rev. Cell Dev. Biol. 2002, 18, 221–245. [Google Scholar] [CrossRef] [PubMed]
- Hogan, R.J.; Mathews, S.A.; Mukhopadhyay, S.; Summersgill, J.T.; Timms, P. Chlamydial Persistence: Beyond the Biphasic Paradigm. Infect. Immun. 2004, 72, 1843–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial Intracellular Survival Strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huai, P.; Li, F.; Chu, T.; Liu, D.; Liu, J.; Zhang, F. Prevalence of genital Chlamydia trachomatis infection in the general population: A meta-analysis. BMC Infect. Dis. 2020, 20, 589. [Google Scholar] [CrossRef]
- Chan, P.A.; Robinette, A.; Montgomery, M.; Almonte, A.; Cu-Uvin, S.; Lonks, J.R.; Chapin, K.C.; Kojic, E.M.; Hardy, E.J. Extragenital Infections Caused by Chlamydia trachomatis and Neisseria gonorrhoeae: A Review of the Literature. Infect. Dis. Obstet. Gynecol. 2016, 2016, 5758387. [Google Scholar] [CrossRef] [Green Version]
- Escarcega-Tame, M.A.; López-Hurtado, M.; Escobedo-Guerra, M.R.; Reyes-Maldonado, E.; Castro-Escarpulli, G.; Guerra-Infante, F.M. Co-infection between genotypes of the human papillomavirus and Chlamydia trachomatis in Mexican women. Int. J. STD AIDS 2020, 31, 1255–1262. [Google Scholar] [CrossRef]
- Lim, R.B.T.; Wong, M.L.; Cook, A.R.; Brun, C.; Chan, R.K.W.; Sen, P.; Chio, M. Determinants of Chlamydia, Gonorrhea, and Coinfection in Heterosexual Adolescents Attending the National Public Sexually Transmitted Infection Clinic in Singapore. Sex. Transm. Dis. 2015, 42, 450–456. [Google Scholar] [CrossRef]
- Harrison, S.A.; Olson, K.; Ratliff, A.E.; Xiao, L.; Van Der Pol, B.; Waites, K.B.; Geisler, W.M. Mycoplasma genitalium Coinfection in Women With Chlamydia trachomatis Infection. Sex. Transm. Dis. 2019, 46, e101–e104. [Google Scholar] [CrossRef]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef]
- World Health Organization. Sexually Transmitted Infections (STIs). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 14 July 2022).
- Land, J.; Van Bergen, J.; Morre, S.; Postma, M. Epidemiology of Chlamydia trachomatis infection in women and the cost-effectiveness of screening. Hum. Reprod. Updat. 2009, 16, 189–204. [Google Scholar] [CrossRef]
- Neinstein, L.S.; Rabinovitz, S. Detection of Chlamydia trachomatis. A study of the direct immunofluorescence technique and a review diagnostic limitation. J. Adolesc. Health Care 1989, 10, 10–15. [Google Scholar] [CrossRef]
- de Haro-Cruz, M.J.; Guadarrama-Macedo, S.I.; López-Hurtado, M.; Escobedo-Guerra, M.R.; Guerra-Infante, F.M. Obtaining an ELISA test based on a recombinant protein of Chlamydia trachomatis. Int. Microbiol. 2019, 22, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Brook, G. The performance of non-NAAT point-of-care (POC) tests and rapid NAAT tests for chlamydia and gonorrhoea infections. An assessment of currently available assays. Sex. Transm. Infect. 2015, 91, 539–544. [Google Scholar] [CrossRef]
- Novak, D.P.; Lindholm, L.; Jonsson, M.; Karlsson, R.B. A Swedish cost-effectiveness analysis of community-based Chlamydia trachomatis PCR testing of postal urine specimens obtained at home. Scand. J. Public Health 2004, 32, 324–332. [Google Scholar] [CrossRef]
- Adamson, P.C.; Loeffelholz, M.J.; Klausner, J.D. Point-of-Care Testing for Sexually Transmitted Infections: A Review of Recent Developments. Arch. Pathol. Lab. Med. 2020, 144, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Harding-Esch, E.; Fuller, S.; Chow, S.-L.; Nori, A.; Harrison, M.; Parker, M.; Piepenburg, O.; Forrest, M.; Brooks, D.; Patel, R.; et al. Diagnostic accuracy of a prototype rapid chlamydia and gonorrhoea recombinase polymerase amplification assay: A multicentre cross-sectional preclinical evaluation. Clin. Microbiol. Infect. 2018, 25, 380.e1–380.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvan, S.P.E.; Von Krogh, G.; Tiveljung, A.; Siwerth, B.-M.; Henriksson, L.; Norén, L.; Asp, A.-K.; Grillner, L. Screening and Genotyping of Genital Chlamydia trachomatis in Urine Specimens From Male and Female Clients of Youth-Health Centers in Stockholm County. Sex. Transm. Dis. 2002, 29, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Parra-Sánchez, M.; Palomares, J.C.; Bernal, S.; González, M.T.; Sivianes, N.; Pérez, L.; Pueyo, I.; Martín-Mazuelos, E. Evaluation of the cobas 4800 CT/NG Test for detecting Chlamydia trachomatis and Neisseria gonorrhoeae DNA in urogenital swabs and urine specimens. Diagn. Microbiol. Infect. Dis. 2012, 74, 338–342. [Google Scholar] [CrossRef]
