Trends in the Bacterial Prevalence and Antibiotic Resistance Patterns in the Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Hospitalized Patients in South India
Abstract
:1. Introduction
2. Results
2.1. Bacterial Prevalence
2.2. Trends in Proportion of the Drug Resistance
2.3. Trends in the Proportion of the Drug Resistance Isolates, Based on Sex and Type of Ward Admission
2.4. Trends in the Proportion of Multi-Drug Resistant Isolates
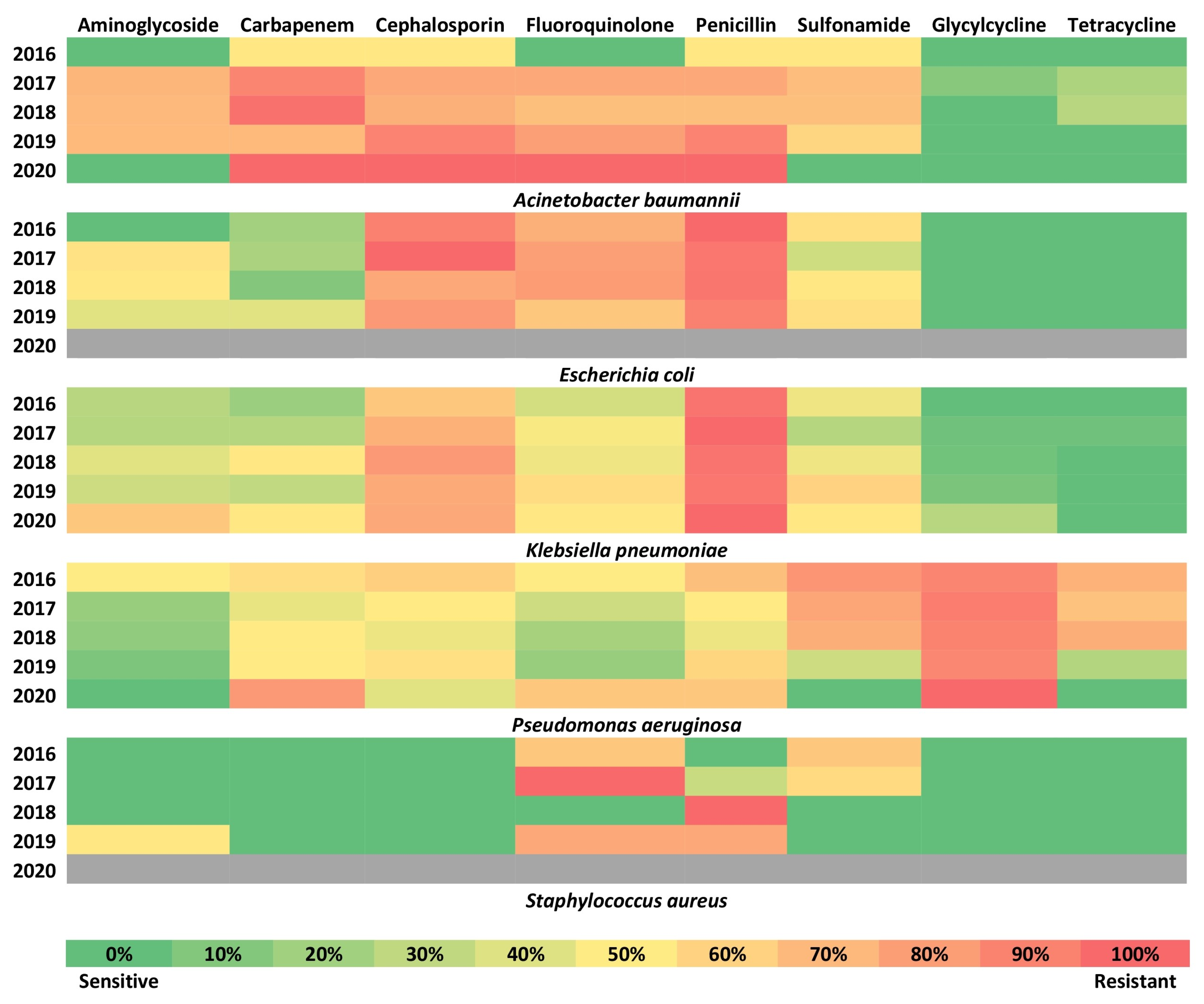
2.5. Trends of the Resistance for Different Drug Classes
2.6. Mortality
3. Discussion
Strength and Limitations
4. Materials and Methods
4.1. Study Population
4.2. Study Definitions
4.3. Microbiological Study
4.4. Antibiotic Susceptibility
4.5. Inclusion and Exclusion Criteria
4.6. Statistical Analysis
4.7. Ethics Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease GOLD Report 2022. pp. 1–165. Available online: https://goldcopd.org/2022-gold-reports-2/ (accessed on 29 September 2022).
