Antimicrobial and Cell-Penetrating Peptides: Understanding Penetration for the Design of Novel Conjugate Antibiotics
Abstract
1. Introduction
2. Mechanisms of Membrane Translocation by Membrane Active Peptides
3. In Vitro Assays for Characterisation of Membrane Active Peptides
3.1. Membrane Disruption Assays
3.1.1. Bacterial Outer and Inner Membrane Permeability Assays (NPN/ONPG)
3.1.2. Calcein Leakage Assay
3.2. Measuring Peptides within Cells
3.2.1. Conjugation of Membrane Active Peptides with Fluorescent Molecules for Cellular Visualisation
3.2.2. Flow Cytometry
3.2.3. Real-Time Luminescence Assay
3.3. Feature and Membrane Selectivity
3.3.1. Antibacterial Activity Assay
3.3.2. Haemolysis Assay
3.3.3. Cytotoxicity Studies
3.4. Investigating Membrane Active Peptides with Biophysical Techniques
3.4.1. Circular Dichroism (CD)
3.4.2. Differential Scanning Calorimetry
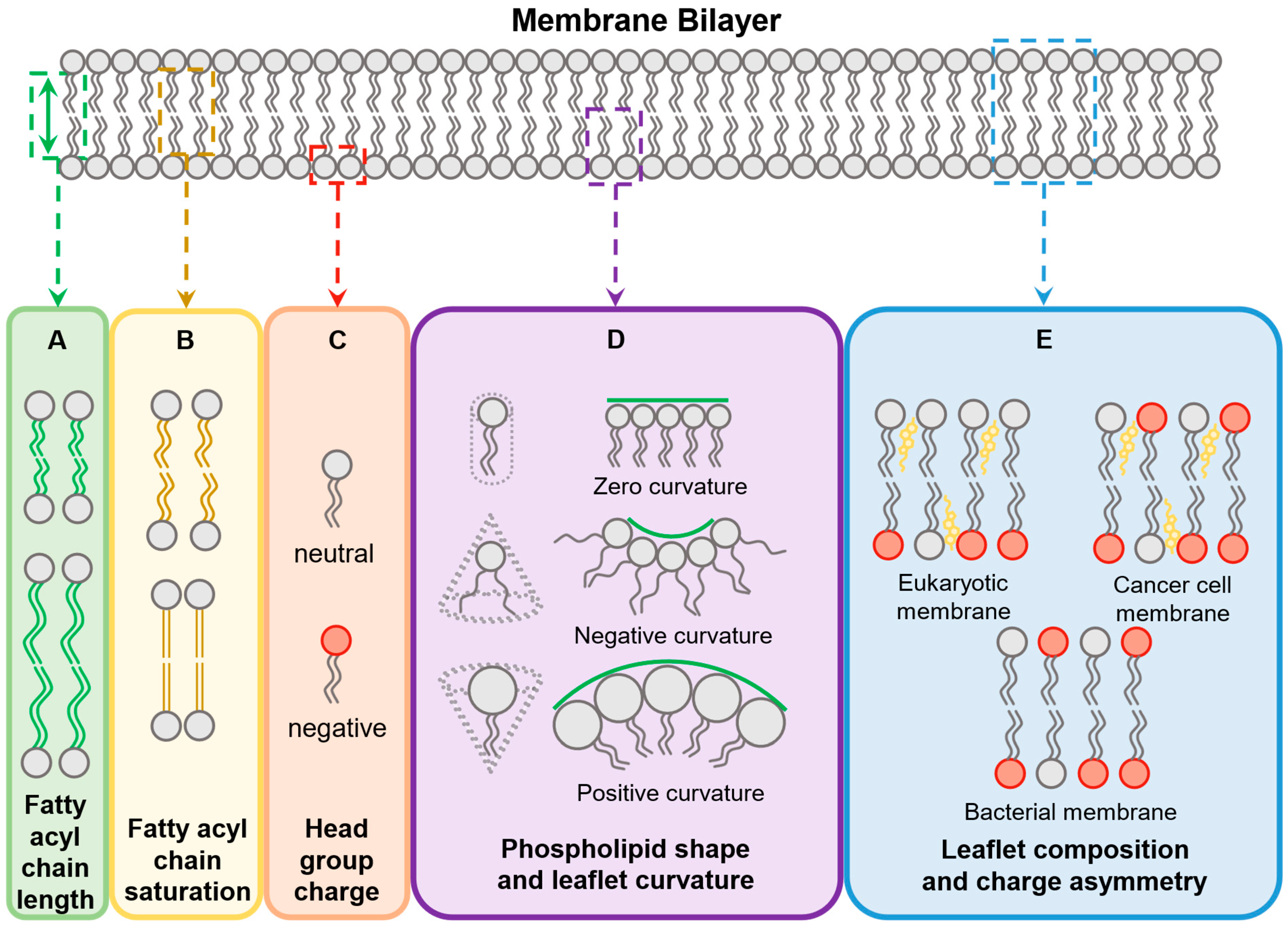
4. Variables Influencing the Biological Activity of Membrane-Active Peptides
4.1. Membrane Features
4.1.1. Phospholipid Composition and Distribution
4.1.2. Fatty Acyl Chains
4.1.3. Membrane Curvature
4.1.4. Presence of Cholesterol
4.1.5. The Role of Sphingomyelin and Ceramide
4.1.6. Glucosaminoglycans
4.1.7. Contribution of Membrane Proteins
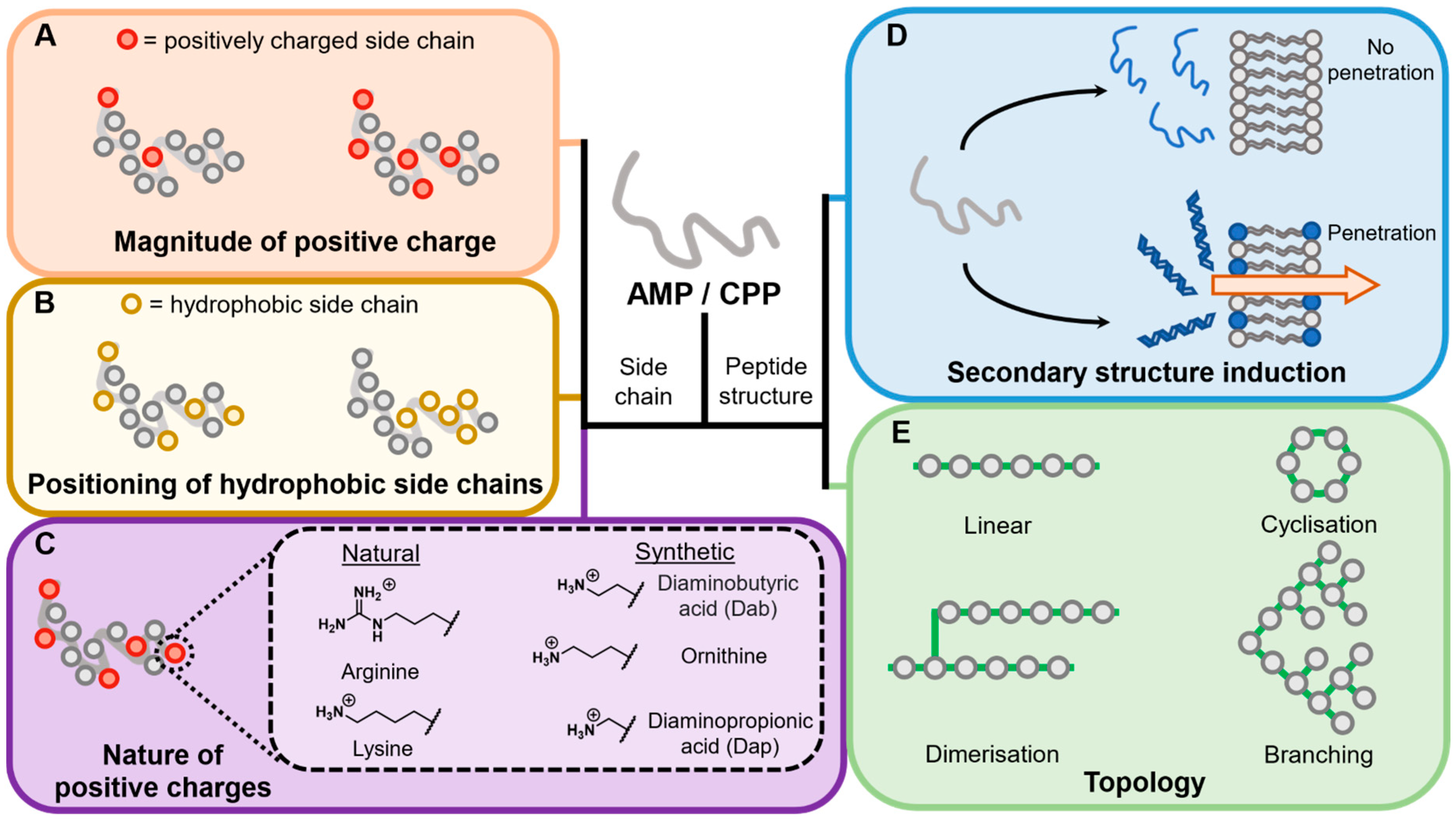
4.2. Peptide Features
4.2.1. Overall Charge
4.2.2. Nature of Amino Acid Side Chains
Positively Charged Side Chains
Natural Side Chain Variants
Hydrophobicity
4.2.3. Induction of Secondary Structure
4.2.4. Stereochemistry
4.2.5. Differential Topological Design
Cyclisation
Dimerisation
Branching
5. Use of Membrane Active Peptides as Vectors for the Development of Novel Antimicrobials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubos, R.J. Studies on a Bactericidal Agent Extracted from a Soil Bacillus: I. Preparation of the agent. Its activity in vitro. J. Exp. Med. 1939, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Kjos, M.; Nes, I.F.; Diep, D.B.; Lotfipour, F. Natural Antimicrobial Peptides from Bacteria: Characteristics and Potential Applications to Fight against Antibiotic Resistance. J. Appl. Microbiol. 2012, 113, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Roy, A. Antimicrobial Peptides as Potential Therapeutic Agents: A Review. Int. J. Pept. Res Ther. 2021, 27, 555–577. [Google Scholar] [CrossRef]
- Brogden, K.A.; Ackermann, M.; McCray, P.B.; Tack, B.F. Antimicrobial Peptides in Animals and Their Role in Host Defences. Int. J. Antimicrob. Agents 2003, 22, 465–478. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant Antimicrobial Peptides: Structures, Functions, and Applications. Bot. Stud. 2021, 62, 5. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Avci, F.G.; Akbulut, B.S.; Ozkirimli, E. Membrane Active Peptides and Their Biophysical Characterization. Biomolecules 2018, 8, 77. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up Immunity: How Antimicrobial Peptides Have Multiple Roles in Immune Defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous Functional Domains of Chemically Synthesized Human Immunodeficiency Virus Tat Trans-Activator Protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Mann, D.A.; Frankel, A.D. Endocytosis and Targeting of Exogenous HIV-1 Tat Protein. EMBO J. 1991, 10, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Vivès, E.; Brodin, P.; Lebleu, B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The Third Helix of the Antennapedia Homeodomain Translocates through Biological Membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Kato, D.; Miyazawa, K.; Ruas, M.; Starborg, M.; Wada, I.; Oka, T.; Sakai, T.; Peters, G.; Hara, E. Features of Replicative Senescence Induced by Direct Addition of Antennapedia-P16INK4A Fusion Protein to Human Diploid Fibroblasts. FEBS Lett. 1998, 427, 203–208. [Google Scholar] [CrossRef]
- Pooga, M.; Soomets, U.; Hällbrink, M.; Valkna, A.; Saar, K.; Rezaei, K.; Kahl, U.; Hao, J.-X.; Xu, X.-J.; Wiesenfeld-Hallin, Z.; et al. Cell Penetrating PNA Constructs Regulate Galanin Receptor Levels and Modify Pain Transmission in Vivo. Nat. Biotechnol. 1998, 16, 857–861. [Google Scholar] [CrossRef]
- Derossi, D.; Calvet, S.; Trembleau, A.; Brunissen, A.; Chassaing, G.; Prochiantz, A. Cell Internalization of the Third Helix of the Antennapedia Homeodomain Is Receptor-Independent. J. Biol. Chem. 1996, 271, 18188–18193. [Google Scholar] [CrossRef]
- Mandal, D.; Nasrolahi Shirazi, A.; Parang, K. Cell-Penetrating Homochiral Cyclic Peptides as Nuclear-Targeting Molecular Transporters. Angew. Chem. Int. Ed. 2011, 50, 9633–9637. [Google Scholar] [CrossRef]
- Islam, M.M.; Odahara, M.; Yoshizumi, T.; Oikawa, K.; Kimura, M.; Su’Etsugu, M.; Numata, K. Cell-Penetrating Peptide-Mediated Transformation of Large Plasmid DNA into Escherichia Coli. ACS Synth. Biol. 2019, 8, 1215–1218. [Google Scholar] [CrossRef]
- Good, L.; Awasthi, S.K.; Dryselius, R.; Larsson, O.; Nielsen, P.E. Bactericidal Antisense Effects of Peptide–PNA Conjugates. Nat. Biotechnol. 2001, 19, 360–364. [Google Scholar] [CrossRef]
- Geller, B.L.; Deere, J.D.; Stein, D.A.; Kroeker, A.D.; Moulton, H.M.; Iversen, P.L. Inhibition of Gene Expression in Escherichia Coli by Antisense Phosphorodiamidate Morpholino Oligomers. Antimicrob. Agents Chemother. 2003, 47, 3233–3239. [Google Scholar] [CrossRef]