- Herbst de Cortina, S.; Bristow, C.C.; Joseph Davey, D.; Klausner, J.D. ASystematic Review of Point of Care Testing for Chlamydia trachomatis, Neisseriagonorrhoeae, and Trichomonas vaginalis. Infect. Dis. Obstet. Gynecol. 2016, 2016, 4386127. [Google Scholar] [CrossRef] [Green Version]
- Frej-Mądrzak, M.; Gryboś, A.; Gryboś, M.; Teryks-Wołyniec, D.; Jama-Kmiecik, A.; Sarowska, J.; Choroszy-Król, I. PCR diagnostics of Chlamydia trachomatis in asymptomatic infection by women. Ginekol. Pol. 2018, 89, 115–119. [Google Scholar] [CrossRef]
- Lesiak-Markowicz, I.; Schötta, A.-M.; Stockinger, H.; Stanek, G.; Markowicz, M. Chlamydia trachomatis serovars in urogenital and ocular samples collected 2014–2017 from Austrian patients. Sci. Rep. 2019, 9, 18327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Guidelines for the Treatment of Chlamydia Trachomatis; WHO: Geneva, Switzerland, 2016; p. 56. Available online: https://www.who.int/publications/i/item/978-92-4-154971-4 (accessed on 16 July 2022).
- Morré, S.A.; Rozendaal, L.; van Valkengoed, I.G.; Boeke, A.J.; van Voorst Vader, P.C.; Schirm, J.; de Blok, S.; van Den Hoek, J.A.; van Doornum, G.J.; Meijer, C.J.; et al. Urogenital Chlamydia trachomatis serovars in men and women with a symptomatic or asymptomatic infection: An association with clinical manifestations? J. Clin. Microbiol. 2000, 38, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Yang, L.; Jiang, Y.; Li, L.; Yi, W.; Lan, L.; Zhang, L. Distribution of Chlamydia Trachomatis Genotypes in Infective Diseases of the Female Lower Genital Tract. Med. Sci. Monit. 2017, 23, 4477–4481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, V.; Cordeiro, D.; Salas, A.I.; Lodhia, Z.; Correia, C.; Isidro, J.; Fernandes, C.; Rodrigues, A.M.; Azevedo, J.; Alves, J.; et al. Chlamydia trachomatis: When the virulence-associated genome backbone imports a prevalence-associated major antigen signature. Microb. Genom. 2019, 5, e000313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelsamed, H.; Peters, J.; Byrne, G.I. Genetic variation in Chlamydia trachomatis and their hosts: Impact on disease severity and tissue tropism. Future Microbiol. 2013, 8, 1129–1146. [Google Scholar] [CrossRef] [Green Version]
- Balasubramaniam, S.D.; Balakrishnan, V.; Oon, C.E.; Kaur, G. Key Molecular Events in Cervical Cancer Development. Medicina 2019, 55, 384. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Cerqueira, F.; Medeiros, R. Chlamydia trachomatis infection: Implications for HPV status and cervical cancer. Arch. Gynecol. Obstet. 2013, 289, 715–723. [Google Scholar] [CrossRef]
- Sabbatucci, M.; Salfa, M.C.; Regine, V.; Pezzotti, P.; Suligoi, B. Estimated burden of Chlamydia trachomatis female infection and consequent severe pelvic inflammatory disease, Italy, 2005–2016. Ann. Dell’istituto Super. Di Sanità 2019, 55, 217–223. [Google Scholar]
- Borrego, M.J.; Gomes, J.P.; Lefebvre, J.F.; Eb, F.; Orfila, J.; Catry, M.A. Genotyping of Portuguese Chlamydia trachomatis urogenital isolates. Sex. Transm. Infect. 1997, 73, 561–563. [Google Scholar] [CrossRef]
- Casillas-Vega, N.; Morfín-Otero, R.; García, S.; Llaca-Díaz, J.; Rodríguez-Noriega, E.; Camacho-Ortiz, A.; Merced Ayala-Castellanos, M.d.l.; Maldonado-Garza, H.J.; Ancer-Rodríguez, J.; Gallegos-Ávila, G.; et al. Frequency and genotypes of Chlamydia trachomatis in patients attending the obstetrics and gynecology clinics in Jalisco, Mexico and correlation with sociodemographic, behavioral, and biological factors. BMC Women’s Health 2017, 17, 83. [Google Scholar] [CrossRef]
- Xie, X.; Yang, M.; Ding, Y.; Chen, J. Microbial infection, inflammation and epithelial ovarian cancer. Oncol. Lett. 2017, 14, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- DrugBank. Available online: https://go.drugbank.com/ (accessed on 8 December 2021).