- Halpin, D.M.G.; Celli, B.R.; Criner, G.J.; Frith, P.; López Varela, M.V.; Salvi, S.; Vogelmeier, C.F.; Chen, R.; Mortimer, K.; Montes de Oca, M.; et al. The GOLD Summit on Chronic Obstructive Pulmonary Disease in Low- and Middle-Income Countries. Int. J. Tuberc. Lung Dis. 2019, 23, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.J.; Mahesh, P.A.; Fordham, J.Z.; Majeed, A. Prevalence of COPD in India: A Systematic Review. Prim. Care Respir. J. 2012, 21, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, S.; Kumar, G.A.; Dhaliwal, R.S.; Paulson, K.; Agrawal, A.; Koul, P.A.; Mahesh, P.A.; Nair, S.; Singh, V.; Aggarwal, A.N.; et al. The Burden of Chronic Respiratory Diseases and Their Heterogeneity across the States of India: The Global Burden of Disease Study 1990–2016. Lancet Glob. Health 2018, 6, e1363–e1374. [Google Scholar] [CrossRef] [Green Version]
- Seemungal, T.A.R.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Wedzicha, J.A. Time Course and Recovery of Exacerbations in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2000, 161, 1608–1613. [Google Scholar] [CrossRef]
- Sethi, S. Bacteria in Exacerbations of Chronic Obstructive Pulmonary Disease: Phenomenon or Epiphenomenon? Proc. Am. Thorac. Soc. 2004, 1, 109–114. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Suwono, B.; Eckmanns, T.; Markwart, R. The Epidemiology of Carbapenem-Non-Susceptible Acinetobacter Species in Europe: Analysis of EARS-Net Data from 2013 to 2017. Antimicrob. Resist. Infect. Control 2020, 9, 1–10. [Google Scholar] [CrossRef]
- World Health Organization. 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021; pp. 1–76. [Google Scholar]
- Dixit, A.; Kumar, N.; Kumar, S.; Trigun, V. Antimicrobial Resistance: Progress in the Decade since Emergence of New Delhi Metallo-β-Lactamase in India. Indian J. Community Med. 2019, 44, 4–8. [Google Scholar]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Ritchie, A.I.; Wedzicha, J.A. Definition, Causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin. Chest Med. 2020, 41, 421–438. [Google Scholar] [CrossRef]
- Cullen, L.; McClean, S. Bacterial Adaptation during Chronic Respiratory Infections. Pathogens 2015, 4, 66–89. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo-Troyano, A.; Sibila, O. The Respiratory Threat Posed by Multidrug Resistant Gram-Negative Bacteria. Respirology 2017, 22, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Abhinav, D.; Anurag, A.; Lalit, S.; Rajeev, T. A Comparative Study of Clinical Characteristics and Bacteriological Sensitivity Pattern of Sputum in Acute Exacerbations of COPD. J. Evol. Med. Dent. Sci. 2019, 8, 1726–1730. [Google Scholar] [CrossRef]
- Bashir, S.; Muzamil, J.; Guru, F.; Mohsin, N.; Nabi, F.; Kanwar, M. Patterns of Infections in Chronic Obstructive Pulmonary Disease Exacerbations and Its Outcome in High Dependency Area, Intensive Care Setting in a Tertiary Care Hospital. Community Acquir. Infect. 2016, 3, 77. [Google Scholar] [CrossRef]
- Firdaus, H.; Khan, N.A.; Fatima, N.; Shameem, M.; Bhargava, R.; Ahmad, Z. Acute Exacerbation of Chronic Obstructive Pulmonary Disease: Phenotypic Screening and Sensitivity of Microbiological Profile. J. Clin. Diagn. Res. 2020, 14, LC15–LC20. [Google Scholar] [CrossRef]
- Kuwal, A.; Singh, S.; Purohit, G.; Agarwal, K.C.; Dutt, N.; Joshi, V. A Prospective Study of Bacteriological Etiology in Hospitalized Acute Exacerbation of COPD Patients: Relationship with Lung Function and Respiratory Failure. Turk. Thorac. J. 2017, 19, 19. [Google Scholar] [CrossRef] [Green Version]
- Mampilly, T.T.; Idikula, M.; Thilakan, A.; Lancy, J. Bacteriological Profile of Chronic Obstructive Pulmonary Disease in Patients Admitted with Acute Exacerbation at a Tertiary Care Centre. J. Med. Sci. Clin. Res. 2019, 7, 64–71. [Google Scholar] [CrossRef]
