The Use of Star Anise-Cinnamon Essential Oil as an Alternative Antibiotic in Prevention of Salmonella Infections in Yellow Chickens
Abstract
:1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration (MIC) of SCEO
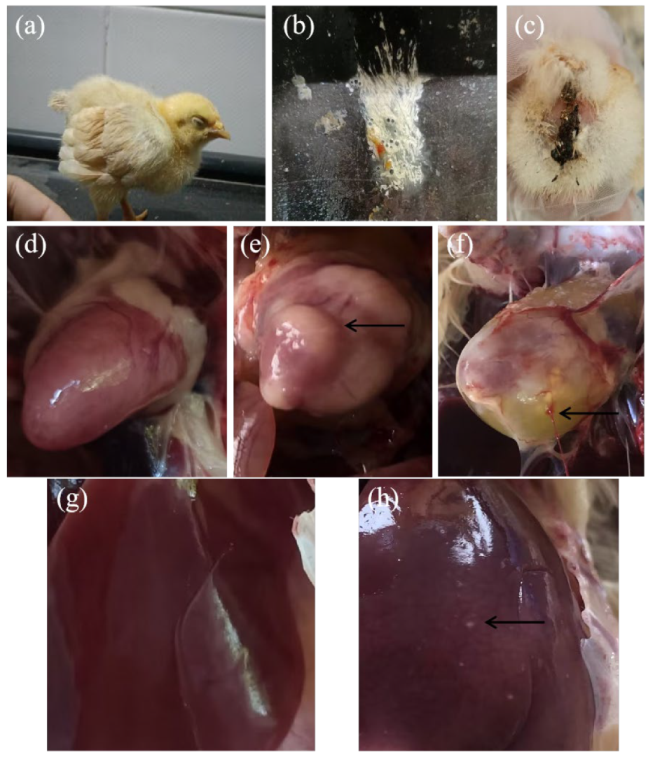
2.2. Clinical Signs and Gross Lesions
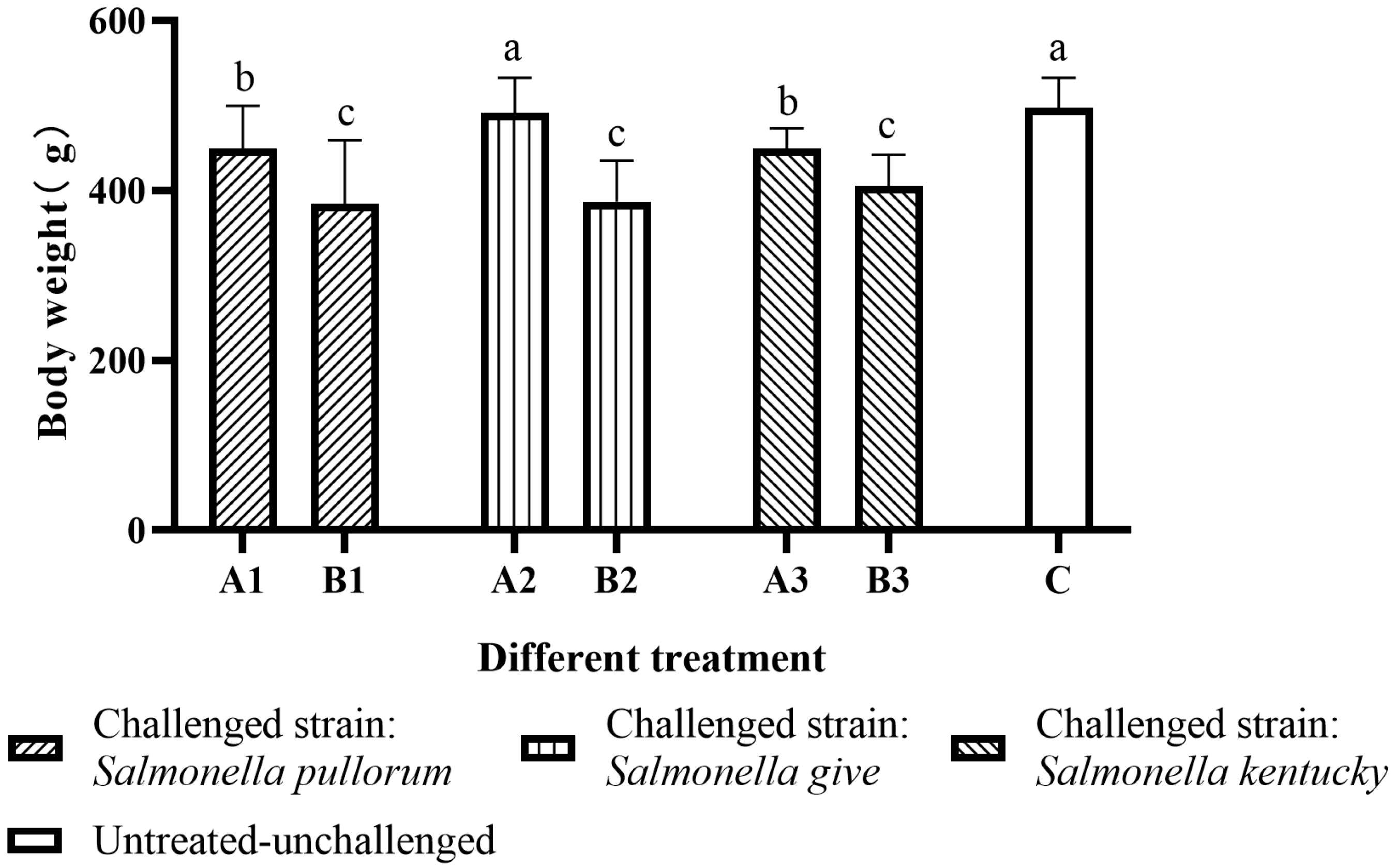
2.3. Body Weight (BW)
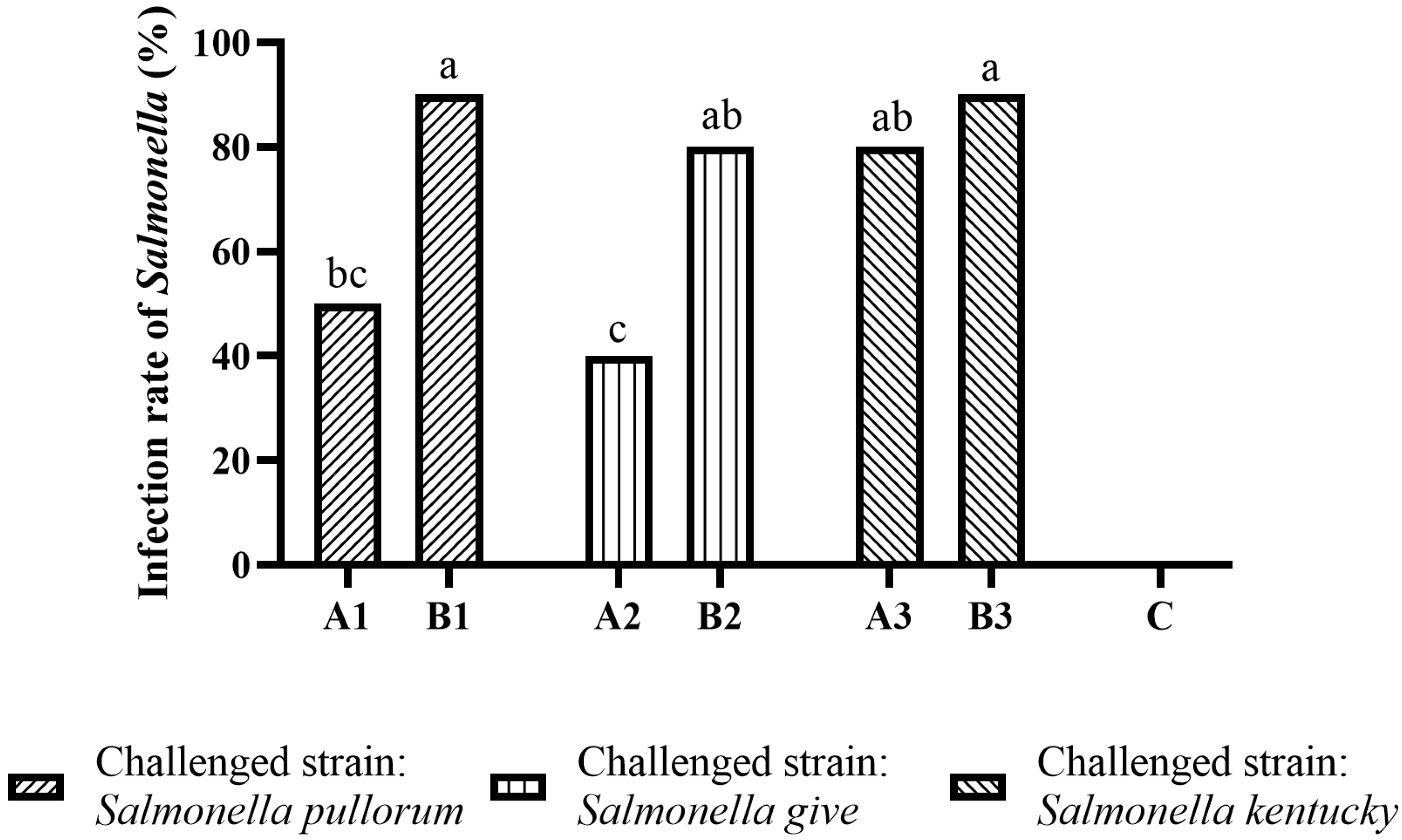
2.4. The Recovery Rate of the Challenged Salmonella in Birds
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of Minimum Inhibitory Concentration (MIC)
4.3. Preparation of Salmonella Inoculum
4.4. Laboratory Challenge-Protection Experiment
4.5. Animals Management
4.6. Clinical Symptoms, Pathological Changes, and Body Weight Observed and Recorded
4.7. Recovery of the Challenged Salmonella
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laidlow, T.A.; Stafford, R.; Jennison, A.V.; Bell, R.; Graham, R.; Graham, T.; Musgrave, N.; Myerson, M.; Kung, N.; Crook, A.; et al. A multi-jurisdictional outbreak of Salmonella typhimurium infections linked to backyard poultry-Australia, 2020. Zoonoses Public Health 2022, 69, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Wang, W.W.; Fan, Q.L.; Ye, J.L.; Zhang, S.; Jiang, S.Q. Effects and interaction of dietary calcium and non-phytate phosphorus for slow-growing yellow-feathered broilers during the starter phase. Animal 2021, 15, 100201. [Google Scholar] [CrossRef] [PubMed]
- Wei, P. Challenge and Development Opportunity of Quality Chicken Industry in China: A Review about 9 Issues on the Topic. China Poult. 2019, 41, 1–6. [Google Scholar]
- Wei, P.; Cui, Z.Z. Prevention and control of avian leukosis and pullorum in local chicken breeds. China Poult. 2015, 37, 1–4. [Google Scholar]
- Shen, X.H.; Zhang, A.Y.; Gu, J.; Zhao, R.H.; Pan, X.C.; Dai, Y.; Yin, L.; Zhang, Q.H.; Hu, X.M.; Wang, H.N.; et al. Evaluating Salmonella pullorum dissemination and shedding patterns and antibody production in infected chickens. BMC Vet. Res. 2022, 18, 240. [Google Scholar] [CrossRef]