- Yavari, N.; Goltermann, L.; Nielsen, P.E. Uptake, Stability, and Activity of Antisense Anti- AcpP PNA-Peptide Conjugates in Escherichia Coli and the Role of SbmA. ACS Chem. Biol. 2021, 16, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Lai, G.H.; Schmidt, N.W.; Sun, V.Z.; Rodriguez, A.R.; Tong, R.; Tang, L.; Cheng, J.; Deming, T.J.; Kamei, D.T.; et al. Translocation of HIV TAT Peptide and Analogues Induced by Multiplexed Membrane and Cytoskeletal Interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 16883–16888. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Comparative Mechanisms of Protein Transduction Mediated by Cell-Penetrating Peptides in Prokaryotes. J. Membr. Biol. 2015, 248, 355–368. [Google Scholar] [CrossRef]
- Gomarasca, M.; Martins, T.F.C.; Greune, L.; Hardwidge, P.R.; Schmidt, M.A.; Rüter, C. Bacterium-Derived Cell-Penetrating Peptides Deliver Gentamicin to Kill Intracellular Pathogens. Antimicrob. Agents Chemother. 2017, 61, e02545-16. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Vitali, A.; Scorciapino, M.A.; Rinaldi, A.C.; Petruzzelli, R.; Brancatisano, F.L.; Esin, S.; Stringaro, A.; Colone, M.; Luzi, C.; et al. PH-Dependent Disruption of Escherichia Coli ATCC 25922 and Model Membranes by the Human Antimicrobial Peptides Hepcidin 20 and 25. FEBS J. 2013, 280, 2842–2854. [Google Scholar] [CrossRef] [PubMed]
- Rajarao, G.K.; Nekhotiaeva, N.; Good, L. Peptide-Mediated Delivery of Green Fluorescent Protein into Yeasts and Bacteria. FEMS Microbiol. Lett. 2002, 215, 267–272. [Google Scholar] [CrossRef]
- Nischan, N.; Herce, H.D.; Natale, F.; Bohlke, N.; Budisa, N.; Cardoso, M.C.; Hackenberger, C.P.R. Covalent Attachment of Cyclic TAT Peptides to GFP Results in Protein Delivery into Live Cells with Immediate Bioavailability. Angew. Chem. Int. Ed. 2015, 54, 1950–1953. [Google Scholar] [CrossRef]
- Watkins, C.L.; Schmaljohann, D.; Futaki, S.; Jones, A.T. Low Concentration Thresholds of Plasma Membranes for Rapid Energy-Independent Translocation of a Cell-Penetrating Peptide. Biochem. J. 2009, 420, 179–189. [Google Scholar] [CrossRef]
- Neundorf, I. Antimicrobial and Cell-Penetrating Peptides: How to Understand Two Distinct Functions despite Similar Physicochemical Properties. Adv. Exp. Med. Biol. 2019, 1117, 93–109. [Google Scholar] [CrossRef]
- Lee, H.-M.; Ren, J.; Tran, K.M.; Jeon, B.-M.; Park, W.-U.; Kim, H.; Lee, K.E.; Oh, Y.; Choi, M.; Kim, D.-S.; et al. Identification of Efficient Prokaryotic Cell-Penetrating Peptides with Applications in Bacterial Biotechnology. Commun. Biol. 2021, 4, 205. [Google Scholar] [CrossRef]
- Eiríksdóttir, E.; Konate, K.; Langel, Ü.; Divita, G.; Deshayes, S. Secondary Structure of Cell-Penetrating Peptides Controls Membrane Interaction and Insertion. Biochim. Biophys. Acta 2010, 1798, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Joanne, P.; Galanth, C.; Goasdoué, N.; Nicolas, P.; Sagan, S.; Lavielle, S.; Chassaing, G.; El Amri, C.; Alves, I.D. Lipid Reorganization Induced by Membrane-Active Peptides Probed Using Differential Scanning Calorimetry. Biochim. Biophys. Acta 2009, 1788, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Splith, K.; Neundorf, I. Antimicrobial Peptides with Cell-Penetrating Peptide Properties and Vice Versa. Eur. Biophys. J. 2011, 40, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Saido-Sakanaka, H.; Ishibashi, J.; Momotani, E.; Amano, F.; Yamakawa, M. In Vitro and in Vivo Activity of Antimicrobial Peptides Synthesized Based on the Insect Defensin. Peptides 2004, 25, 19–27. [Google Scholar] [CrossRef]
- Lung, F.D.T.; Wang, K.S.; Liao, Z.J.; Hsu, S.K.; Song, F.Y.; Liou, C.C.; Wu, Y.S. Discovery of Potent Antimicrobial Peptide Analogs of Ixosin-B. Bioorg. Med. Chem. Lett. 2012, 22, 4185–4188. [Google Scholar] [CrossRef]
- Wagstaff, J.M.; Balmforth, M.; Lewis, N.; Dods, R.; Rowland, C.; Van Rietschoten, K.; Chen, L.; Harrison, H.; Skynner, M.J.; Dawson, M.; et al. An Assay for Periplasm Entry Advances the Development of Chimeric Peptide Antibiotics. ACS Infect. Dis. 2020, 6, 2355–2361. [Google Scholar] [CrossRef]
- Lee, H.; Lim, S.I.; Shin, S.H.; Lim, Y.; Koh, J.W.; Yang, S. Conjugation of Cell-Penetrating Peptides to Antimicrobial Peptides Enhances Antibacterial Activity. ACS Omega 2019, 4, 15694–15701. [Google Scholar] [CrossRef]
- Inoue, G.; Toyohara, D.; Mori, T.; Muraoka, T. Critical Side Chain Effects of Cell-Penetrating Peptides for Transporting Oligo Peptide Nucleic Acids in Bacteria. ACS Appl. Bio Mater. 2021, 4, 3462–3468. [Google Scholar] [CrossRef]
- Funderburg, N.; Lederman, M.M.; Feng, Z.; Drage, M.G.; Jadlowsky, J.; Harding, C.V.; Weinberg, A.; Sieg, S.F. Human β-Defensin-3 Activates Professional Antigen-Presenting Cells via Toll-like Receptors 1 and 2. Proc. Natl. Acad. Sci. USA 2007, 104, 18631–18635. [Google Scholar] [CrossRef]
- Manabe, T.; Kawasaki, K. D-Form KLKLLLLLKLK-NH2 Peptide Exerts Higher Antimicrobial Properties than Its L-Form Counterpart via an Association with Bacterial Cell Wall Components. Sci. Rep. 2017, 7, 43384. [Google Scholar] [CrossRef]