- Estirado, C.; Ceccato, A.; Guerrero, M.; Huerta, A.; Cilloniz, C.; Vilaró, O.; Gabarrús, A.; Gea, J.; Crisafulli, E.; Soler, N.; et al. Microorganisms Resistant to Conventional Antimicrobials in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Respir. Res. 2018, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, M.K.; Makhlouf, H.A.; Hasan, A.A.; Rashed, H.G.; Khalifa, H.S. Bacteriological Profile of Critically Ill Patients with Chronic Obstructive Pulmonary Disease in Respiratory Intensive Care Unit in Assuit University Hospital. Egypt. J. Bronchol. 2019, 13, 343–348. [Google Scholar] [CrossRef]
- Mythri, B.A.; Patil, A.B.; Prathibha, J.; Gana, P. Aerobic Bacteriological Profile of Acute Exacerbations of Chronic Obstructive Pulmonary Disease in a Tertiary Care Hospital. Indian J. Microbiol. Res. 2020, 7, 293–298. [Google Scholar] [CrossRef]
- Rashid, M.H.U.; Ahmed, I. Pattern of Sputum Bacteriology in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. J. Enam Med. Coll. 2018, 8, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Erkan, L.; Uzun, O.; Findik, S.; Katar, D.; Sanic, A.; Atici, A.G. Role of Bacteria in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Int. J. COPD 2008, 3, 463. [Google Scholar] [CrossRef]
- Hassan, A.T.; Mohamed, S.A.A.; Mohamed, M.S.E.; El-Mokhtar, M.A. Acute Exacerbations of Chronic Obstructive Pulmonary Disease: Etiological Bacterial Pathogens and Antibiotic Resistance in Upper Egypt. Egypt. J. Bronchol. 2016, 10, 283–290. [Google Scholar] [CrossRef]
- Ma, X.; Cui, J.; Wang, J.; Chang, Y.; Fang, Q.; Bai, C.; Zhou, X.; Zhou, H.; Feng, H.; Wang, Y.; et al. Multicentre Investigation of Pathogenic Bacteria and Antibiotic Resistance Genes in Chinese Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. J. Int. Med. Res. 2015, 43, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Tackling Antimicrobial Resistance (AMR) Together. Working Paper 5.0: Enhancing the Focus on Gender and Equity; World Health Organization: Geneva, Switzerland, 2018; pp. 1–23. [Google Scholar]
- Jones, N.; Mitchell, J.; Cooke, P.; Baral, S.; Arjyal, A.; Shrestha, A.; King, R. Gender and Antimicrobial Resistance: What Can We Learn From Applying a Gendered Lens to Data Analysis Using a Participatory Arts Case Study? Front. Glob. Womens Health 2022, 3, 745862. [Google Scholar] [CrossRef] [PubMed]
- ReAct Scoping the Significance of Gender for Antibiotic Resistance. 2019; pp. 1–23. Available online: https://www.reactgroup.org/wp-content/uploads/2020/09/Scoping-the-Significance-of-Gender-for-Antibiotic-Resistance-IDS-ReAct-Report-October-2020.pdf (accessed on 24 October 2022).
- ReAct Burden of Antibiotic Resistance on Women’s Health. A Fact Sheet from ReAct-Action on Antibiotic Resistance. 2008; pp. 1–4. Available online: https://www.reactgroup.org (accessed on 24 October 2022).
- Van den Bogaard, A.E. Antibiotic Resistance of Faecal Escherichia Coli in Poultry, Poultry Farmers and Poultry Slaughterers. J. Antimicrob. Chemother. 2001, 47, 763–771. [Google Scholar] [CrossRef]
- Schröder, W.; Sommer, H.; Gladstone, B.P.; Foschi, F.; Hellman, J.; Evengard, B.; Tacconelli, E. Gender Differences in Antibiotic Prescribing in the Community: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2016, 71, 1800–1806. [Google Scholar] [CrossRef] [Green Version]
- Laxminarayan, R.; Chaudhury, R.R. Antibiotic Resistance in India: Drivers and Opportunities for Action. PLoS Med. 2016, 13, e1001974. [Google Scholar] [CrossRef] [Green Version]
- ICMR Antimicrobial Resistance Research and Surveillance Network-ICMR Annual Report. 2020; pp. 1–187. Available online: https://iamrsn.icmr.org.in/index.php/resources/amr-icmr-data (accessed on 24 October 2022).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. 2016; pp. 1–80. Available online: https://amr-review.org (accessed on 29 September 2022).