- Bakheit, A.H.H.; Al-Hadiya, B.M.H.; Abd-Elgalil, A.A. Chapter One—Azithromycin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J.; Haber, V.E.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.C.; Cunha, B.A. Tetracyclines. Med. Clin. N. Am. 1995, 79, 789–801. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Amsden, G.W. Erythromycin, clarithromycin, and azithromycin: Are the differences real? Clin. Ther. 1996, 18, 56–72. [Google Scholar] [CrossRef]
- Lamp, K.C.; Bailey, E.M.; Rybak, M.J. Ofloxacin Clinical Pharmacokinetics. Clin. Pharmacokinet. 1992, 22, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Al-Hadiya, B.M.; Abd-Elgalil, A.A. Azithromycin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 1–40. [Google Scholar]
- PubChem. PubChem Compound Summary for CID 447043, Zithromax; PubChem, National Center for Biotechnology Information, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Zithromax (accessed on 15 November 2022).
- PubChem. Azithromycin Action Pathway. In PubChem Pathway Summary for Pathway—PathBank; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004; Volume SMP0000247. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Azithromycin (accessed on 15 November 2022).
- PubChem. PubChem Compound Summary for CID 54671203, Doxycycline; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Doxycycline (accessed on 15 November 2022).
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR. Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Lanjouw, E.; Ouburg, S.; de Vries, H.; Stary, A.; Radcliffe, K.; Unemo, M. 2015 European guideline on the management of Chlamydia trachomatis infections. Int. J. STD AIDS 2016, 27, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Pitt, R.A.; Alexander, S.; Horner, P.J.; Ison, C.A. Presentation of clinically suspected persistent chlamydial infection: A case series. Int. J. STD AIDS 2013, 24, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Manavi, K.; Hettiarachchi, N.; Hodson, J. Comparison of doxycycline with azithromycin in treatment of pharyngeal chlamydia infection. Int. J. STD AIDS 2016, 27, 1303–1308. [Google Scholar] [CrossRef]
- Reveneau, N.; Crane, D.D.; Fischer, E.; Caldwell, H.D. Bactericidal activity of first-choice antibiotics against gamma interferon-induced persistent infection of human epithelial cells by Chlamydia trachomatis. Antimicrob. Agents Chemother. 2005, 49, 1787–1793. [Google Scholar] [CrossRef] [Green Version]
- PubChem. PubChem Compound Summary for CID 12560, Erythromycin; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Erythromycin (accessed on 15 November 2022).
- Pitsouni, E.; Iavazzo, C.; Athanasiou, S.; Falagas, M.E. Single-dose azithromycin versus erythromycin or amoxicillin for Chlamydia trachomatis infection during pregnancy: A meta-analysis of randomised controlled trials. Int. J. Antimicrob. Agents 2007, 30, 213–221. [Google Scholar] [CrossRef]
- PubChem. PubChem Compound Summary for CID 54675776, Tetracycline; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetracycline (accessed on 15 November 2022).
- PubChem. PubChem Compound Summary for CID 149096, Levofloxacin; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Levofloxacin (accessed on 15 November 2022).
- PubChem. PubChem Compound Summary for CID 33613, Amoxicillin; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amoxicillin (accessed on 15 November 2022).
- Belko, J.; Urueta, G.; Emre, U. Amoxicillin overdose manifested by hematuria and acute renal failure. Pediatr. Infect. Dis. J. 1995, 14, 917–918. [Google Scholar] [PubMed]
- Kacmar, J.; Cheh, E.; Montagno, A.; Peipert, J.F. A Randomized Trial of Azithromycin Versus Amoxicillin for the Treatment of Chlamydia trachomatis in pregnancy. Infect. Dis. Obstet. Gynecol. 2001, 9, 197–202. [Google Scholar] [CrossRef]
- Rahangdale, L.; Guerry, S.; Bauer, H.M.; Packel, L.; Rhew, M.; Baxter, R.; Chow, J.; Bolan, G. An Observational Cohort Study of Chlamydia trachomatis Treatment in Pregnancy. Sex. Transm. Dis. 2006, 33, 106–110. [Google Scholar] [CrossRef]
- PubChem. PubChem Compound Summary for CID 54704426, Tetracycline Hydrochloride; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetracycline-HCl (accessed on 15 November 2022).
- PubChem. PubChem Compound Summary for CID 410087, Povidone-Iodine; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Povidone-iodine (accessed on 15 November 2022).
- PubChem. PubChem Compound Summary for CID 24470, Silver Nitrate; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Silver-Nitrate (accessed on 15 November 2022).
- PubChem. PubChem Compound Summary for CID 5959, Chloramphenicol; PubChem, National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chloramphenicol (accessed on 15 November 2022).