- Falciani, C.; Lozzi, L.; Pollini, S.; Luca, V.; Carnicelli, V.; Brunetti, J.; Lelli, B.; Bindi, S.; Scali, S.; Di Giulio, A.; et al. Isomerization of an Antimicrobial Peptide Broadens Antimicrobial Spectrum to Gram-Positive Bacterial Pathogens. PLoS ONE 2012, 7, e46259. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.; Boman, A.; Wåhlin, B.; Drain, C.M.; Andreu, D.; Boman, H.G.; Merrifield, R.B. All-D Amino Acid-Containing Channel-Forming Antibiotic Peptides. Proc. Natl. Acad. Sci. USA 1990, 87, 4761–4765. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, K.; Kida, Y.; Zhang, Y.; Shimizu, T.; Kuwano, K. Antimicrobial Activity and Stability to Proteolysis of Small Linear Cationic Peptides with D-Amino Acid Substitutions. Microbiol. Immunol. 2002, 46, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hao, D.; Chen, Y.; Xu, Y.; Tan, J.; Huang, Y.; Li, F.; Chen, Y. Inhibitory Effects and Mechanisms of Physiological Conditions on the Activity of Enantiomeric Forms of an α-Helical Antibacterial Peptide against Bacteria. Peptides 2011, 32, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Vasil, A.I.; Rehaume, L.; Mant, C.T.; Burns, J.L.; Vasil, M.L.; Hancock, R.E.W.; Hodges, R.S. Comparison of Biophysical and Biologic Properties of α-Helical Enantiomeric Antimicrobial Peptides. Chem. Biol. Drug Des. 2006, 67, 162–173. [Google Scholar] [CrossRef]
- Desgranges, S.; Ruddle, C.C.; Burke, L.P.; McFadden, T.M.; O’Brien, J.E.; Fitzgerald-Hughes, D.; Humphreys, H.; Smyth, T.P.; Devocelle, M. β-Lactam-Host Defence Peptide Conjugates as Antibiotic Prodrug Candidates Targeting Resistant Bacteria. RSC Adv. 2012, 2, 2480–2492. [Google Scholar] [CrossRef]
- Jacob, B.; Rajasekaran, G.; Kim, E.Y.; Park, I.-S.; Bang, J.-K.; Shin, S.Y. The Stereochemical Effect of SMAP-29 and SMAP-18 on Bacterial Selectivity, Membrane Interaction and Anti-Inflammatory Activity. Amino Acids 2016, 48, 1241–1251. [Google Scholar] [CrossRef]
- Gronewold, A.; Horn, M.; Ranđelović, I.; Tóvári, J.; Muñoz Vázquez, S.; Schomäcker, K.; Neundorf, I. Characterization of a Cell-Penetrating Peptide with Potential Anticancer Activity. ChemMedChem 2017, 12, 42–49. [Google Scholar] [CrossRef]
- Falla, T.J.; Karunaratne, D.N.; Hancock, R.E.W. Mode of Action of the Antimicrobial Peptide Indolicidin. J. Biol. Chem. 1996, 271, 19298–19303. [Google Scholar] [CrossRef]
- Yang, C.-H.; Chen, Y.-C.; Peng, S.-Y.; Tsai, A.P.-Y.; Lee, T.J.-F.; Yen, J.-H.; Liou, J.-W. An Engineered Arginine-Rich α-Helical Antimicrobial Peptide Exhibits Broad-Spectrum Bactericidal Activity against Pathogenic Bacteria and Reduces Bacterial Infections in Mice. Sci. Rep. 2018, 8, 14602. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Tokuda, H.; Funakoshi, S.; Fujii, N.; Miyajima, K. Orientational and Aggregational States of Magainin 2 in Phospholipid Bilayers. Biochemistry 1994, 33, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Ramada, M.H.S.; Brand, G.D.; Abrão, F.Y.; Oliveira, M.; Filho, J.L.C.; Galbieri, R.; Gramacho, K.P.; Prates, M.V.; Bloch, C. Encrypted Antimicrobial Peptides from Plant Proteins. Sci. Rep. 2017, 7, 13263. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing Antimicrobial Peptides: Form Follows Function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Agüero-Chapin, G.; Galpert-Cañizares, D.; Domínguez-Pérez, D.; Marrero-Ponce, Y.; Pérez-Machado, G.; Teijeira, M.; Antunes, A. Emerging Computational Approaches for Antimicrobial Peptide Discovery. Antibiotics 2022, 11, 936. [Google Scholar] [CrossRef]
- de Oliveira, E.C.L.; da Costa, K.S.; Taube, P.S.; Lima, A.H.; de Souza de Sales Junior, C. Biological Membrane-Penetrating Peptides: Computational Prediction and Applications. Front. Cell. Infect. Microbiol. 2022, 12, 838259. [Google Scholar] [CrossRef]
- Liu, B.R.; Huang, Y.W.; Lee, H.J. Mechanistic Studies of Intracellular Delivery of Proteins by Cell-Penetrating Peptides in Cyanobacteria. BMC Microbiol. 2013, 13, 57. [Google Scholar] [CrossRef]
- Fuchs, S.M.; Raines, R.T. Pathway for Polyarginine Entry into Mammalian Cells. Biochemistry 2004, 43, 2438–2444. [Google Scholar] [CrossRef]
- Alves, I.D.; Jiao, C.Y.; Aubry, S.; Aussedat, B.; Burlina, F.; Chassaing, G.; Sagan, S. Cell Biology Meets Biophysics to Unveil the Different Mechanisms of Penetratin Internalization in Cells. Biochim. Biophys. Acta 2010, 1798, 2231–2239. [Google Scholar] [CrossRef]
- Lee, H.-J.; Huang, Y.-W.; Chiou, S.-H.; Aronstam, R.S. Polyhistidine Facilitates Direct Membrane Translocation of Cell-Penetrating Peptides into Cells. Sci. Rep. 2019, 9, 9398. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Cong, L.; Yan, B.; Jiang, Y. The Role of Cell-penetrating Peptides in Potential Anti-cancer Therapy. Clin. Transl. Med. 2022, 12, e822. [Google Scholar] [CrossRef]
- Szabó, I.; Yousef, M.; Soltész, D.; Bató, C.; Mező, G.; Bánóczi, Z. Redesigning of Cell-Penetrating Peptides to Improve Their Efficacy as a Drug Delivery System. Pharmaceutics 2022, 14, 907. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.M.; Horton, K.L.; Kelley, S.O. Cell-Penetrating Peptides as Delivery Vehicles for Biology and Medicine. Org. Biomol. Chem. 2008, 6, 2242–2255. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.P.; Guimarães, M.S.; Rabelo, J.; Belén, L.H.; Perecin, C.J.; Farías, J.G.; Santos, J.H.P.M.; Rangel-Yagui, C.O. Recent Advances in the Design of Antimicrobial Peptide Conjugates. J. Mater. Chem. B 2022, 10, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Ebenhan, T.; Gheysens, O.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M.M. Antimicrobial Peptides: Their Role as Infection-Selective Tracers for Molecular Imaging. Biomed. Res. Int. 2014, 2014, 867381. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yoneyama, S.; Murase, O.; Miyajima, K. Transbilayer Transport of Ions and Lipids Coupled with Mastoparan X Translocation. Biochemistry 1996, 35, 8450–8456. [Google Scholar] [CrossRef] [PubMed]