- Manesh, A.; Varghese, G.M. Rising Antimicrobial Resistance: An Evolving Epidemic in a Pandemic. Lancet Microbe 2021, 2, e419–e420. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Apisarnthanarak, A.; Khan, E.; Suwantarat, N.; Ghafur, A.; Tambyah, P.A. Carbapenem-Resistant Acinetobacter Baumannii and Enterobacteriaceae in South and Southeast Asia. Clin. Microbiol. Rev. 2017, 30, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Gandra, S.; Gupta, P.; Mehta, Y.; Laxminarayan, R.; Sengupta, S. Clinical Outcome of Dual Colistin- and Carbapenem-Resistant Klebsiella Pneumoniae Bloodstream Infections: A Single-Center Retrospective Study of 75 Cases in India. Am. J. Infect. Control 2017, 45, 1289–1291. [Google Scholar] [CrossRef]
- Prescott, E.; Bjerg, A.M.; Andersen, P.K.; Lange, P.; Vestbo, J. Gender Difference in Smoking Effects on Lung Function and Risk of Hospitalization for COPD: Results Front a Danish Longitudinal Population Study. Eur. Respir. J. 1997, 10, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Ringbaek, T.; Seersholm, N.; Viskum, K. Standardised Mortality Rates in Females and Males with COPD and Asthma. Eur. Respir. J. 2005, 25, 891–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiligianni, I.; Rodríguez, M.R.; Lisspers, K.; LeeTan, T.; Infantino, A. Call to Action: Improving Primary Care for Women with COPD. NPJ Prim. Care Respir. Med. 2017, 27, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merkus, P.J.F.M.; Ten Have-Opbroek, A.A.W.; Quanjer, P.H. Human Lung Growth: A Review. Pediatr. Pulmonol. 1996, 21, 383–397. [Google Scholar] [CrossRef]
- Tam, A.; Churg, A.; Wright, J.L.; Zhou, S.; Kirby, M.; Coxson, H.O.; Lam, S.; Man, S.F.P.; Sin, D.D. Sex Differences in Airway Remodeling in a Mouse Model of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 825–834. [Google Scholar] [CrossRef]