- Donachie, A.; Melillo, T.; Bubba, L.; Hartman, H.; Borg, M.L. National outbreak of Salmonella give linked to a local food manufacturer in Malta, October 2016. Epidemiol. Infect. 2018, 146, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Gast, R.K.; Jones, D.R.; Guraya, R.; Anderson, K.E.; Karcher, D.M. Research Note: Horizontal transmission and internal organ colonization by Salmonella enteritidis a nd Salmonella kentucky in experimentally infected laying hens in indoor cage-free housing. Poult. Sci. 2020, 99, 6071–6074. [Google Scholar] [CrossRef]
- Liang, J.Z. The Detection of Salmonella in the Breeding Companies and the Experimental Research on Effects of SYF against Salmonella Infection. Master’s Thesis, Guangxi University, Nanning, China, 2019. [Google Scholar]
- Wang, M. Analysis of Salmonella Contaminations in the Production Chain of the Breeding Farms and the Isolation and Identification of the Bacteriophages of Salmonella pullorum. Master’s Thesis, Guangxi University, Nanning, China, 2021. [Google Scholar]
- Wang, C. Investigation of Salmonella Contamination in GuangXi Quality-Chicken Breeding Farms and Research of the Antimicrobials Alternatives. Master’s Thesis, Guangxi University, Nanning, China, 2022. [Google Scholar]
- Xu, Z.H.; Wang, M.; Zhou, C.Y.; Gu, G.M.; Liang, J.Z.; Hou, X.J.; Wang, M.L.; Wei, P. Prevalence and antimicrobial resistance of retail-meat-borne Salmonella in southern China during the years 2009–2016: The diversity of contamination and the resistance evolution of multidrug-resistant isolates. Int. J. Food Microbiol. 2020, 333, 108790. [Google Scholar] [CrossRef]
- Mahtab, U.T.; Jyoti, C.A.; Ameer, K.; Matin, Z.B.R.; Saikat, M.; Bin, E.T.; Kuldeep, D.; Hossain, R.M.K.; Márió, G.; Khayam, S.M.U.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar]
- Chen, L.; Du, K.; Bai, X.; Shao, J.H.; Tang, T.; Xia, S.Q.; Mei, H.; Wang, J.; Jia, X.B.; Lai, S.J. Transcriptomics Analysis Reveals the Immune Response Mechanism of Rabbits with Diarrhea Fed an Antibiotic-Free Diet. Animals 2021, 11, 2994. [Google Scholar] [CrossRef]