- Sherrard, J.; Jensen, J.S. Chlamydia treatment failure after repeat courses of azithromycin and doxycycline. Int. J. STD AIDS 2019, 30, 1025–1027. [Google Scholar] [CrossRef]
- Huemer, M.; Shambat, S.M.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Ljubin-Sternak, S.; Mestrovic, T.; Vilibic-Cavlek, T.; Mlinaric-Galinovic, G.; Sviben, M.; Markotic, A.; Skerk, V. In vitro susceptibility of urogenital Chlamydia trachomatis strains in a country with high azithromycin consumption rate. Folia Microbiol. Praha 2013, 58, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Hathorn, E.; Opie, C.; Goold, P. What is the appropriate treatment for the management of rectal Chlamydia trachomatis in men and women? Sex. Transm. Infect. 2012, 88, 352. [Google Scholar] [CrossRef] [PubMed]
- Wyrick, P.B.; Knight, S.T. Pre-exposure of infected human endometrial epithelial cells to penicillin in vitro renders Chlamydia trachomatis refractory to azithromycin. J. Antimicrob. Chemother. 2004, 54, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestrovic, T.; Ljubin-Sternak, S. Molecular mechanisms of Chlamydia trachomatis resistance to antimicrobial drugs. Front. Biosci. 2018, 23, 656–670. [Google Scholar] [CrossRef] [Green Version]
- Scurtu, L.G.; Jinga, V.; Simionescu, O. Fascinating Molecular and Immune Escape Mechanisms in the Treatment of STIs (Syphilis, Gonorrhea, Chlamydia, and Herpes Simplex). Int. J. Mol. Sci. 2022, 23, 3550. [Google Scholar] [CrossRef] [PubMed]
- Benamri, I.; Azzouzi, M.; Sanak, K.; Moussa, A.; Radouani, F. An overview of genes and mutations associated with Chlamydiae species’ resistance to antibiotics. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 59. [Google Scholar] [CrossRef]
- Wyrick, P.B. Chlamydia trachomatis Persistence In Vitro: An Overview. J. Infect. Dis. 2010, 201, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Panzetta, M.E.; Valdivia, R.H.; Saka, H.A. Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo. Front. Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef]
- Mpiga, P.; Ravaoarinoro, M. Chlamydia trachomatis persistence: An update. Microbiol. Res. 2006, 161, 9–19. [Google Scholar] [CrossRef]
- Beatty, W.L.; Morrison, R.P.; Byrne, G.I. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect. Immun. 1995, 63, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Shemer-Avni, Y.; Wallach, D.; Sarov, I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect. Immun. 1988, 56, 2503–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanada, H.; Ikeda-Dantsuji, Y.; Naito, M.; Nagayama, A. Infection of human fibroblast-like synovial cells with Chlamydia trachomatis results in persistent infection and interleukin-6 production. Microb. Pathog. 2003, 34, 57–63. [Google Scholar] [CrossRef]
- Clements, J.D.; Harper, A.; Pogson, C.I.; Jones, M.L.; Pearce, J.H. Chlamydial Development Is Adversely Affected by Minor Changes in Amino Acid Supply, Blood Plasma Amino Acid Levels, and Glucose Deprivation. Infect. Immun. 2000, 68, 1457–1464. [Google Scholar]
- Dreses-Werringloer, U.; Padubrin, I.; Jürgens-Saathoff, B.; Hudson, A.P.; Zeidler, H.; Köhler, L. Persistence of Chlamydia trachomatis Is Induced by Ciprofloxacin and Ofloxacin In Vitro. Antimicrob. Agents Chemother. 2000, 44, 3288–3297. [Google Scholar] [CrossRef] [Green Version]
- Johnson, F.W.A.; Hobson, D. The effect of penicillin on genital strains of Chlamydia trachomatis in tissue culture. J. Antimicrob. Chemother. 1977, 3, 49–56. [Google Scholar] [CrossRef]
- Shima, K.; Ledig, S.; Loeper, N.; Schiefer, A.; Pfarr, K.; Hoerauf, A.; Graspeuntner, S.; Rupp, J. Effective inhibition of rifampicin-resistant Chlamydia trachomatis by the novel DNA-dependent RNA polymerase inhibitor corallopyronin A. Int. J. Antimicrob. Agents 2018, 52, 523–524. [Google Scholar] [CrossRef]