- Ekström, M.P.; Jogréus, C.; Ström, K.E. Comorbidity and Sex-Related Differences in Mortality in Oxygen-Dependent Chronic Obstructive Pulmonary Disease. PLoS ONE 2012, 7, e35806. [Google Scholar] [CrossRef]
- Celli, B.; Vestbo, J.; Jenkins, C.R.; Jones, P.W.; Ferguson, G.T.; Calverley, P.M.A.; Yates, J.C.; Anderson, J.A.; Willits, L.R.; Wise, R.A. Sex Differences in Mortality and Clinical Expressions of Patients with Chronic Obstructive Pulmonary Disease: The TORCH Experience. Am. J. Respir. Crit. Care Med. 2011, 183, 317–322. [Google Scholar] [CrossRef]
- Torres-Duque, C.A.; García-Rodriguez, M.C.; González-García, M. Is Chronic Obstructive Pulmonary Disease Caused by Wood Smoke a Different Phenotype or a Different Entity? Arch. Bronconeumol. 2016, 52, 425–431. [Google Scholar] [CrossRef]
- Torres-Duque, C.; Severiche-Bueno, F.; González-García, M. Chronic Obstructive Pulmonary Disease Related to Wood and Other Biomass Smoke: A Different Phenotype or Specific Diseases?—A Current Conspectus. In Chronic Obstructive Pulmonary Disease—A Current Conspectus; Chung Ong, K., Ed.; IntechOpen: London, UK, 2021; p. 120. ISBN 978-1-83968-927-7. [Google Scholar]
- Shallcross, L.; Beckley, N.; Rait, G.; Hayward, A.; Petersen, I. Antibiotic Prescribing Frequency amongst Patients in Primary Care: A Cohort Study Using Electronic Health Records. J. Antimicrob. Chemother. 2017, 72, 1818–1824. [Google Scholar] [CrossRef] [Green Version]
- Pereira, B.; Kulkarni, S. Antibiotic Misuse and Improper Practices in India: Identifying the Scope to Improve through a Narrative Review. Int. J. Risk Saf. Med. 2021, 33, 357–364. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Bacterial Infection in Chronic Obstructive Pulmonary Disease in 2000: A State-of-the-Art Review. Clin. Microbiol. Rev. 2001, 14, 336–363. [Google Scholar] [CrossRef] [PubMed]