- Zhao, R.; Ben, A.L.; Wei, M.X.; Ruan, M.; Gu, H.Y.; Yang, L. Essential oil obtained from Thlaspi arvense L. leaves and seeds using microwave-assisted hydrodistillation and extraction in situ by vegetable oil and its antifungal activity against Penicillium expansum. LWT 2022, 165, 113718. [Google Scholar] [CrossRef]
- Lal, M.; Begum, T.; Gogoi, R.; Sarma, N.; Munda, S.; Pandey, S.K.; Baruah, J.; Tamang, R.; Saikia, S. Anethole rich Clausena heptaphylla (Roxb.) Wight & Arn., essential oil pharmacology and genotoxic efficiencies. Sci. Rep. 2022, 12, 9978. [Google Scholar]
- Alibi, S.; Selma, W.B.; Mansour, H.B.; Navas, J. Activity of Essential Oils Against Multidrug-Resistant Salmonella enteritidis. Curr. Microbiol. 2022, 79, 273. [Google Scholar] [CrossRef]
- El-Ashram, S.; Abdelhafez, G.A. Effects of phytogenic supplementation on productive performance of broiler chickens. J. Appl. Poult. Res. 2020, 29, 852–862. [Google Scholar] [CrossRef]
- Rashid, Z.; Mirani, Z.A.; Zehra, S.; Gilani, S.M.H.; Ashraf, A.; Azhar, A.; Al-Ghanim, K.; Al-Misned, F.; Al-Mulahim, N.; Mahboob, S. Enhanced modulation of gut microbial dynamics affecting body weight in birds triggered by natural growth promoters administered in conventional feed. Saudi J. Biol. Sci. 2020, 27, 2747–2755. [Google Scholar] [CrossRef]
- Saif, Y.M. Diseases of Poultry, 12th ed.; John Wiley & Sons: New York, NY, USA, 2009; pp. 619–674. [Google Scholar]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Tosi, G.; Massi, P.; Pistelli, L.; Mancianti, F. In vitro antimicrobial activity of essential oils against Salmonella enterica serotypes Enteritidis and Typhimurium strains isolated from poultry. Molecules 2019, 24, 900. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhao, Y.; Chen, X.; Li, W.; Li, W.; Du, J.; Wang, L. Effects of Cinnamon Essential Oil on Oxidative Damage and Outer Membrane Protein Genes of Salmonella enteritidis Cells. Foods 2022, 11, 2234. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.; Elnesr, S.S.; Abd El-Hack, M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef]