- Pouny, Y.; Rapaport, D.; Mor, A.; Nicolas, P.; Shai, Y. Interaction of Antimicrobial Dermaseptin and Its Fluorescently Labeled Analogs with Phospholipid Membranes. Biochemistry 1992, 31, 12416–12423. [Google Scholar] [CrossRef]
- Lee, M.T.; Hung, W.C.; Chen, F.Y.; Huang, H.W. Many-Body Effect of Antimicrobial Peptides: On the Correlation between Lipid’s Spontaneous Curvature and Pore Formation. Biophys. J. 2005, 89, 4006–4016. [Google Scholar] [CrossRef]
- Lundberg, P.; Langel, Ü. A Brief Introduction to Cell-Penetrating Peptides. J. Mol. Recognit. 2003, 16, 227–233. [Google Scholar] [CrossRef]
- Zorko, M.; Langel, Ü. Studies of Cell-Penetrating Peptides by Biophysical Methods. Q. Rev. Biophys. 2022, 55, e3. [Google Scholar] [CrossRef]
- Loh, B.; Grant, C.; Hancock, R.E. Use of the Fluorescent Probe 1-N-Phenylnaphthylamine to Study the Interactions of Aminoglycoside Antibiotics with the Outer Membrane of Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 1984, 26, 546–551. [Google Scholar] [CrossRef]
- Eriksson, M.; Nielsen, P.E.; Good, L. Cell Permeabilization and Uptake of Antisense Peptide-Peptide Nucleic Acid (PNA) into Escherichia Coli. J. Biol. Chem. 2002, 277, 7144–7147. [Google Scholar] [CrossRef] [PubMed]
- Gravel, J.; Paradis-Bleau, C.; Schmitzer, A.R. Adaptation of a Bacterial Membrane Permeabilization Assay for Quantitative Evaluation of Benzalkonium Chloride as a Membrane-Disrupting Agent. Med. Chem. Commun. 2017, 8, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Han, F.F.; Gao, Y.H.; Luan, C.; Xie, Y.G.; Liu, Y.F.; Wang, Y.Z. Comparing Bacterial Membrane Interactions and Antimicrobial Activity of Porcine Lactoferricin-Derived Peptides. J. Dairy Sci. 2013, 96, 3471–3487. [Google Scholar] [CrossRef]
- Salassi, S.; Simonelli, F.; Bartocci, A.; Rossi, G. A Martini Coarse-Grained Model of the Calcein Fluorescent Dye. J. Phys. D Appl. Phys. 2018, 51, 384002. [Google Scholar] [CrossRef]
- Janosi, L.; Gorfe, A.A. Simulating POPC and POPC/POPG Bilayers: Conserved Packing and Altered Surface Reactivity. J. Chem. Theory Comput. 2010, 6, 3267–3273. Available online: https://pubs.acs.org/doi/pdf/10.1021/ct100381g (accessed on 22 July 2022). [CrossRef]
- Yang, S.-T.; Shin, S.Y.; Hahm, K.-S.; Kim, J.I. Different Modes in Antibiotic Action of Tritrpticin Analogs, Cathelicidin-Derived Trp-Rich and Pro/Arg-Rich Peptides. Biochim. Biophys. Acta 2006, 1758, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Machida, S.; Niimi, S.; Shi, X.; Ando, Y.; Yu, Y. Design of a Novel Membrane-Destabilizing Peptide Selectively Acting on Acidic Liposomes. Biosci. Biotechnol. Biochem. 2000, 64, 985–994. [Google Scholar] [CrossRef]
- Patel, K.D.; Mohid, S.A.; Dutta, A.; Arichthota, S.; Bhunia, A.; Haldar, D.; Sarojini, V. Synthesis and Antibacterial Study of Cell-Penetrating Peptide Conjugated Trifluoroacetyl and Thioacetyl Lysine Modified Peptides. Eur. J. Med. Chem. 2021, 219, 113447. [Google Scholar] [CrossRef]
- McHenry, A.J.; Sciacca, M.F.M.; Brender, J.R.; Ramamoorthy, A. Does Cholesterol Suppress the Antimicrobial Peptide Induced Disruption of Lipid Raft Containing Membranes? Biochim. Biophys. Acta 2012, 1818, 3019–3024. [Google Scholar] [CrossRef]
- Hedegaard, S.F.; Derbas, M.S.; Lind, T.K.; Kasimova, M.R.; Christensen, M.V.; Michaelsen, M.H.; Campbell, R.A.; Jorgensen, L.; Franzyk, H.; Cárdenas, M.; et al. Fluorophore Labeling of a Cell-Penetrating Peptide Significantly Alters the Mode and Degree of Biomembrane Interaction. Sci. Rep. 2018, 8, 6327. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Miszkiewicz, J.; Trylska, J. Conformational Changes of Anoplin, w-Mreb1–9, and (Kff)3 k Peptides near the Membranes. Int. J. Mol. Sci. 2020, 21, 9672. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, K.; Islam, M.M.; Horii, Y.; Yoshizumi, T.; Numata, K. Screening of a Cell-Penetrating Peptide Library in Escherichia Coli: Relationship between Cell Penetration Efficiency and Cytotoxicity. ACS Omega 2018, 3, 16489–16499. [Google Scholar] [CrossRef]
- Cavaco, M.; Pérez-Peinado, C.; Valle, J.; Silva, R.D.M.; Correia, J.D.G.; Andreu, D.; Castanho, M.A.R.B.; Neves, V. To What Extent Do Fluorophores Bias the Biological Activity of Peptides? A Practical Approach Using Membrane-Active Peptides as Models. Front. Bioeng. Biotechnol. 2020, 8, 552035. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.; Christensen, M.V.; Staerk, D.; Franzyk, H.; Nielsen, H.M. Fluorophore Labeling of a Cell-Penetrating Peptide Induces Differential Effects on Its Cellular Distribution and Affects Cell Viability. Biochim. Biophys. Acta 2017, 1859, 2483–2494. [Google Scholar] [CrossRef]
- Tünnemann, G.; Martin, R.M.; Haupt, S.; Patsch, C.; Edenhofer, F.; Cardoso, M.C. Cargo-Dependent Mode of Uptake and Bioavailability of TAT-Containing Proteins and Peptides in Living Cells. FASEB J. 2006, 20, 1775–1784. [Google Scholar] [CrossRef]