- Kyd, J.; McGrath, J.; Krishnamurthy, A. Mechanisms of Bacterial Resistance to Antibiotics in Infections of COPD Patients. Curr. Drug Targets 2011, 12, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Barbeta, E.; Ielpo, A.; Torres, A. Management of Severe Acute Exacerbations of COPD: An Updated Narrative Review. Multidiscip. Respir. Med. 2018, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Cabello, H.; Torres, A.; Celis, R.; El-Ebiary, M.; Puig De La Bellacasa, J.; Xaubet, A.; González, J.; Agustí, C.; Soler, N. Bacterial Colonization of Distal Airways in Healthy Subjects and Chronic Lung Disease: A Bronchoscopic Study. Eur. Respir. J. 1997, 10, 1137–1144. [Google Scholar] [CrossRef]
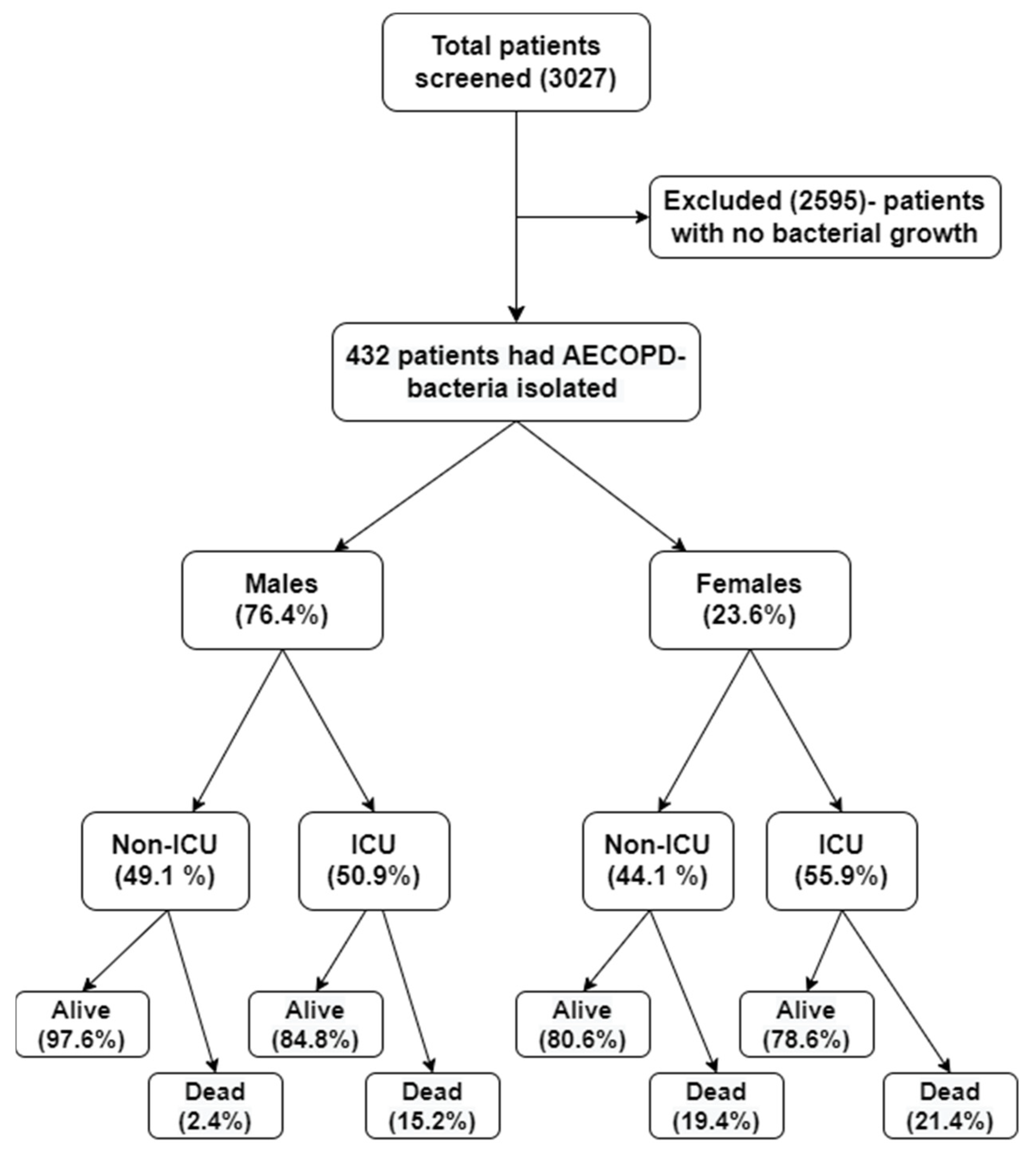
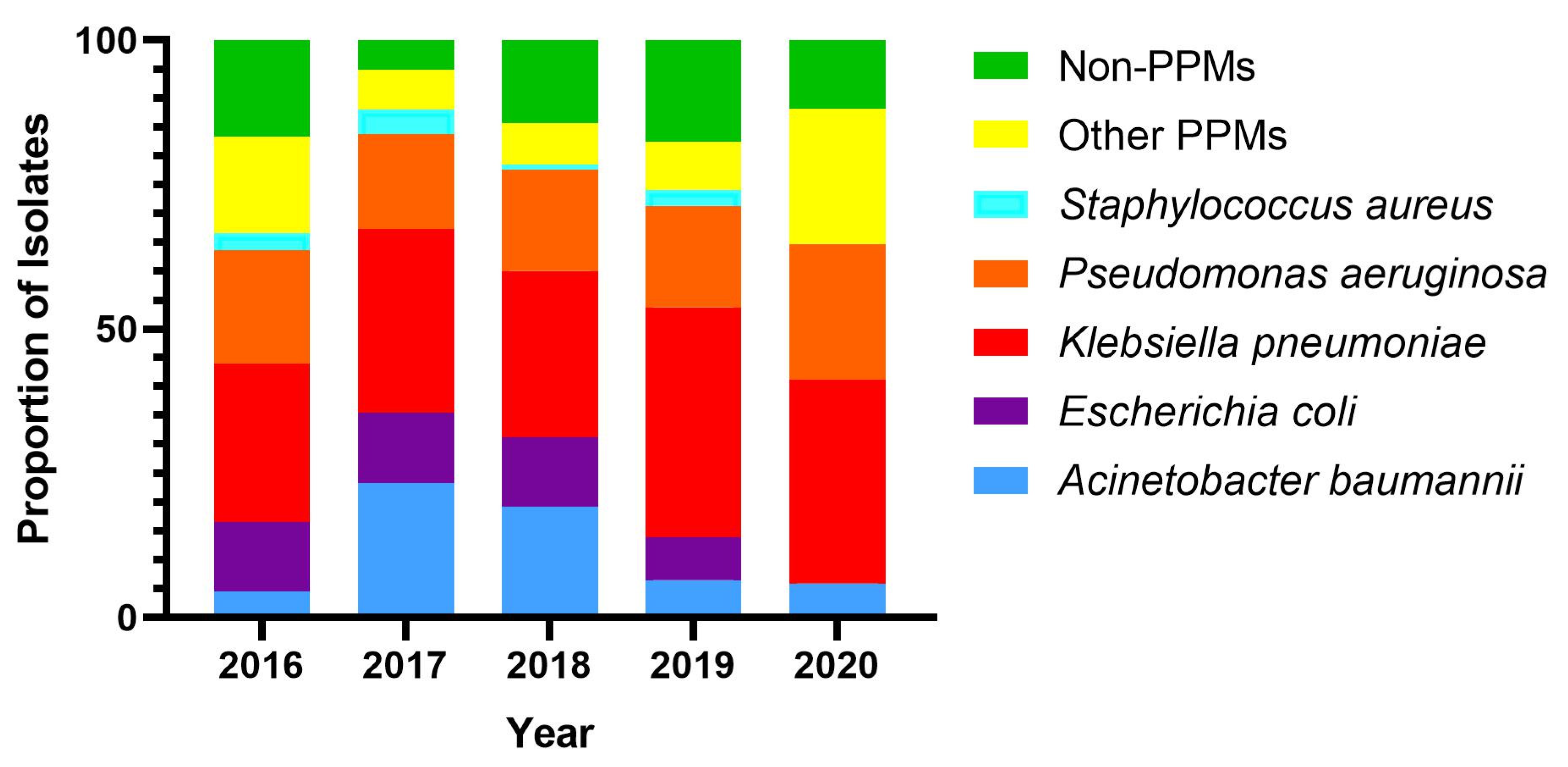

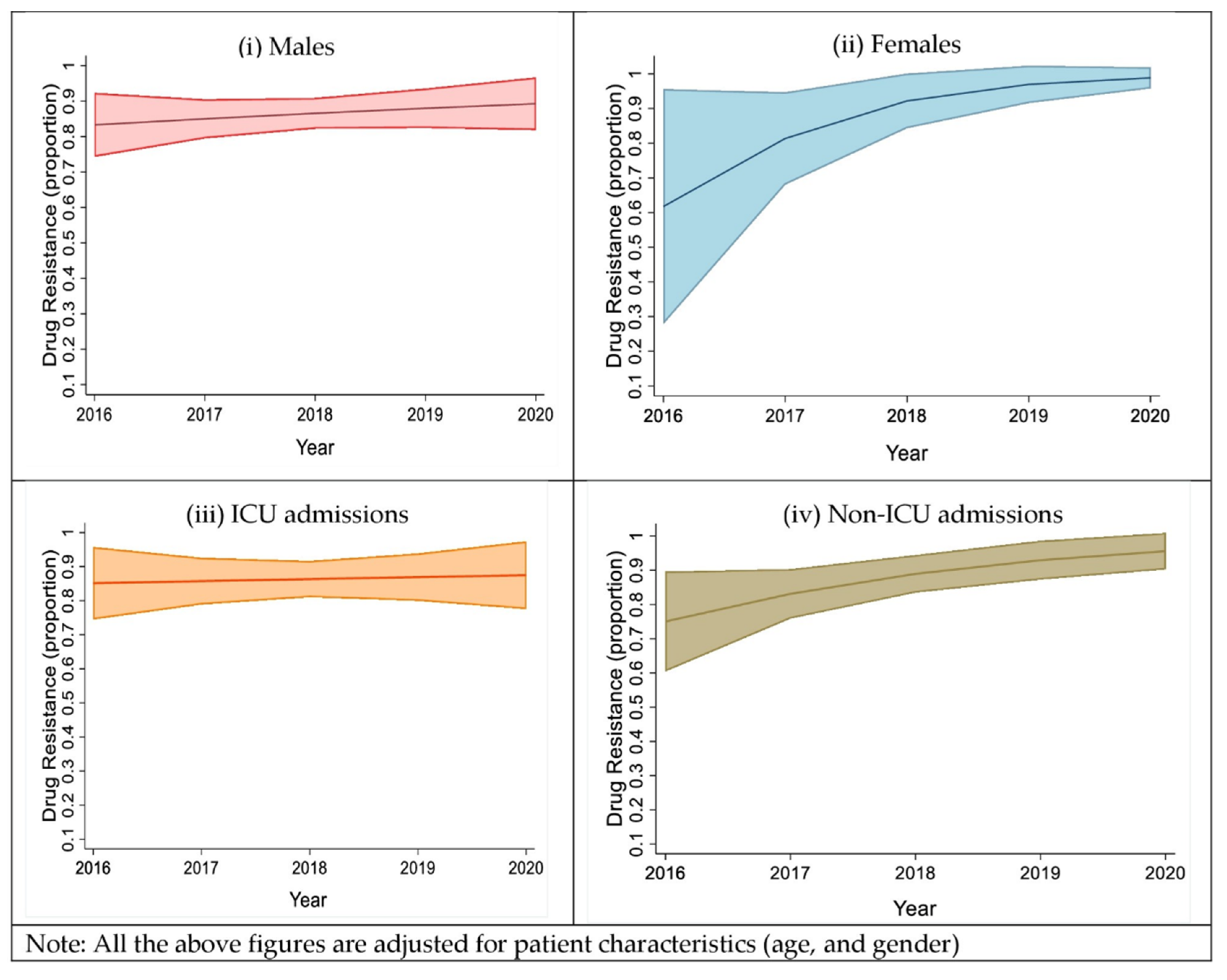
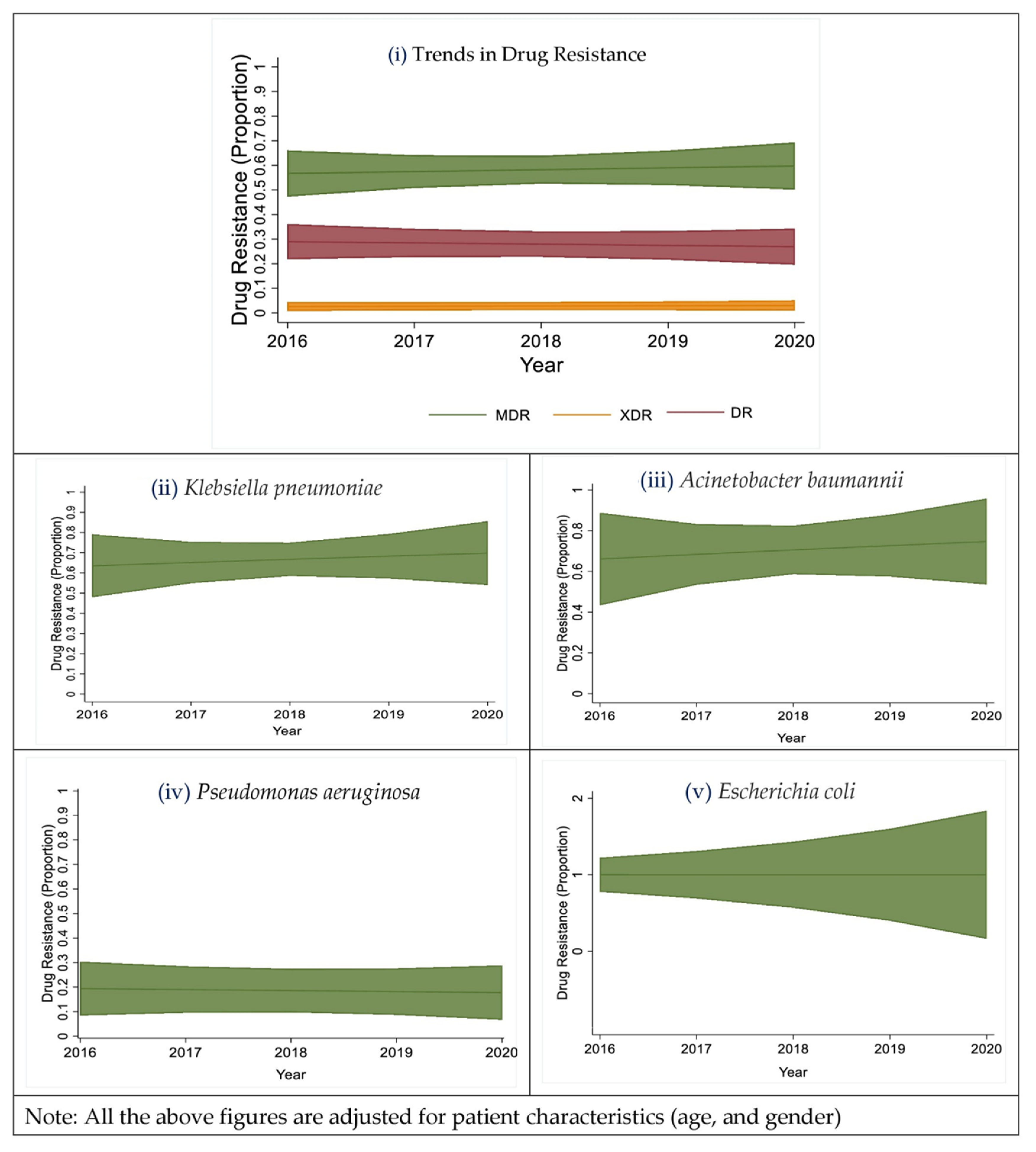
| Total (n = 432) | Male (n = 330) | Female (n = 102) | p-Value | |
|---|---|---|---|---|
| Age (Mean ± SD) | 66 (60 to 75) | 66.8 ± 10.7 | 65.6 ± 11.3 | 0.413 * |
| Length of hospital stay (Median (IQR) | 6.0 (4.0 to 10) | 6.0 (4.0 to 10) | 7.0 (4.0 to 9.75) | 0.668 * |
| Non-ICU (n, %) | 207 (47.9) | 162 (49.1) | 45 (44.1) | 0.380 † |
| ICU (n, %) | 225 (52.1) | 168 (50.9) | 57 (55.9) | |
| Alive (n, %) | 384 (88.9) | 302 (91.5) | 82 (80.4) | 0.002 † |
| Dead (n, %) | 48 (11.1) | 28 (8.5) | 20 (19.6) | |
| Potentially pathogenic microorganisms (PPMs) | ||||
| Yes (n, %) | 381 (88.2) | 297 (90) | 84 (82.4) | 0.036 † |
| No (n, %) | 51 (11.8) | 33 (10) | 18 (17.6) | |
| Drug resistance | ||||
| Yes (n, %) | 292 (87.2%) | 226 (86.3%) | 66 (90.4%) | |