- Loeper, N.; Graspeuntner, S.; Ledig, S.; Kaufhold, I.; Hoellen, F.; Schiefer, A.; Henrichfreise, B.; Pfarr, K.; Hoerauf, A.; Shima, K.; et al. Elaborations on Corallopyronin A as a Novel Treatment Strategy Against Genital Chlamydial Infections. Front. Microbiol. 2019, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Traore, Y.; Jimenez, C.; Ho, E.A. Autophagy induction and PDGFR-β knockdown by siRNA-encapsulated nanoparticles reduce chlamydia trachomatis infection. Sci. Rep. 2019, 9, 1306. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Otero, C.; Bahnan, W.; Vielfort, K.; Silver, J.; Singh, P.; Elbir, H.; Almqvist, F.; Bergström, S.; Gylfe, Å. A 2-Pyridone Amide Inhibitor of Transcriptional Activity in Chlamydia trachomatis. Antimicrob. Agents Chemother. 2021, 65, e01826-20. [Google Scholar] [CrossRef]
- Hamarsheh, O.; Amro, A.; Al-Zeer, M. In Vitro Antibacterial Activity of Selected Palestinian Medicinal Plants against Chlamydia trachomatis. Microbiol. Res. 2021, 12, 656–662. [Google Scholar] [CrossRef]
- Lam, H.N.; Lau, T.; Lentz, A.; Sherry, J.; Cabrera-Cortez, A.; Hug, K.; Lalljie, A.; Engel, J.; Lokey, R.S.; Auerbuch, V. Developing Cyclic Peptomers as Broad-Spectrum Type III Secretion System Inhibitors in Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2021, 65, e01690-20. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Strange, N.; Phillips, M.J.; Krause, A.L.; Heywood, A.; Gamble, A.B.; Huston, W.M.; Tyndall, J.D. Optimization of peptide-based inhibitors targeting the HtrA serine protease in Chlamydia: Design, synthesis and biological evaluation of pyridone-based and N-Capping group-modified analogues. Eur. J. Med. Chem. 2021, 224, 113692. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Rubanik, L.; Lobov, A.; Poleshchuk, N.; Baikova, I.; Kapustina, Y.; Petrova, A.; Korzun, T.; Lopatina, T.; Fedorova, A.; et al. Synthesis of erythrodiol C-ring derivatives and their activity against Chlamydia trachomatis. Steroids 2021, 175, 108912. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Duarte, D.; Vale, N. Drug Repurposing in Cancer Therapy: Influence of Patient’s Genetic Background in Breast Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 4280. [Google Scholar] [CrossRef] [PubMed]
- Itoh, R.; Kurihara, Y.; Yoshimura, M.; Hiromatsu, K. Bortezomib Eliminates Persistent Chlamydia trachomatis Infection through Rapid and Specific Host Cell Apoptosis. Int. J. Mol. Sci. 2022, 23, 7434. [Google Scholar] [CrossRef]
- Schautteet, K.; De Clercq, E.; Vanrompay, D. Chlamydia trachomatis Vaccine Research through the Years. Infect. Dis. Obstet. Gynecol. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Brunham, R.C.; Rappuoli, R. Chlamydia trachomatis control requires a vaccine. Vaccine 2013, 31, 1892–1897. [Google Scholar] [CrossRef] [Green Version]
- Ababneh, M.; Ghazal, E.; khalifeh, M. Immunogenicity of an Adenoviral Vector Vaccine (rAd-MOMP-CpG) against Chlamydia Trachomatis. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Poston, T.B.; Gottlieb, S.L.; Darville, T. Status of vaccine research and development of vaccines for Chlamydia trachomatis infection. Vaccine 2019, 37, 7289–7294. [Google Scholar] [CrossRef]
- Bell, S.D.; Nichols, R.L.; Haddad, N.A. The immunology of the trachoma agent with a preliminary report on field trials on vaccine. Investig. Ophthalmol. 1963, 2, 471–481. [Google Scholar]
- Sowa, S.; Sowa, J.; Collier, L.H.; Blyth, W.A. Trachoma vaccine field trials in The Gambia. Epidemiol. Infect. 1969, 67, 699–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-p.; Thomas Grayston, J.; Russell Alexander, E. Trachoma Vaccine Studies in Monkeys. Am. J. Ophthalmol. 1967, 63, 1615–1630. [Google Scholar] [CrossRef]
- Maza, L.M.d.l.; Zhong, G.; Brunham, R.C.; Papasian, C.J. Update on Chlamydia trachomatis Vaccinology. Clin. Vaccine Immunol. 2017, 24, e00543-16. [Google Scholar]