- Harreither, E.; Rydberg, H.A.; Åmand, H.L.; Jadhav, V.; Fliedl, L.; Benda, C.; Esteban, M.A.; Pei, D.; Borth, N.; Grillari-Voglauer, R.; et al. Characterization of a Novel Cell Penetrating Peptide Derived from Human Oct4. Cell Regen. 2014, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, D.; Yokoi, Y.; Inoue, G.; Muraoka, T.; Mori, T. Abiotic Factors Promote Cell Penetrating Peptide Permeability in Enterobacteriaceae Models. Front. Microbiol. 2019, 10, 2534. [Google Scholar] [CrossRef]
- Dhanda, G.; Mukherjee, R.; Basak, D.; Haldar, J. Small-Molecular Adjuvants with Weak Membrane Perturbation Potentiate Antibiotics against Gram-Negative Superbugs. ACS Infect. Dis. 2022, 8, 1086–1097. [Google Scholar] [CrossRef]
- Illien, F.; Rodriguez, N.; Amoura, M.; Joliot, A.; Pallerla, M.; Cribier, S.; Burlina, F.; Sagan, S. Quantitative Fluorescence Spectroscopy and Flow Cytometry Analyses of Cell-Penetrating Peptides Internalization Pathways: Optimization, Pitfalls, Comparison with Mass Spectrometry Quantification. Sci. Rep. 2016, 6, 36938. [Google Scholar] [CrossRef]
- Brand, G.D.; Ramada, M.H.S.; Genaro-Mattos, T.C.; Bloch, C. Towards an Experimental Classification System for Membrane Active Peptides. Sci. Rep. 2018, 8, 1194. [Google Scholar] [CrossRef]
- Zeiders, S.M.; Chmielewski, J. Antibiotic-Cell-Penetrating Peptide Conjugates Targeting Challenging Drug-Resistant and Intracellular Pathogenic Bacteria. Chem. Biol. Drug Des. 2021, 98, 762–778. [Google Scholar] [CrossRef]
- Reinhardt, A.; Horn, M.; Pieper Gen Schmauck, J.; Bröhl, A.; Giernoth, R.; Oelkrug, C.; Schubert, A.; Neundorf, I. Novel Imidazolium Salt-Peptide Conjugates and Their Antimicrobial Activity. Bioconjug. Chem. 2014, 25, 2166–2174. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between Hemolytic Activity, Cytotoxicity and Systemic in Vivo Toxicity of Synthetic Antimicrobial Peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex Vivo Red Blood Cell Hemolysis Assay for the Evaluation of PH-Responsive Endosomolytic Agents for Cytosolic Delivery of Biomacromolecular Drugs. J. Vis. Exp. 2013, 9, 50166. [Google Scholar] [CrossRef]
- Yang, S.-T.; Lee, J.Y.; Kim, H.-J.; Eu, Y.-J.; Shin, S.Y.; Hahm, K.-S.; Kim, J.I. Contribution of a Central Proline in Model Amphipathic Alpha-Helical Peptides to Self-Association, Interaction with Phospholipids, and Antimicrobial Mode of Action. FEBS J. 2006, 273, 4040–4054. [Google Scholar] [CrossRef]
- Zhang, S.-K.; Song, J.; Gong, F.; Li, S.-B.; Chang, H.-Y.; Xie, H.-M.; Gao, H.-W.; Tan, Y.-X.; Ji, S.-P. Design of an α-Helical Antimicrobial Peptide with Improved Cell-Selective and Potent Anti-Biofilm Activity. Sci. Rep. 2016, 6, 27394. [Google Scholar] [CrossRef]
- Takada, H.; Tsuchiya, K.; Demizu, Y. Helix-Stabilized Cell-Penetrating Peptides for Delivery of Antisense Morpholino Oligomers: Relationships among Helicity, Cellular Uptake, and Antisense Activity. Bioconjug. Chem. 2022, 33, 1311–1318. [Google Scholar] [CrossRef]
- Riahifard, N.; Mozaffari, S.; Aldakhil, T.; Nunez, F.; Alshammari, Q.; Alshammari, S.; Yamaki, J.; Parang, K.; Tiwari, R. Design, Synthesis, and Evaluation of Amphiphilic Cyclic and Linear Peptides Composed of Hydrophobic and Positively-Charged Amino Acids as Antibacterial Agents. Molecules 2018, 23, 2722. [Google Scholar] [CrossRef]
- Luther, A.; Urfer, M.; Zahn, M.; Müller, M.; Wang, S.Y.; Mondal, M.; Vitale, A.; Hartmann, J.B.; Sharpe, T.; Monte, F.L.; et al. Chimeric Peptidomimetic Antibiotics against Gram-Negative Bacteria. Nature 2019, 576, 452–458. [Google Scholar] [CrossRef]
- Liu, J.; Afshar, S. In Vitro Assays: Friends or Foes of Cell-Penetrating Peptides. Int. J. Mol. Sci. 2020, 21, 4719. [Google Scholar] [CrossRef]
- Haney, E.F.; Nathoo, S.; Vogel, H.J.; Prenner, E.J. Induction of Non-Lamellar Lipid Phases by Antimicrobial Peptides: A Potential Link to Mode of Action. Chem. Phys. Lipids 2010, 163, 82–93. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugishita, K.; Ishibe, N.; Ueha, M.; Nakata, S.; Miyajima, K.; Epand, R.M. Relationship of Membrane Curvature to the Formation of Pores by Magainin 2. Biochemistry 1998, 37, 11856–11863. [Google Scholar] [CrossRef] [PubMed]
- (Binder, H.; Lindblom, G. Charge-Dependent Translocation of the Trojan Peptide Penetratin across Lipid Membranes. Biophys. J. 2003, 85, 982–995. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Domadia, P.N.; Bhunia, A.; Ramamoorthy, A.; Bhattacharjya, S. Structure, Interactions, and Antibacterial Activities of MSI-594 Derived Mutant Peptide MSI-594F5A in Lipopolysaccharide Micelles: Role of the Helical Hairpin Conformation in Outer-Membrane Permeabilization. J. Am. Chem. Soc. 2010, 132, 18417–18428. [Google Scholar] [CrossRef]
- McMahon, H.T.; Gallop, J.L. Membrane Curvature and Mechanisms of Dynamic Cell Membrane Remodelling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef]
- Morrow, M.R.; Whitehead, J.P.; Lu, D. Chain-Length Dependence of Lipid Bilayer Properties near the Liquid Crystal to Gel Phase Transition. Biophys. J. 1992, 63, 18–27. [Google Scholar] [CrossRef]
- Ciobanasu, C.; Siebrasse, J.P.; Kubitscheck, U. Cell-Penetrating HIV1 TAT Peptides Can Generate Pores in Model Membranes. Biophys. J. 2010, 99, 153–162. [Google Scholar] [CrossRef]