| No (n, %) | 43 (12.8%) | 36 (13.7%) | 7 (9.6%) | 0.348 † |
| Charlson’s comorbidity index (CCI) (Median (IQR)) | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 0.289 * |
| Comorbidities | ||||
| Diabetes mellitus (n, %) | 110 (25.5) | 78 (23.6) | 32 (31.4) | 0.117 † |
| Heart disease (n, %) | 64 (14.8) | 53 (16.1) | 11 (10.8) | 0.190 † |
| Kidney disease (n, %) | 50 (11.6) | 42 (12.7) | 8 (7.8) | 0.178 † |
| Liver disease (n, %) | 17 (3.9) | 16 (4.8) | 1 (1) | 0.079 † |
| Corpulmonale (n, %) | 99 (22.9) | 63 (19.1) | 36 (35.3) | <0.001 † |
| Hypertension (n, %) | 146 (33.8) | 99 (30) | 47 (46.1) | 0.003 † |
| Obesity (n, %) | 24 (5.6) | 13 (3.9) | 11 (10.8) | 0.008 † |
| Pulmonary Hypertension (n, %) | 89 (20.6) | 67 (20.3) | 22 (21.6) | 0.782 † |
| Sepsis (n, %) | 33 (7.6) | 25 (7.6) | 8 (7.8) | 0.929 † |
| Dependent | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (Univariable) | HR (Multivariable) | HR (Univariable) | HR (Multivariable) | |
| Bacterial Species | ||||
| Klebsiella pneumoniae | Reference | Reference | Reference | Reference |
| Pseudomonas aeruginosa | 1.60 (0.66–3.88) | 1.63 (0.67–3.99) | 1.60 (0.66–3.88) | 1.94 (0.77–4.92) |
| Acinetobacter baumannii | 2.27 (0.96–5.35) | 2.64 (1.08–6.43) * | 2.27 (0.96–5.35) | 2.88 (1.18–7.03) * |
| Escherichia coli | 1.77 (0.60–5.18) | 1.82 (0.61–5.40) | 1.77 (0.60–5.18) | 1.79 (0.60–5.32) |
| Staphylococcus aureus | 2.43 (0.53–11.11) | 2.50 (0.54–11.62) | 2.43 (0.53–11.11 | 2.50 (0.54–11.60) |
| Gender | ||||
| Male | Reference | Reference | Reference | Reference |
| Female | 2.62 (1.35–5.08) * | 2.89 (1.47–5.70) * | 2.62 (1.35–5.08) * | 2.71 (1.36–5.37) * |
| Age in years (Mean (SD)) | 1.02 (0.99–1.05) | 0.99 (0.95–1.04) | 1.02 (0.99–1.05) | 0.99 (0.94–1.04) |