- Phillips, S.; Quigley, B.L.; Timms, P. Seventy Years of Chlamydia Vaccine Research—Limitations of the Past and Directions for the Future. Front. Microbiol. 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Theodor, I.; Peterson, E.M.; Maza, L.M.d.l. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect. Immun. 1997, 65, 3361–3369. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.; Whittum-Hudson, J.; Schachter, J.; Caldwell, H.D.; Prendergast, R.A. Oral immunization with chlamydial major outer membrane protein (MOMP). Investig. Ophthalmol. Vis. Sci. 1988, 29, 1847–1853. [Google Scholar]
- Vasilevsky, S.; Greub, G.; Nardelli-Haefliger, D.; Baud, D. Genital Chlamydia trachomatis: Understanding the Roles of Innate and Adaptive Immunity in Vaccine Research. Clin. Microbiol. Rev. 2014, 27, 346–370. [Google Scholar] [CrossRef] [Green Version]
- Aslam, S.; Ahmad, S.; Noor, F.; Ashfaq, U.A.; Shahid, F.; Rehman, A.; Tahir Ul Qamar, M.; Alatawi, E.A.; Alshabrmi, F.M.; Allemailem, K.S. Designing a Multi-Epitope Vaccine against Chlamydia trachomatis by Employing Integrated Core Proteomics, Immuno-Informatics and In Silico Approaches. Biology 2021, 10, 997. [Google Scholar] [CrossRef]
- Aslam, M.; Shehroz, M.; Ali, F.; Zia, A.; Pervaiz, S.; Shah, M.; Hussain, Z.; Nishan, U.; Zaman, A.; Afridi, S.G.; et al. Chlamydia trachomatis core genome data mining for promising novel drug targets and chimeric vaccine candidate’s identification. Comput. Biol. Med. 2021, 136, 104701. [Google Scholar] [CrossRef]
- Shiragannavar, S.; Madagi, S.; Hosakeri, J.; Barot, V. In silico vaccine design against Chlamydia trachomatis infection. Netw. Model. Anal. Heal. Inform. Bioinform. 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Dimitrov, I.; Flower, D.R.; Doytchinova, I. AllerTOP—A server for in silico prediction of allergens. BMC Bioinform. 2013, 14 (Suppl. 6), S4. [Google Scholar] [CrossRef] [PubMed]
| Genital Tract | Symptoms |
|---|---|
| Uncomplicated infection | |
| Female | Abnormal vaginal discharge; dysuria; post-coital and intermenstrual bleeding |
| Male | Urethral discharge; dysuria; testicular pain |
| Persistent infection | |
| Female | Pelvic inflammatory disease; ectopic pregnancy; salpingitis; tubal factor infertility |
| Male | Epididymitis |
| Non-genital Tract | |
| Rectal infection | Rectal discharge; rectal pain; blood in the stools |
| Oropharyngeal infection | Pharyngitis and mild sore throat |
| Pregnancy complications | Preterm birth and low birth weight |
| Perinatal transmission | Neonatal conjunctivitis and/or nasopharyngeal infection; ocular discharge and swollen eyelids |
| Drug | Chemical Structure | Main Information |
|---|---|---|
| Azithromycin |  | Mechanism of action: Inhibition of bacterial protein synthesis by interrupting the transpeptidation/translocation pathway due to its binding to the bacterial 50S ribosomal subunit’s 23S rRNA (ribosomal RNA). Absorption: Following oral administration, peak plasma concentrations occur after 2–3 h. Maximum concentration (Cmax): 0.4 mg.L−1). When administered intravenously, peak plasma concentration is reported to be 3–4 mg.L−1 after 1 h infusion. Distribution: Mostly distributed in the body (except in the brain and cerebrospinal fluid) the volume of distribution (Vd) is about 31.1 L.kg−1. It is well tolerated within cells (phagocytes, e.g.,) allowing high efficacy in Ct infection treatment. Metabolism: Although its metabolic pathway has yet to be explored, it is known that azithromycin undergoes some hepatic metabolism. Route of elimination: Biliary excretion is the major route of elimination; 12.2% of the drug is eliminated in the urine after intravenous (IV) administration and 4.5% when administered orally. Half-life: The elimination half-life (t1/2) is 40–68 h due to extensive tissue retention. |
