Detection of an Enterococcus faecium Carrying a Double Copy of the PoxtA Gene from Freshwater River, Italy
Abstract
:1. Introduction
2. Results
2.1. Detection of Oxazolidinone Resistance Genes in Florfenicol-Resistant Enterococci and Antimicrobial Susceptibility Profiles
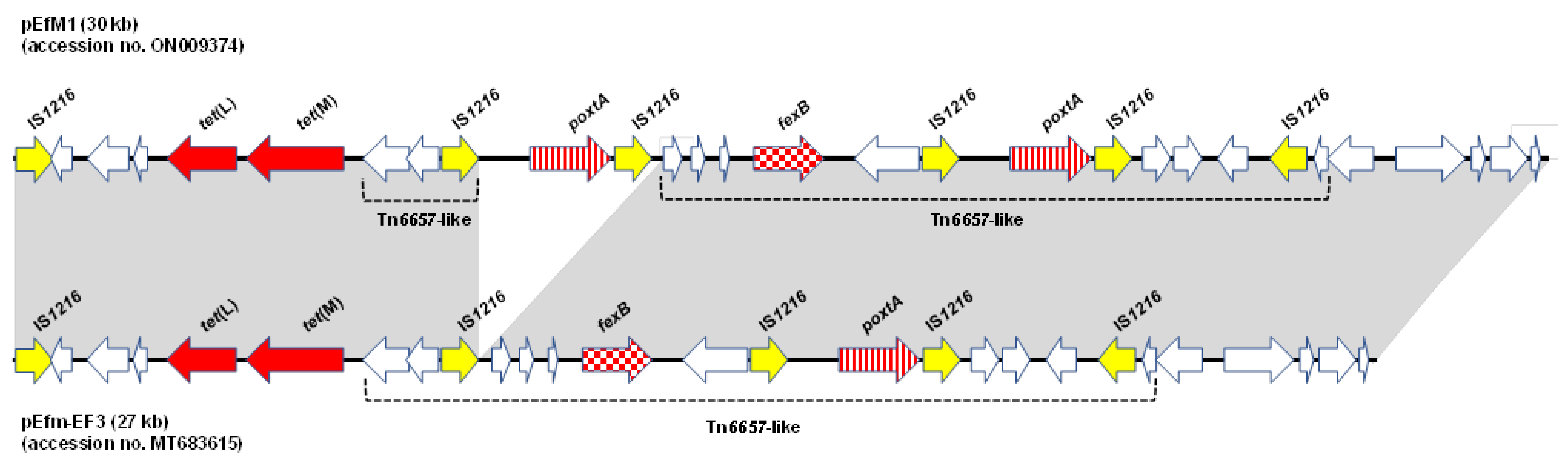
2.2. Location of PoxtA Gene and Detection of Circular Forms
2.3. Transferability in Filter Mating Experiments and in Aquaria Microcosm Assays
2.4. WGS Analysis
3. Materials and Methods
3.1. Sampling, Sample Processing, and Enterococcal Isolation
3.2. Genotypic and Phenotypic Characterization
3.3. S1-PFGE, Southern Blotting, and Hybridization Assays
3.4. Detection of Circular Forms
3.5. Mating Experiments
3.6. WGS and Sequence Analysis
3.7. Nucleotide Sequence Accession Numbers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byappanahalli, M.N.; Nevers, M.B.; Korajkic, A.; Staley, Z.R.; Harwood, V.J. Enterococci in the Environment. Microbiol. Mol. Biol. Rev. 2012, 76, 685–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Solache, M.; Rice, L.B. The Enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [Green Version]
- Bender, J.K.; Cattoir, V.; Hegstad, K.; Coque, T.M.; Westh, H.; Hammerum, A.M.; Schaffer, K.; Burns, K.; Murchan, S.; Novais, C.; et al. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: Towards a common nomenclature. Drug Resist. Updat. 2018, 40, 25–39. [Google Scholar] [CrossRef]
- Wilson, D.N.; Schluenzen, F.; Harms, J.M.; Starosta, A.L.; Connell, S.R.; Fucini, P. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc. Natl. Acad. Sci. USA 2008, 105, 13339–13344. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, R.D.; Schreckenberger, P.; Graham, M.B.; Kelkar, S.; DenBesten, K.; Quinn, J.P. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001, 357, 1179. [Google Scholar] [CrossRef]
- Mendes, R.E.; Deshpande, L.; Streit, J.M.; Sader, H.S.; Castanheira, M.; Hogan, P.A.; Flamm, R.K. ZAAPS programme results for 2016: An activity and spectrum analysis of linezolid using clinical isolates from medical centres in 42 countries. J. Antimicrob. Chemother. 2018, 73, 1880–1887. [Google Scholar] [CrossRef] [Green Version]
- Brenciani, A.; Morroni, G.; Schwarz, S.; Giovanetti, E. Oxazolidinones: Mechanisms of resistance and mobile genetic elements involved. J. Antimicrob. Chemother. 2022, 77, 2596–2621. [Google Scholar] [CrossRef]
- Long, K.S.; Poehlsgaard, J.; Kehrenberg, C.; Schwarz, S.; Vester, B. The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob. Agents Chemother. 2006, 50, 2500–2505. [Google Scholar] [CrossRef]
- Crowe-McAuliffe, C.; Murina, V.; Turnbull, K.J.; Huch, S.; Kasari, M.; Takada, H.; Nersisyan, L.; Sundsfjord, A.; Hegstad, K.; Atkinson, G.C.; et al. Structural basis for PoxtA-mediated resistance to phenicol and oxazolidinone antibiotics. Nat. Commun. 2022, 13, 1860. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Zhang, W.; Du, X.-D.; Krüger, H.; Feßler, A.T.; Ma, S.; Zhu, Y.; Wu, C.; Shen, J.; Wang, Y. Mobile oxazolidinone resistance genes in Gram-positive and Gram-negative bacteria. Clin. Microbiol. Rev. 2021, 34, e0018820. [Google Scholar] [CrossRef] [PubMed]