- Ciobanasu, C.; Rzeszutek, A.; Kubitscheck, U.; Willumeit, R. NKCS, a Mutant of the NK-2 Peptide, Causes Severe Distortions and Perforations in Bacterial, But Not Human Model Lipid Membranes. Molecules 2015, 20, 6941–6958. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Thennarasu, S.; Lee, D.-K.; Tan, A.; Maloy, L. Solid-State NMR Investigation of the Membrane-Disrupting Mechanism of Antimicrobial Peptides MSI-78 and MSI-594 Derived from Magainin 2 and Melittin. Biophys. J. 2006, 91, 206–216. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J.; Mainali, L.; Raguz, M. High Cholesterol/Low Cholesterol: Effects in Biological Membranes: A Review. Cell Biochem. Biophys. 2017, 75, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.R.; McHenry, A.J.; Ramamoorthy, A. Does Cholesterol Play a Role in the Bacterial Selectivity of Antimicrobial Peptides? Front. Immunol. 2012, 3, 195. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.; Rowat, A.C.; Brief, E.; Hsueh, Y.W.; Thewalt, J.L.; Zuckermann, M.J.; Ipsen, J.H. Universal Behavior of Membranes with Sterols. Biophys. J. 2006, 90, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Pae, J.; Säälik, P.; Liivamägi, L.; Lubenets, D.; Arukuusk, P.; Langel, Ü.; Pooga, M. Translocation of Cell-Penetrating Peptides across the Plasma Membrane Is Controlled by Cholesterol and Microenvironment Created by Membranous Proteins. J. Control. Release 2014, 192, 103–113. [Google Scholar] [CrossRef]
- Verdurmen, W.P.R.; Thanos, M.; Ruttekolk, I.R.; Gulbins, E.; Brock, R. Cationic Cell-Penetrating Peptides Induce Ceramide Formation via Acid Sphingomyelinase: Implications for Uptake. J. Control. Release 2010, 147, 171–179. [Google Scholar] [CrossRef]
- Wallbrecher, R.; Ackels, T.; Olea, R.A.; Klein, M.J.; Caillon, L.; Schiller, J.; Bovée-Geurts, P.H.; van Kuppevelt, T.H.; Ulrich, A.S.; Spehr, M.; et al. Membrane Permeation of Arginine-Rich Cell-Penetrating Peptides Independent of Transmembrane Potential as a Function of Lipid Composition and Membrane Fluidity. J. Control. Release 2017, 256, 68–78. [Google Scholar] [CrossRef]
- Sahoo, H.; Schwille, P. Influence of Glycosaminoglycans on Lipid Dynamics in Supported Phospholipid Bilayers. Soft Matter 2013, 9, 3859–3865. [Google Scholar] [CrossRef]
- Laremore, T.N.; Zhang, F.; Dordick, J.S.; Liu, J.; Linhardt, R.J. Recent Progress and Applications in Glycosaminoglycan and Heparin Research. Curr. Opin. Chem. Biol. 2009, 13, 633–640. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Nadai, R.; Kimura, H.; Nishitsuji, K.; Uchimura, K.; Sakai-Kato, K.; Kawakami, K.; Shigenaga, A.; Kawakami, T.; Otaka, A.; et al. Enthalpy-Driven Interactions with Sulfated Glycosaminoglycans Promote Cell Membrane Penetration of Arginine Peptides. Biochim. Biophys. Acta 2016, 1858, 1339–1349. [Google Scholar] [CrossRef]
- Johansson, A.C.V.; Lindahl, E. Protein Contents in Biological Membranes Can Explain Abnormal Solvation of Charged and Polar Residues. Proc. Natl. Acad. Sci. USA 2009, 106, 15684–15689. [Google Scholar] [CrossRef]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A Novel Human Antibiotic Peptide Secreted by Sweat Glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef]
- Paulmann, M.; Arnold, T.; Linke, D.; Özdirekcan, S.; Kopp, A.; Gutsmann, T.; Kalbacher, H.; Wanke, I.; Schuenemann, V.J.; Habeck, M.; et al. Structure-Activity Analysis of the Dermcidin-Derived Peptide DCD-1L, an Anionic Antimicrobial Peptide Present in Human Sweat. J. Biol. Chem. 2012, 287, 8434–8443. [Google Scholar] [CrossRef]
- Wadhwani, P.; Reichert, J.; Bürck, J.; Ulrich, A.S. Antimicrobial and Cell-Penetrating Peptides Induce Lipid Vesicle Fusion by Folding and Aggregation. Eur. Biophys. J. 2012, 41, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lättig-Tünnemann, G.; Prinz, M.; Hoffmann, D.; Behlke, J.; Palm-Apergi, C.; Morano, I.; Herce, H.D.; Cardoso, M.C. Backbone Rigidity and Static Presentation of Guanidinium Groups Increases Cellular Uptake of Arginine-Rich Cell-Penetrating Peptides. Nat. Commun. 2011, 2, 453. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.J.; Kim, D.T.; Steinman, L.; Fathman, C.G.; Rothbard, J.B.; Rothbard, C.G.; Polyarginine, J.B. Polyarginine Enters Cells More Efficiently than Other Polycationic Homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The Design, Synthesis, and Evaluation of Molecules That Enable or Enhance Cellular Uptake: Peptoid Molecular Transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13003–13008. [Google Scholar] [CrossRef]
- Lan, Y.; Langlet-Bertin, B.; Abbate, V.; Vermeer, L.S.; Kong, X.; Sullivan, K.E.; Leborgne, C.; Scherman, D.; Hider, R.C.; Drake, A.F.; et al. Incorporation of 2,3-Diaminopropionic Acid into Linear Cationic Amphipathic Peptides Produces PH-Sensitive Vectors. ChemBioChem 2010, 11, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.W.; Tai, K.P.; Kamdar, K.; Mishra, A.; Lai, G.H.; Zhao, K.; Ouellette, A.J.; Wong, G.C.L. Arginine in α-Defensins: Differential Effects on Bactericidal Activity Correspond to Geometry of Membrane Curvature Generation and Peptide-Lipid Phase Behavior. J. Biol. Chem. 2012, 287, 21866–21872. [Google Scholar] [CrossRef]