- Naz, F.; Kumar, M.; Koley, T.; Sharma, P.; Haque, M.A.; Kapil, A.; Kumar, M.; Kaur, P.; Ethayathulla, A.S. Screening of plant-based natural compounds as an inhibitor of FtsZ from Salmonella Typhi using the computational, biochemical and in vitro cell-based studies. Int. J. Biol. Macromol. 2022, 219, 428–437. [Google Scholar] [CrossRef]
- Ilić, D.P.; Stanojević, L.P.; Troter, D.Z.; Stanojević, J.S.; Danilović, B.R.; Nikolić, V.D.; Nikolić, L.B. Improvement of the yield and antimicrobial activity of fennel (Foeniculum vulgare Mill.) essential oil by fruit milling. Ind. Crop. Prod. 2019, 142, 111854. [Google Scholar] [CrossRef]
- Gast, R.K.; Regmi, P.; Guraya, R.; Jones, D.R.; Anderson, K.E.; Karcher, D.M. Colonization of internal organs by Salmonella enteritidis in experimentally infected laying hens of four commercial genetic lines in conventional cages and enriched colony housing. Poult. Sci. 2019, 98, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Liang, J.Z.; Jiang, W.W.; He, X.S.; Liu, S.H.; Wei, P. The effect of a selected yeast fraction on the prevention of pullorum disease and fowl typhoid in commercial breeder chickens. Poult. Sci. 2020, 99, 101–110. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Abd El-Mageed, T.A.; Soliman, S.M.; Khafaga, A.F.; Swelum, A.A.; Ahmed, A.E.; Alshammari, F.A.; Abd El-Hack, M.E. The control of poultry salmonellosis using organic agents: An updated overview. Poult. Sci. 2022, 101, 101716. [Google Scholar] [CrossRef] [PubMed]
- Göser, V.; Kehl, A.; Röder, J.; Hensel, M. Role of the ESCRT-III complex in controlling integrity of the Salmonella-containing vacuole. Cell. Microbiol. 2020, 22, e13176. [Google Scholar] [CrossRef]
- Li, Q.C.; Wang, X.; Xia, J.; Yuan, Y.; Yin, C.; Xu, L.J.; Li, Y.; Jiao, X.A. Salmonella-containing vacuole development in avian cells and characteristic of cigR in Salmonella enterica serovar Pullorum replication within macrophages. Vet. Microbiol. 2018, 223, 65–71. [Google Scholar] [CrossRef]