| CCI (Mean (SD)) | 1.19 (0.96–1.48) | 1.31 (0.95–1.80) | 1.19 (0.96–1.48) | 1.33 (0.97–1.83) |
| Drug resistance | ||||
| No | - | - | Reference | Reference |
| Yes | - | - | 2.01 (0.48–8.37) | 2.36 (0.53–10.51) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleem Ullah, M.; Malamardi, S.; Siddaiah, J.B.; A, T.; Prashant, A.; Vishwanath, P.; Riley, L.W.; Madhivanan, P.; Mahesh, P.A. Trends in the Bacterial Prevalence and Antibiotic Resistance Patterns in the Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Hospitalized Patients in South India. Antibiotics 2022, 11, 1577. https://doi.org/10.3390/antibiotics11111577
Kaleem Ullah M, Malamardi S, Siddaiah JB, A T, Prashant A, Vishwanath P, Riley LW, Madhivanan P, Mahesh PA. Trends in the Bacterial Prevalence and Antibiotic Resistance Patterns in the Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Hospitalized Patients in South India. Antibiotics. 2022; 11(11):1577. https://doi.org/10.3390/antibiotics11111577
Chicago/Turabian StyleKaleem Ullah, Mohammed, Sowmya Malamardi, Jayaraj Biligere Siddaiah, Tejashree A, Akila Prashant, Prashant Vishwanath, Lee W. Riley, Purnima Madhivanan, and Padukudru Anand Mahesh. 2022. "Trends in the Bacterial Prevalence and Antibiotic Resistance Patterns in the Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Hospitalized Patients in South India" Antibiotics 11, no. 11: 1577. https://doi.org/10.3390/antibiotics11111577
APA StyleKaleem Ullah, M., Malamardi, S., Siddaiah, J. B., A, T., Prashant, A., Vishwanath, P., Riley, L. W., Madhivanan, P., & Mahesh, P. A. (2022). Trends in the Bacterial Prevalence and Antibiotic Resistance Patterns in the Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Hospitalized Patients in South India. Antibiotics, 11(11), 1577. https://doi.org/10.3390/antibiotics11111577