- Fioriti, S.; Morroni, G.; Coccitto, S.N.; Brenciani, A.; Antonelli, A.; Di Pilato, V.; Baccani, I.; Pollini, S.; Cucco, L.; Morelli, A.; et al. Detection of oxazolidinone resistance genes and characterization of genetic environments in enterococci of swine origin, Italy. Microorganims 2020, 8, 2021. [Google Scholar] [CrossRef] [PubMed]
- Coccitto, S.N.; Cinthi, M.; Fioriti, S.; Morroni, G.; Simoni, S.; Vignaroli, C.; Garofalo, C.; Mingoia, M.; Brenciani, A.; Giovanetti, E. Linezolid-resistant Enterococcus gallinarum isolate of swine origin carrying cfr, optrA and poxtA genes. J. Antimicrob. Chemother. 2022, 77, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Fioriti, S.; Coccitto, S.N.; Cedraro, N.; Simoni, S.; Morroni, G.; Brenciani, A.; Mangiaterra, G.; Vignaroli, C.; Vezzulli, L.; Biavasco, F.; et al. Linezolid resistance genes in enterococci isolated from sediment and zooplankton in two Italian coastal areas. Appl. Environ. Microbiol. 2021, 87, e02958-20. [Google Scholar] [CrossRef]
- Biggel, M.; Nüesch-Inderbinen, M.; Jans, C.; Stevens, M.J.A.; Stephan, R. Genetic context of optrA and poxtA in florfenicol-resistant enterococci isolated from flowing surface water in Switzerland. Antimicrob. Agents Chemother. 2021, 65, e0108321. [Google Scholar] [CrossRef]
- Ruiz-Ripa, L.; Feßler, A.T.; Hanke, D.; Sanz, S.; Olarte, C.; Eichhorn, I.; Schwarz, S.; Torres, C. Detection of poxtA- and optrA-carrying E. faecium isolates in air samples of a Spanish swine farm. J. Glob. Antimicrob. Resist. 2020, 22, 28–31. [Google Scholar] [CrossRef]
- Wang, Y.; Lia, X.; Fu, Y.; Chen, Y.; Wang, Y.; Ye, D.; Wang, C.; Hu, X.; Zhou, L.; Du, J.; et al. Association of florfenicol residues with the abundance of oxazolidinone resistance genes in livestock manures. J. Hazard. Mater. 2020, 399, 123059. [Google Scholar] [CrossRef]
- Cinthi, M.; Coccitto, S.N.; Fioriti, S.; Morroni, G.; Simoni, S.; Vignaroli, C.; Magistrali, C.F.; Albini, E.; Brenciani, A.; Giovanetti, E. Occurrence of a plasmid cocarrying cfr(D) and poxtA2 linezolid resistance genes in Enterococcus faecalis and Enterococcus casseliflavus from porcine manure, Italy. J. Antimicrob. Chemother. 2022, 77, 598–603. [Google Scholar] [CrossRef]
- Subbiah, M.; Mitchell, S.M.; Ullman, J.L.; Call, D.R. β-lactams and florfenicol antibiotics remain bioactive in soils while ciprofloxacin, neomycin, and tetracycline are neutralized. Appl. Environ. Microbiol. 2011, 77, 7255–7260. [Google Scholar] [CrossRef]
- European Medicines Agency. European Surveillance of Veterinary Antimicrobial Consumption, 2018. Sales of Veterinary Antimicrobial Agents in 30 European Countries in 2016; EMA/275982/2018; European Medicines Agency: London, UK, 2018. [Google Scholar]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a Potential Hotspot for Antibiotic Resistance Dissemination by Horizontal Gene Transfer Events. Vet Sci. 2020, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Sant’Ana, A.S. Manure-borne pathogens as an important source of water contamination: An update on the dynamics of pathogen survival/transport as well as practical risk mitigation strategies. Int. J. Hyg. Environ. Health 2020, 227, 113524. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.M.; Antonelli, A.; Brenciani, A.; Di Pilato, V.; Morroni, G.; Pollini, S.; Fioriti, S.; Giovanetti, E.; Rossolini, G.M. Characterization of Tn6349, a novel mosaic transposon carrying poxtA, cfr and other resistance determinants, inserted in the chromosome of an ST5-MRSA-II strain of clinical origin. J. Antimicrob. Chemother. 2019, 74, 2870–2875. [Google Scholar] [PubMed]