- Ezzat, K.; EL Andaloussi, S.; Zaghloul, E.M.; Lehto, T.; Lindberg, S.; Moreno, P.M.D.; Viola, J.R.; Magdy, T.; Abdo, R.; Guterstam, P.; et al. PepFect 14, a Novel Cell-Penetrating Peptide for Oligonucleotide Delivery in Solution and as Solid Formulation. Nucleic Acids Res. 2011, 39, 5284–5298. [Google Scholar] [CrossRef]
- Vaara, M. Novel Derivatives of Polymyxins. J. Antimicrob. Chemother. 2013, 68, 1213–1219. [Google Scholar] [CrossRef]
- Vaara, M.; Siikanen, O.; Apajalahti, J.; Fox, J.; Frimodt-Møller, N.; He, H.; Poudyal, A.; Li, J.; Nation, R.L.; Vaara, T. A Novel Polymyxin Derivative That Lacks the Fatty Acid Tail and Carries Only Three Positive Charges Has Strong Synergism with Agents Excluded by the Intact Outer Membrane. Antimicrob. Agents Chemother. 2010, 54, 3341–3346. [Google Scholar] [CrossRef]
- Mant, C.T.; Jiang, Z.; Gera, L.; Davis, T.; Nelson, K.L.; Bevers, S.; Hodges, R.S. De Novo Designed Amphipathic α-Helical Antimicrobial Peptides Incorporating Dab and Dap Residues on the Polar Face To Treat the Gram-Negative Pathogen, Acinetobacter Baumannii. J. Med. Chem. 2019, 62, 3354–3366. [Google Scholar] [CrossRef] [PubMed]
- Rothbard, J.B.; Jessop, T.C.; Lewis, R.S.; Murray, B.A.; Wender, P.A. Role of Membrane Potential and Hydrogen Bonding in the Mechanism of Translocation of Guanidinium-Rich Peptides into Cells. J. Am. Chem. Soc. 2004, 126, 9506–9507. [Google Scholar] [CrossRef]
- Jobin, M.-L.; Blanchet, M.; Henry, S.; Chaignepain, S.; Manigand, C.; Castano, S.; Lecomte, S.; Burlina, F.; Sagan, S.; Alves, I.D. The Role of Tryptophans on the Cellular Uptake and Membrane Interaction of Arginine-Rich Cell Penetrating Peptides. Biochim. Biophys. Acta 2015, 1848, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Staubitz, P.; Peschel, A.; Nieuwenhuizen, W.F.; Otto, M.; Götz, F.; Jung, G.; Jack, R.W. Structure–Function Relationships in the Tryptophan-Rich, Antimicrobial Peptide Indolicidin. J. Pept. Sci. 2001, 7, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Q.; Yuan, X.; Cao, Y.; Yuan, Y.; Yin, H.; Ding, X.; Zhu, Z.; Luo, S.-Z. How Charge Distribution Influences the Function of Membrane-Active Peptides: Lytic or Cell-Penetrating? Int. J. Biochem. Cell Biol. 2017, 83, 71–75. [Google Scholar] [CrossRef]
- Qian, Z.; Liu, T.; Liu, Y.-Y.; Briesewitz, R.; Barrios, A.M.; Jhiang, S.M.; Pei, D. Efficient Delivery of Cyclic Peptides into Mammalian Cells with Short Sequence Motifs. ACS Chem. Biol. 2013, 8, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Tsubery, H.; Ofek, I.; Cohen, S.; Fridkin, M. The Functional Association of Polymyxin B with Bacterial Lipopolysaccharide Is Stereospecific: Studies on Polymyxin B Nonapeptide. Biochemistry 2000, 39, 11837–11844. [Google Scholar] [CrossRef]
- Hoyer, J.; Schatzschneider, U.; Schulz-Siegmund, M.; Neundorf, I. Dimerization of a Cell-Penetrating Peptide Leads to Enhanced Cellular Uptake and Drug Delivery. Beilstein J. Org. Chem. 2012, 8, 1788–1797. [Google Scholar] [CrossRef]
- Sommer, P.; Fluxa, V.S.; Darbre, T.; Reymond, J.-L. Proteolysis of Peptide Dendrimers. ChemBioChem 2009, 10, 1527–1536. [Google Scholar] [CrossRef]
- McLaughlin, M.I.; van der Donk, W.A. The Fellowship of the Rings: Macrocyclic Antibiotic Peptides Reveal an Anti-Gram-Negative Target. Biochemistry 2020, 59, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowich-Knipp, S.J.; Jois, D.S.; Siahaan, T.J. The Effect of Conformation on the Solution Stability of Linear vs. Cyclic RGD Peptides. J. Pept. Res. 1999, 53, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, G.A.; Blattes, E.; Buschor, S.; Biswas, R.; Kammer, S.M.; Darbre, T.; Reymond, J.-L. Designed Cell Penetrating Peptide Dendrimers Efficiently Internalize Cargo into Cells. Chem. Commun. 2014, 50, 7254–7257. [Google Scholar] [CrossRef]
- Pires, J.; Siriwardena, T.N.; Stach, M.; Tinguely, R.; Kasraian, S.; Luzzaro, F.; Leib, S.L.; Darbre, T.; Reymond, J.-L.; Endimiani, A. In Vitro Activity of the Novel Antimicrobial Peptide Dendrimer G3KL against Multidrug-Resistant Acinetobacter Baumannii and Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 7915–7918. [Google Scholar] [CrossRef] [PubMed]
- Stach, M.; Maillard, N.; Kadam, R.U.; Kalbermatter, D.; Meury, M.; Page, M.G.P.; Fotiadis, D.; Darbre, T.; Reymond, J.-L. Membrane Disrupting Antimicrobial Peptide Dendrimers with Multiple Amino Termini. Med. Chem. Commun. 2012, 3, 86–89. [Google Scholar] [CrossRef]
- Takeshima, K.; Chikushi, A.; Lee, K.K.; Yonehara, S.; Matsuzaki, K. Translocation of Analogues of the Antimicrobial Peptides Magainin and Buforin across Human Cell Membranes. J. Biol. Chem. 2003, 278, 1310–1315. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Willyard, C. The Drug-Resistant Bacteria That Pose the Greatest Health Threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Hansford, K.A.; Gong, Y.; Butler, M.S.; Muldoon, C.; Huang, J.X.; Ramu, S.; Silva, A.B.; Cheng, M.; Kavanagh, A.M.; et al. Protein-Inspired Antibiotics Active against Vancomycin- and Daptomycin-Resistant Bacteria. Nat. Commun. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Umstätter, F.; Domhan, C.; Hertlein, T.; Ohlsen, K.; Mühlberg, E.; Kleist, C.; Zimmermann, S.; Beijer, B.; Klika, K.D.; Haberkorn, U.; et al. Vancomycin Resistance Is Overcome by Conjugation of Polycationic Peptides. Angew. Chem. Int. Ed. 2020, 59, 8823–8827. [Google Scholar] [CrossRef]