- Cheng, Y.; Pedroso, A.A.; Porwollik, S.; McClelland, M.; Lee, M.D.; Kwan, T.; Zamperini, K.; Soni, V.; Sellers, H.S.; Russell, S.M.; et al. rpoS-Regulated core genes involved in the competitive fitness of Salmonella enterica Serovar Kentucky in the intestines of chickens. Appl. Environ. Microbiol. 2015, 81, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Lang, M.; Montjarret, A.; Duteil, E.; Bedoux, G. Cinnamomum cassia and Syzygium aromaticum Essential Oils Reduce the Colonization of Salmonella typhimurium in an In Vivo Infection Model Using Caenorhabditis elegans. Molecules 2021, 26, 5598. [Google Scholar] [CrossRef]
- Al baqir, A.; Hussein, A.; Ghanem, I.; Megahed, M. Characterization of Paratyphoid Salmonellae Isolated from Broiler Chickens at Sharkia Governorate, Egypt. Zagazig Vet. J. 2019, 47, 183–192. [Google Scholar] [CrossRef]
- Moharreri, M.; Vakili, R.; Oskoueian, E.; Rajabzadeh, G. Effects of microencapsulated essential oils on growth performance and biomarkers of inflammation in broiler chickens challenged with Salmonella enteritidis. J. Saudi Soc. Agric. Sci. 2022, 21, 349–357. [Google Scholar] [CrossRef]
- Huang, C.M.; Lee, T.T. Immunomodulatory effects of phytogenics in chickens and pigs—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, S.; Zhao, C.; Zhang, Z.; Nie, D.; Tang, W.; Li, Y. The Chemical Composition and Antibacterial and Antioxidant Activities of Five Citrus Essential Oils. Molecules 2022, 27, 7044. [Google Scholar] [CrossRef]

| Salmonella Strains | Dilution (SCEO 1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | 1:256 | 1:512 | |
| Salmonella pullorum | − | − | − | + | + | + | + | + | + |
| Salmonella give | − | + | + | + | + | + | + | + | + |
| Salmonella kentucky | − | + | + | + | + | + | + | + | + |
| Groups | Treatments | Challenge Strains | No. of Birds | |
|---|---|---|---|---|
| A | A1 | SCEO (1.25 mL/L supplemented in the water) | Salmonella pullorum | 10 |
| A2 | Salmonella give | 10 | ||
| A3 | Salmonella kentucky | 10 | ||
| B | B1 | No SCEO | Salmonella pullorum | 10 |
| B2 | Salmonella give | 10 | ||
| B3 | Salmonella kentucky | 10 | ||
| C | Untreated/unchallenged | No SCEO | - | 10 |