| Doxycycline | 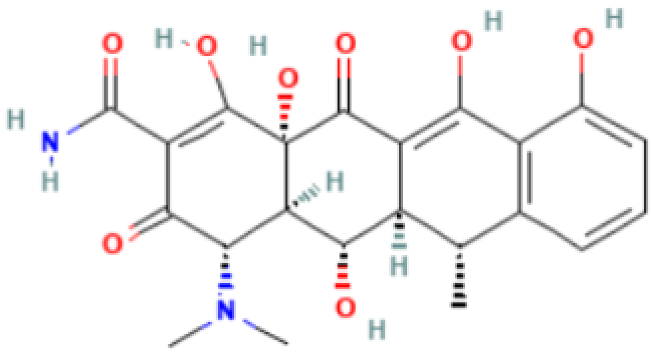 | Mechanism of action: It prevents aminoacyl-tRNA (aa-tRNA) from binding to the ribosomal site, hence inhibiting bacterial protein synthesis, namely the elongation phase. Absorption: Peak plasma concentration of approximately 3.0–5.0 μg.mL−1 occur 2–3 h after oral administration and 4–10 µg.mL−1 within 30 min after IV dosing. Distribution: Despite the scarcity of data, it is known that the drug is widely distributed in tissues and body fluids, including cerebrospinal fluid. Metabolism: It has not been studied yet. Route of elimination: Most of the drug is excreted through the kidneys, with a small fraction being eliminated in the bile. It can also be excreted in feces. Half-life: 18–22 h. |
| Tetracycline | 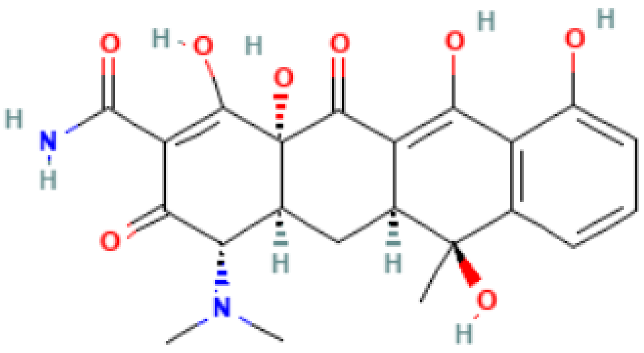 | Mechanism of action: Inhibition of the ribosome subunits association by binding to the 30S ribosomal subunit via passive diffusion in bacterial membrane porin channels, hence interfering with protein synthesis. Absorption: Following oral administration, peak plasma concentrations of 3–5 μg.mL−1 within 2 h. Intramuscular (IM) administration has low bioavailability (<40%), followed by oral (60–80%) and IV administration (100%). Distribution: Limited information available. This drug’s class of antibiotics has a solubility-dependent distribution in the tissues and body fluids. Metabolism: Not metabolized. Route of elimination: It is excreted in the urine (30%) and feces (20–60%) at high concentrations in its biologically active form. Half-life: 6–12 h. |
| Erythromycin | 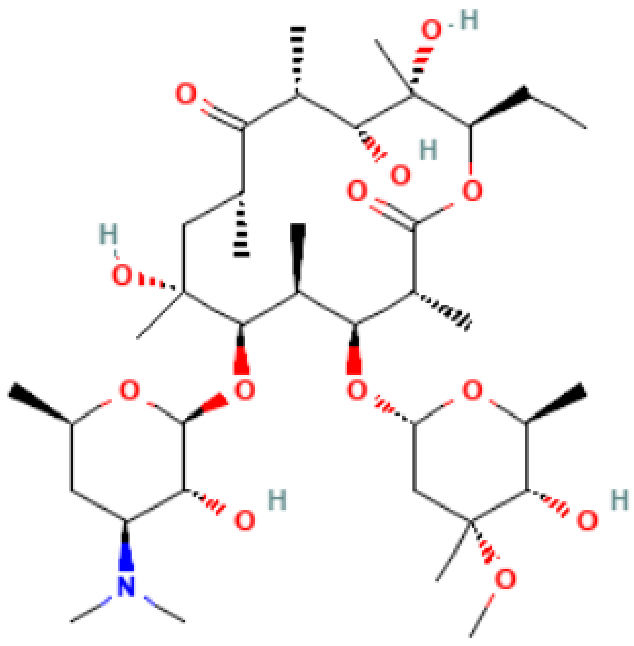 | Mechanism of action: It inhibits transpeptidation/translocation and the assembly of the 50S ribosomal subunit, preventing bacterial protein synthesis. Absorption: Despite the interindividual heterogeneity in absorption, the peak plasma concentration is reported to be 1.8 μg.L−1 after 1.2 h of an orally administered dose. Its bioavailability ranges from 18–45%. Distribution: Found in most body fluids and accumulated in leucocytes and inflammatory liquid (Vd: 1.5 L.kg−1). This drug is well diffused in meningitis, as the blood-brain barrier (BBB) is easily penetrated (inflamed tissues). Metabolism: It undergoes hepatic first-pass metabolism after an oral dose. CYP3A4 enzyme partially metabolizes it to N-desmethylerythromycin. In acidic conditions, it is also hydrolyzed to anhydro forms (inactive against bacteria). Route of elimination: It is excreted in the bile. After an oral dosage, less than 5% is eliminated in the urine. Half-life: 2.4–3.1 h. |
| Levofloxacin | 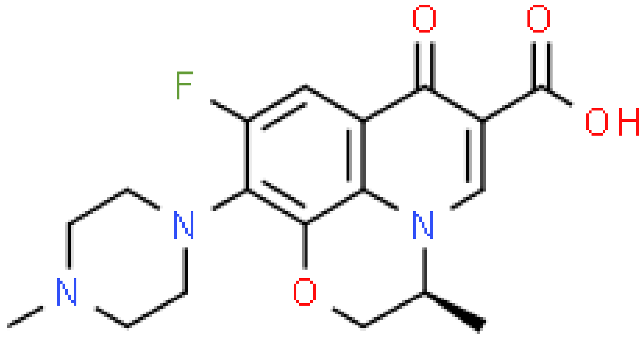 | Mechanism of action: It prevents normal cell division by acting on the DNA (deoxyribonucleic acid) gyrase and topoisomerase IV, enzymes responsible for avoiding excessive supercoiling of DNA during replication or transcription. Absorption: Peak plasma concentrations of 11.5 µg.mL−1 within 2–3 h following oral administration. Bioavailability is approximately 99%. Distribution: Extensive distribution in body fluids and inflammatory exudates. Vd ranges between 1.09 and 1.26 L.kg−1 after an orally administered dose. Metabolism: Levofloxacin metabolism in humans occurs by demethylation and oxidation originating the metabolites: desmethyl-levofloxacin and levofloxacin-N-oxide. Route of elimination: After oral administration, approximately 87% is excreted in urine and less than 4% in feces. Half-life: 6–8 h. |