- Antonoplis, A.; Zang, X.; Wegner, T.; Wender, P.A.; Cegelski, L. Vancomycin–Arginine Conjugate Inhibits Growth of Carbapenem-Resistant E. Coli and Targets Cell-Wall Synthesis. ACS Chem. Biol. 2019, 14, 2065–2070. [Google Scholar] [CrossRef]
- Shi, W.; Chen, F.; Zou, X.; Jiao, S.; Wang, S.; Hu, Y.; Lan, L.; Tang, F.; Huang, W. Design, Synthesis, and Antibacterial Evaluation of Vancomycin-LPS Binding Peptide Conjugates. Bioorg. Med. Chem. Lett. 2021, 45, 128122. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, S.; Xian, W.; Schmidt, N.W.; Kordbacheh, S.; Lieng, J.; Wang, J.; Zarmer, S.; Germain, S.S.; Voyen, L.; Thulin, J.; et al. Designing Hybrid Antibiotic Peptide Conjugates To Cross Bacterial Membranes. Bioconjug. Chem. 2017, 28, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Jelinkova, P.; Splichal, Z.; Jimenez, A.M.J.; Haddad, Y.; Mazumdar, A.; Sur, V.P.; Milosavljevic, V.; Kopel, P.; Buchtelova, H.; Guran, R.; et al. Novel Vancomycin-Peptide Conjugate as Potent Antibacterial Agent against Vancomycin-Resistant Staphylococcus Aureus. Infect. Drug Resist. 2018, 11, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; O’Brien-Simpson, N.M.; Holden, J.A.; Otvos, L.; Reynolds, E.C.; Separovic, F.; Hossain, M.A.; Wade, J.D. Covalent Conjugation of Cationic Antimicrobial Peptides with a β-Lactam Antibiotic Core. Pept. Sci. 2018, 110, e24059. [Google Scholar] [CrossRef]
- Yamauchi, R.; Kawano, K.; Yamaoka, Y.; Taniguchi, A.; Yano, Y.; Takasu, K.; Matsuzaki, K. Development of Antimicrobial Peptide–Antibiotic Conjugates to Improve the Outer Membrane Permeability of Antibiotics Against Gram-Negative Bacteria. ACS Infect. Dis. 2022, 8, 2339–2347. [Google Scholar] [CrossRef]
- Huo, S.; Chen, C.; Lyu, Z.; Zhang, S.; Wang, Y.; Nie, B.; Yue, B. Overcoming Planktonic and Intracellular Staphylococcus Aureus-Associated Infection with a Cell-Penetrating Peptide-Conjugated Antimicrobial Peptide. ACS Infect. Dis. 2020, 6, 3147–3162. [Google Scholar] [CrossRef]
- Frimodt-Møller, J.; Campion, C.; Nielsen, P.E.; Løbner-Olesen, A. Translocation of Non-Lytic Antimicrobial Peptides and Bacteria Penetrating Peptides across the Inner Membrane of the Bacterial Envelope. Curr. Genet. 2022, 68, 83–90. [Google Scholar] [CrossRef]
- Gerstmans, H.; Rodríguez-Rubio, L.; Lavigne, R.; Briers, Y. From Endolysins to Artilysin®s: Novel Enzyme-Based Approaches to Kill Drug-Resistant Bacteria. Biochem. Soc. Trans. 2016, 44, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, Y.; Dai, J.; Zhou, J.; Yu, R.; Zhang, C. Design SMAP29-LysPA26 as a Highly Efficient Artilysin against Pseudomonas Aeruginosa with Bactericidal and Antibiofilm Activity. Microbiol. Spectr. 2021, 9, e0054621. [Google Scholar] [CrossRef] [PubMed]
- Defraine, V.; Schuermans, J.; Grymonprez, B.; Govers, S.K.; Aertsen, A.; Fauvart, M.; Michiels, J.; Lavigne, R.; Briers, Y. Efficacy of Artilysin Art-175 against Resistant and Persistent Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2016, 60, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]


| “Cargo” * | Vector * | Reference |
|---|---|---|
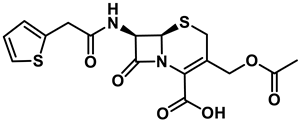 Cephalothin | 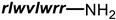 D-Bac8C(Leu2,5) ** | [46] |
 Vancomycin | 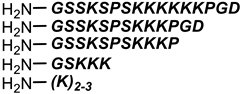 Polylysine Variants | [151] |
 Hexa-arginine (R6) | [152] | |
 Hecate | [156] | |
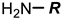 Arginine | [153] | |
 LL-15, RR-6 and cyclo-KC-10 | [154] | |
 Tobramycin |  Penetratin (top) and variants | [155] |
 Cephalosporin core |  CA(1–7)M(2–9)NH2, MS1-78, Chex1-Arg20 | [157] |
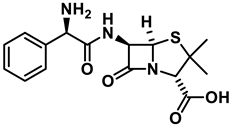 Ampicillin | 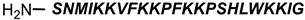 9P2-2 | [158] |
 Magainin and M15 |  Nona-arginine (R9) | [37] |
 KR-12 |  Tat peptide | [159] |
| Histone Deacetylase (enzyme) *** | [78] | |
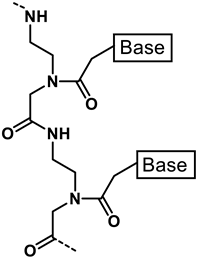 PNA | 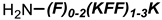 KFF motif peptide and variants | [19,21] |
 Endolysins (enzyme) *** |  SMAP-29 | [162,163] |
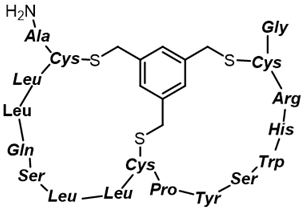 Bi-cyclic peptide (“Bicycle”) |  KFF motif peptide and DRAMP 1853 | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjicharalambous, A.; Bournakas, N.; Newman, H.; Skynner, M.J.; Beswick, P. Antimicrobial and Cell-Penetrating Peptides: Understanding Penetration for the Design of Novel Conjugate Antibiotics. Antibiotics 2022, 11, 1636. https://doi.org/10.3390/antibiotics11111636
Hadjicharalambous A, Bournakas N, Newman H, Skynner MJ, Beswick P. Antimicrobial and Cell-Penetrating Peptides: Understanding Penetration for the Design of Novel Conjugate Antibiotics. Antibiotics. 2022; 11(11):1636. https://doi.org/10.3390/antibiotics11111636
Chicago/Turabian StyleHadjicharalambous, Andreas, Nikolaos Bournakas, Hector Newman, Michael J. Skynner, and Paul Beswick. 2022. "Antimicrobial and Cell-Penetrating Peptides: Understanding Penetration for the Design of Novel Conjugate Antibiotics" Antibiotics 11, no. 11: 1636. https://doi.org/10.3390/antibiotics11111636
APA StyleHadjicharalambous, A., Bournakas, N., Newman, H., Skynner, M. J., & Beswick, P. (2022). Antimicrobial and Cell-Penetrating Peptides: Understanding Penetration for the Design of Novel Conjugate Antibiotics. Antibiotics, 11(11), 1636. https://doi.org/10.3390/antibiotics11111636