| Items (g/kg) | Starter 3 | Grower A 4 | Grower B 5 |
|---|---|---|---|
| Corn | 596 | 688 | 750 |
| Soybean meal (78.0 g/kg) | 342 | 266 | 214 |
| Soybean oil | 22 | 9 | 0 |
| Limestone (370 g/kg) | 12.7 | 13.4 | 13.2 |
| Calcium hydrogen phosphate | 18 | 15 | 15 |
| Sodium chloride | 2.5 | 2.5 | 2.5 |
| Methionine (998 g/kg) | 2.5 | 2 | 1.6 |
| Lysine HCL (780 g/kg) | 2 | 1.8 | 1.4 |
| Vitamin premix 1 | 0.3 | 0.3 | 0.3 |
| Mineral premix 2 | 1 | 1 | 1 |
| Choline chloride (500 g/kg) | 1 | 1 | 1 |
| Total | 1000 | 1000 | 1000 |
| Calculated nutrient content | |||
| Metabolizable energy (kcal/g) | 2950 | 2950 | 2920 |
| Crude protein (g/kg) | 198 | 171 | 152 |
| Calcium (g/kg) | 0.95 | 0.95 | 0.95 |
| Available phosphorus (g/kg) | 4.5 | 4.5 | 4 |
| Lysine (g/kg) | 12.1 | 10 | 8.6 |
| Methionine (g/kg) | 5.4 | 4.6 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Xu, Z.; Chen, W.; Zhou, C.; Wang, C.; Wang, M.; Liang, J.; Wei, P. The Use of Star Anise-Cinnamon Essential Oil as an Alternative Antibiotic in Prevention of Salmonella Infections in Yellow Chickens. Antibiotics 2022, 11, 1579. https://doi.org/10.3390/antibiotics11111579
Li C, Xu Z, Chen W, Zhou C, Wang C, Wang M, Liang J, Wei P. The Use of Star Anise-Cinnamon Essential Oil as an Alternative Antibiotic in Prevention of Salmonella Infections in Yellow Chickens. Antibiotics. 2022; 11(11):1579. https://doi.org/10.3390/antibiotics11111579
Chicago/Turabian StyleLi, Changcheng, Ziheng Xu, Wenyan Chen, Chenyu Zhou, Can Wang, Min Wang, Jingzhen Liang, and Ping Wei. 2022. "The Use of Star Anise-Cinnamon Essential Oil as an Alternative Antibiotic in Prevention of Salmonella Infections in Yellow Chickens" Antibiotics 11, no. 11: 1579. https://doi.org/10.3390/antibiotics11111579
APA StyleLi, C., Xu, Z., Chen, W., Zhou, C., Wang, C., Wang, M., Liang, J., & Wei, P. (2022). The Use of Star Anise-Cinnamon Essential Oil as an Alternative Antibiotic in Prevention of Salmonella Infections in Yellow Chickens. Antibiotics, 11(11), 1579. https://doi.org/10.3390/antibiotics11111579