- Shan, X.; Li, X.-S.; Wang, N.; Schwarz, S.; Zhang, S.-M.; Li, D.; Du, X.-D. Studies on the role of IS1216E in the formation and dissemination of poxtA-carrying plasmids in an Enterococcus faecium clade A1 isolate. J. Antimicrob. Chemother. 2020, 75, 3126–3130. [Google Scholar] [CrossRef]
- Chandler, M.; Mahillon, J. Insertion Sequences Revisited. In Mobile DNA II; Craig, N., Craigie, R., Gellert, M., Lambowitz, A., Eds.; ASM Press: Washington, DC, USA, 2001; pp. 305–366. [Google Scholar]
- Clewell, D.B.; Weaver, K.E.; Dunny, G.M.; Coque, T.M.; Francia, M.V.; Hayes, F. Extrachromosomal and mobile elements in enterococci: Transmission, maintenance, and epidemiology. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Di Sante, L.; Morroni, G.; Brenciani, A.; Vignaroli, C.; Antonelli, A.; D’Andrea, M.M.; Di Cesare, A.; Giovanetti, E.; Varaldo, P.E.; Rossolini, G.M.; et al. pHTβ-promoted mobilization of non-conjugative resistance plasmids from Enterococcus faecium to Enterococcus faecalis. J. Antimicrob. Chemother. 2017, 72, 2447–2453. [Google Scholar] [CrossRef]
- Brenciani, A.; Fioriti, S.; Morroni, G.; Cucco, L.; Morelli, A.; Pezzotti, G.; Paniccià, M.; Antonelli, A.; Magistrali, C.F.; Rossolini, G.M.; et al. Detection in Italy of a porcine Enterococcus faecium isolate carrying the novel phenicol-oxazolidinone-tetracycline resistance gene poxtA. J. Antimicrob. Chemother. 2019, 74, 817–818. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Barton, B.M.; Harding, G.P.; Zuccarelli, A.J. A general method for detecting and sizing large plasmids. Anal. Biochem. 1995, 226, 235–240. [Google Scholar] [CrossRef]
- Brenciani, A.; Morroni, G.; Pollini, S.; Tiberi, E.; Mingoia, M.; Varaldo, P.E.; Rossolini, G.M.; Giovanetti, E. Characterization of novel conjugative multiresistance plasmids carrying cfr from linezolid-resistant Staphylococcus epidermidis clinical isolates from Italy. J. Antimicrob. Chemother. 2016, 71, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Werner, G.; Klare, I.; Witte, W. Arrangement of the vanA gene cluster in enterococci of different ecological origin. FEMS. Microbiol. Lett. 1997, 155, 55–61. [Google Scholar] [CrossRef]
- Prieto, A.M.; van Schaik, W.; Rogers, M.R.C.; Coque, T.M.; Baquero, F.; Corander, J.; Willems, R.J.L. Global emergence and dissemination of enterococci as nosocomial pathogens: Attack of the clones? Front. Microbiol. 2016, 26, 788. [Google Scholar]