| Amoxicillin | 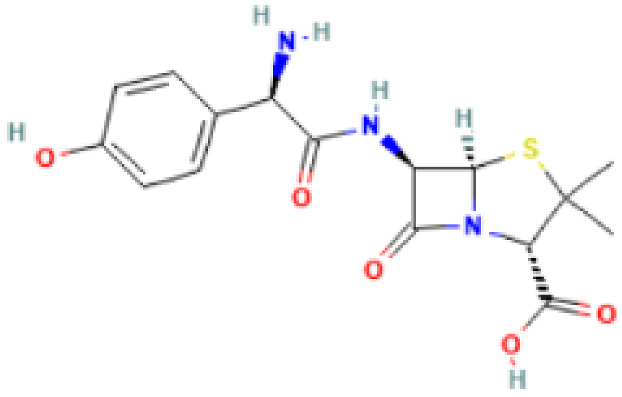 | Mechanism of action: It inhibits penicillin-binding proteins, which are responsible for glycosyltransferase and transpeptidase reactions that lead to cross-linking of D-alanine and D-aspartic acid in bacterial cell walls. This affects the formation and repair of the cell wall, resulting in cell lysis. Absorption: A 250 mg of oral dose reaches peak plasma concentrations of 3.93 mg.L−1 after 1.31 h. Bioavailability is approximately 60%. Distribution: Distribution into liver, lungs, prostate, muscle, and bone is reported in several studies. Vd has been measured to be 27.7 L. Metabolism: It has several metabolic pathways, from hydroxylation, oxidative deamination to decarboxylation. Route of elimination: 70–78% of the drug is eliminated in the urine. Half-life: 1 h. |
| Chloramphenicol |  | Mechanism of action: It binds to the L16 protein of the 50S ribosomal subunit, preventing the transfer of amino acids to growing peptide chains and subsequent protein synthesis. Absorption: Topical application to the eye may also be intraocular and little systemic absorption. Distribution: It has no volume of distribution. Metabolism: It is not metabolized. Route of elimination: Not very clear information. Half-life: 1.5–3.5 h. |
| Povidone-iodine | 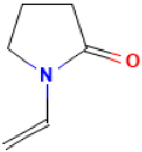 | Mechanism of action: It is a complex that gradually releases free iodine at the application site. Free iodine penetrates the cell wall, resulting in disruption of protein and nucleic acid structure and synthesis. Absorption: Topical application; it is not absorbed. Distribution: It has no volume of distribution. Metabolism: It is not metabolized. Route of elimination: It is not eliminated. Half-life: Not applicable. |
| Silver Nitrate | 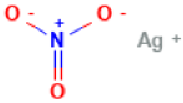 | Pharmacokinetics information is not available. |
| Type of Ct Infection | Treatment Options |
|---|---|
| Uncomplicated genital chlamydia | Doxycycline 100 mg orally twice a day for 7 days Azithromycin 1 g orally as a single dose |
| Tetracycline 500 mg orally four times a day for 7 days | |
| Erythromycin 500 mg orally four times a day for 7 days | |
| Levofloxacin 500 mg orally once daily for 7 days | |
| Anorectal chlamydial infection | Doxycycline 100 mg orally twice a day for 7 days over |
| Azithromycin 1 g orally as a single dose | |
| Genital chlamydial infection in pregnant women | Azithromycin 1 g orally as a single dose |
| Amoxicillin 500 mg orally three times a day for 7 days | |
| Erythromycin 500 mg orally four times a day for 7 days | |
| Lymphogranuloma venereum (LGV) | Doxycycline 100 mg orally twice daily for 21 days Azithromycin 1 g orally, weekly for 3 weeks Erythromycin 500 mg orally four times a day for 21 days |
| Ophthalmia neonatorum | |
| Conjunctivitis | Azithromycin 20 mg/kg/day orally, one dose daily for 3 days |
| Erythromycin 50 mg/kg/day orally, in four divided doses daily for 14 days | |
| Ocular prophylaxis | Tetracycline hydrochloride 1% eye ointment |
| Erythromycin 0.5% eye ointment | |
| Povidone iodine 2.5% solution | |
| Silver nitrate 1% solution | |
| Chloramphenicol 1% eye ointment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.; Marques, L.; Vieira-Baptista, P.; Sousa, C.; Vale, N. Therapeutic Options for Chlamydia trachomatis Infection: Present and Future. Antibiotics 2022, 11, 1634. https://doi.org/10.3390/antibiotics11111634
Rodrigues R, Marques L, Vieira-Baptista P, Sousa C, Vale N. Therapeutic Options for Chlamydia trachomatis Infection: Present and Future. Antibiotics. 2022; 11(11):1634. https://doi.org/10.3390/antibiotics11111634
Chicago/Turabian StyleRodrigues, Rafaela, Lara Marques, Pedro Vieira-Baptista, Carlos Sousa, and Nuno Vale. 2022. "Therapeutic Options for Chlamydia trachomatis Infection: Present and Future" Antibiotics 11, no. 11: 1634. https://doi.org/10.3390/antibiotics11111634
APA StyleRodrigues, R., Marques, L., Vieira-Baptista, P., Sousa, C., & Vale, N. (2022). Therapeutic Options for Chlamydia trachomatis Infection: Present and Future. Antibiotics, 11(11), 1634. https://doi.org/10.3390/antibiotics11111634