- Monticelli, J.; Knezevich, A.; Luzzati, R.; Di Bella, S. Clinical management of non-faecium non-faecalis vancomycin-resistant enterococci infection. Focus on Enterococcus gallinarum and Enterococcus casseliflavus/flavescens. J. Infect. Chemother. 2018, 24, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, G. Detection and Various Environmental Factors of Antibiotic Resistance Gene Horizontal Transfer. Environ. Res. 2022, 212, 113267. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Coman, C.; Szekeres, E.; Teban-Man, A.; Carpa, R.; Butiuc-Keul, A. Molecular typing reveals environmental dispersion of antibiotic-resistant enterococci under anthropogenic pressure. Antibiotics 2022, 11, 1213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, W.; Schwarz, S.; Xu, Q.; Yang, Q.; Wang, L.; Liu, S.; Zhang, W. Characterization of an MDR Lactobacillus salivarius isolate harbouring the phenicol-oxazolidinone-tetracycline resistance gene poxtA. J. Antimicrob. Chemother. 2022, 77, 2125–2129. [Google Scholar] [CrossRef]
- Shan, X.; Yang, M.; Wang, N.; Schwarz, S.; Li, D.; Du, X.-D. Plasmid fusion and recombination events that occurred during conjugation of poxtA-carrying plasmids in enterococci. Microbiol. Spectrum. 2022, 10, e01505-21. [Google Scholar] [CrossRef]
| Recipient | Transfer Frequency | Transconjugants e MIC (mg/L) of: f | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FFC | CHL | LZD | TZD | TE | ERY | ||||
| Filter mating experiments | |||||||||
| E. faecium 64/3 | 5.8 × 10−2 | 64 | 32 | 4 | 0.5 | 128 | 128 | ||
| E. faecium Ef1 | 5 × 10−3 | 128 | 64 | 8 | 1 | 128 | >128 | ||
| Aquaria microcosm assays | |||||||||
| Aquarium A a | 24 h | E. faecium 64/3 | 6.3 × 10−5 | 64 | 32 | 4 | 0.5 | 128 | >128 |
| 48 h | E. faecium 64/3 | 1.8 × 10−7 | 64 | 32 | 4 | 0.5 | 128 | >128 | |
| 96 h | E. faecium 64/3 | ND d | |||||||
| Aquarium B b | 24 h | E. faecium 64/3 | 5 × 10−4 | 64 | 32 | 4 | 0.5 | 128 | >128 |
| 48 h | E. faecium 64/3 | 2.7 × 10−7 | 64 | 32 | 4 | 0.5 | 128 | >128 | |
| 96 h | E. faecium 64/3 | ND | |||||||
| Aquarium C c | 24 h | E. faecium 64/3 | 9.2 × 10−4 | 64 | 32 | 4 | 0.5 | 128 | >128 |
| 48 h | E. faecium 64/3 | 2.9 × 10−7 | 64 | 32 | 4 | 0.5 | 128 | >128 | |
| 96 h | E. faecium 64/3 | ND | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinthi, M.; Coccitto, S.N.; Morroni, G.; D’Achille, G.; Brenciani, A.; Giovanetti, E. Detection of an Enterococcus faecium Carrying a Double Copy of the PoxtA Gene from Freshwater River, Italy. Antibiotics 2022, 11, 1618. https://doi.org/10.3390/antibiotics11111618
Cinthi M, Coccitto SN, Morroni G, D’Achille G, Brenciani A, Giovanetti E. Detection of an Enterococcus faecium Carrying a Double Copy of the PoxtA Gene from Freshwater River, Italy. Antibiotics. 2022; 11(11):1618. https://doi.org/10.3390/antibiotics11111618
Chicago/Turabian StyleCinthi, Marzia, Sonia Nina Coccitto, Gianluca Morroni, Gloria D’Achille, Andrea Brenciani, and Eleonora Giovanetti. 2022. "Detection of an Enterococcus faecium Carrying a Double Copy of the PoxtA Gene from Freshwater River, Italy" Antibiotics 11, no. 11: 1618. https://doi.org/10.3390/antibiotics11111618
APA StyleCinthi, M., Coccitto, S. N., Morroni, G., D’Achille, G., Brenciani, A., & Giovanetti, E. (2022). Detection of an Enterococcus faecium Carrying a Double Copy of the PoxtA Gene from Freshwater River, Italy. Antibiotics, 11(11), 1618. https://doi.org/10.3390/antibiotics11111618







