Pharmacokinetics of Vancomycin among Patients with Chemotherapy-Associated Febrile Neutropenia: Which Would Be the Best Dosing to Obtain Appropriate Exposure?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
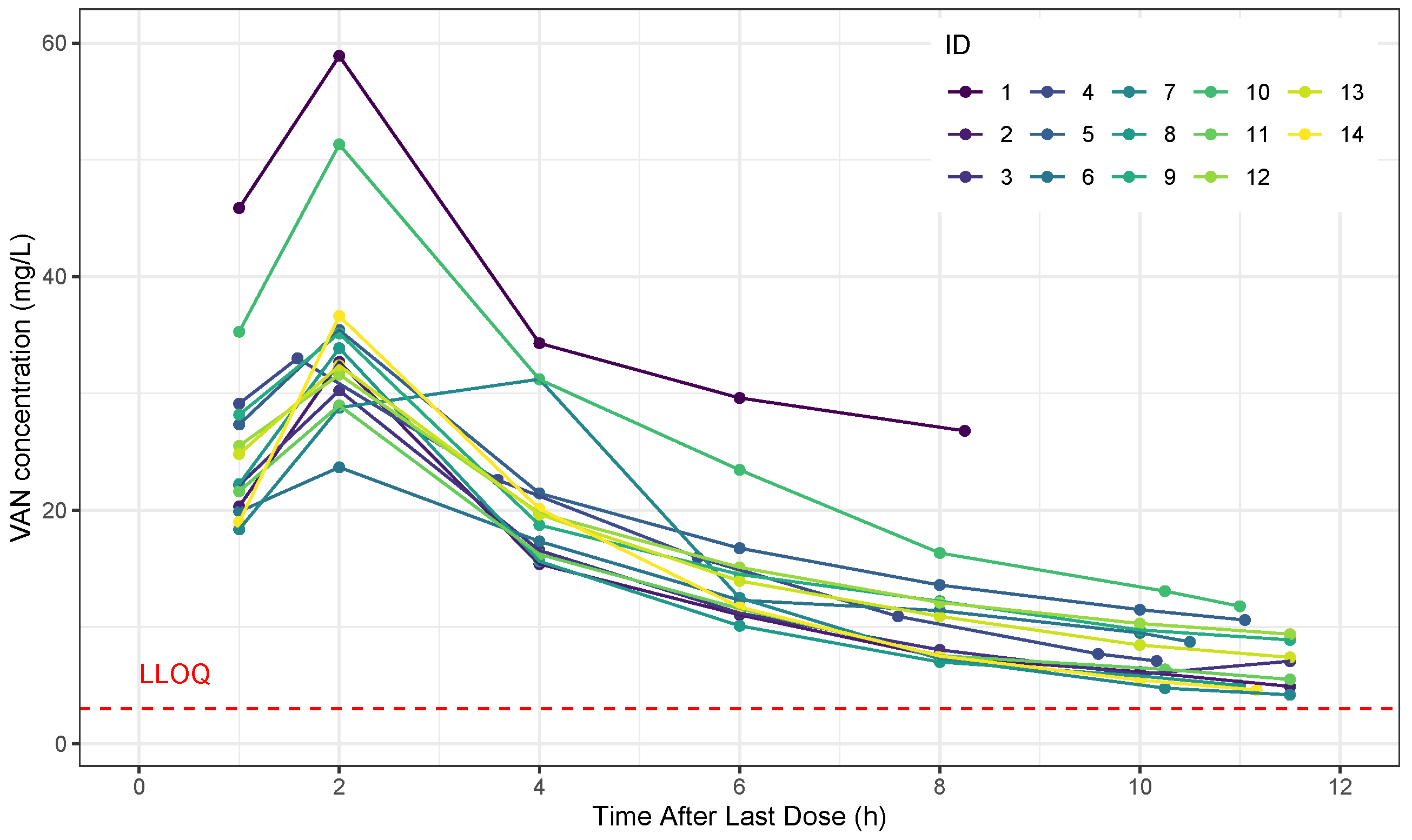
2.2. Administration and Determination of Concentrations
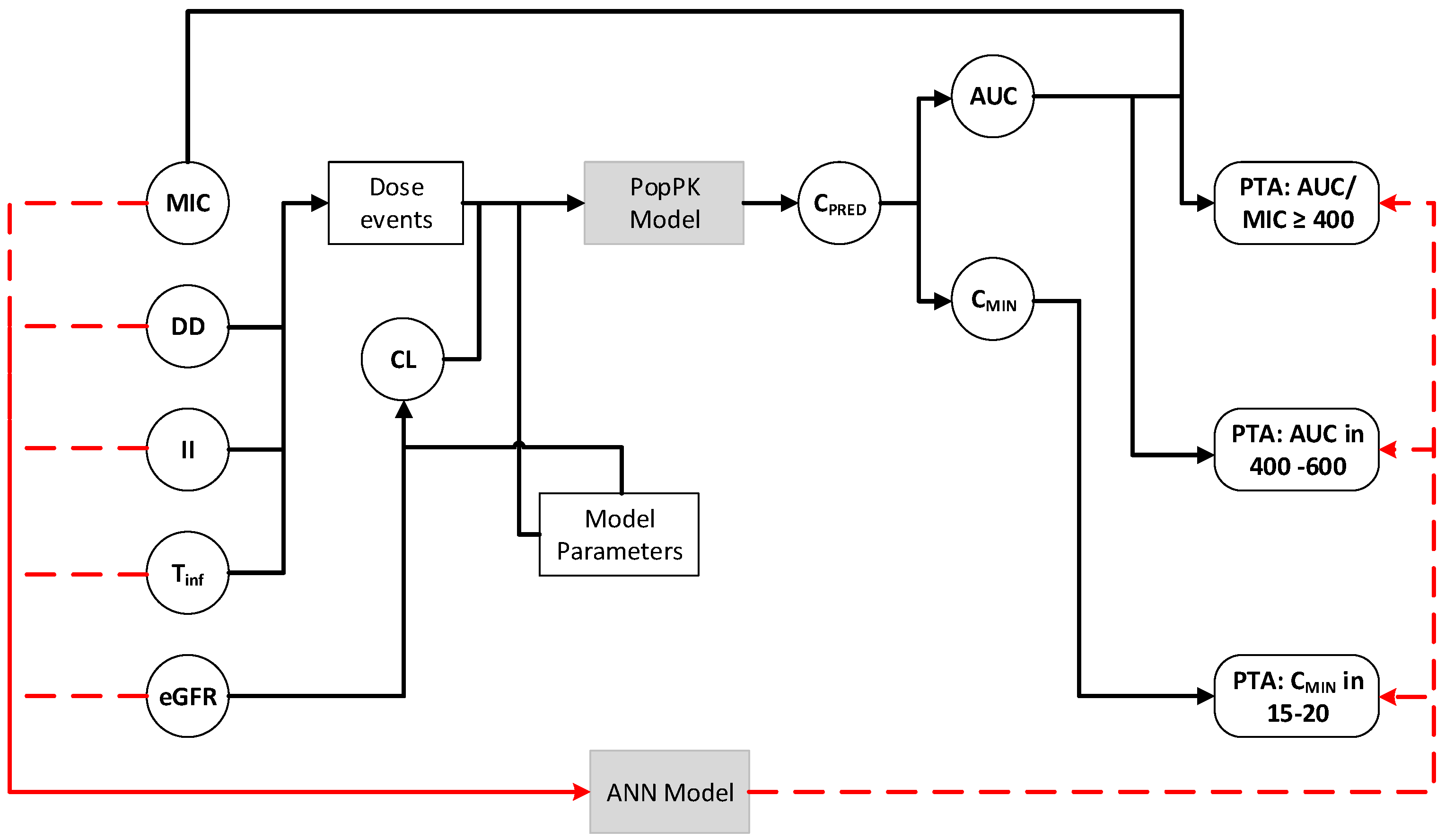
2.3. Pharmacokinetic Modeling
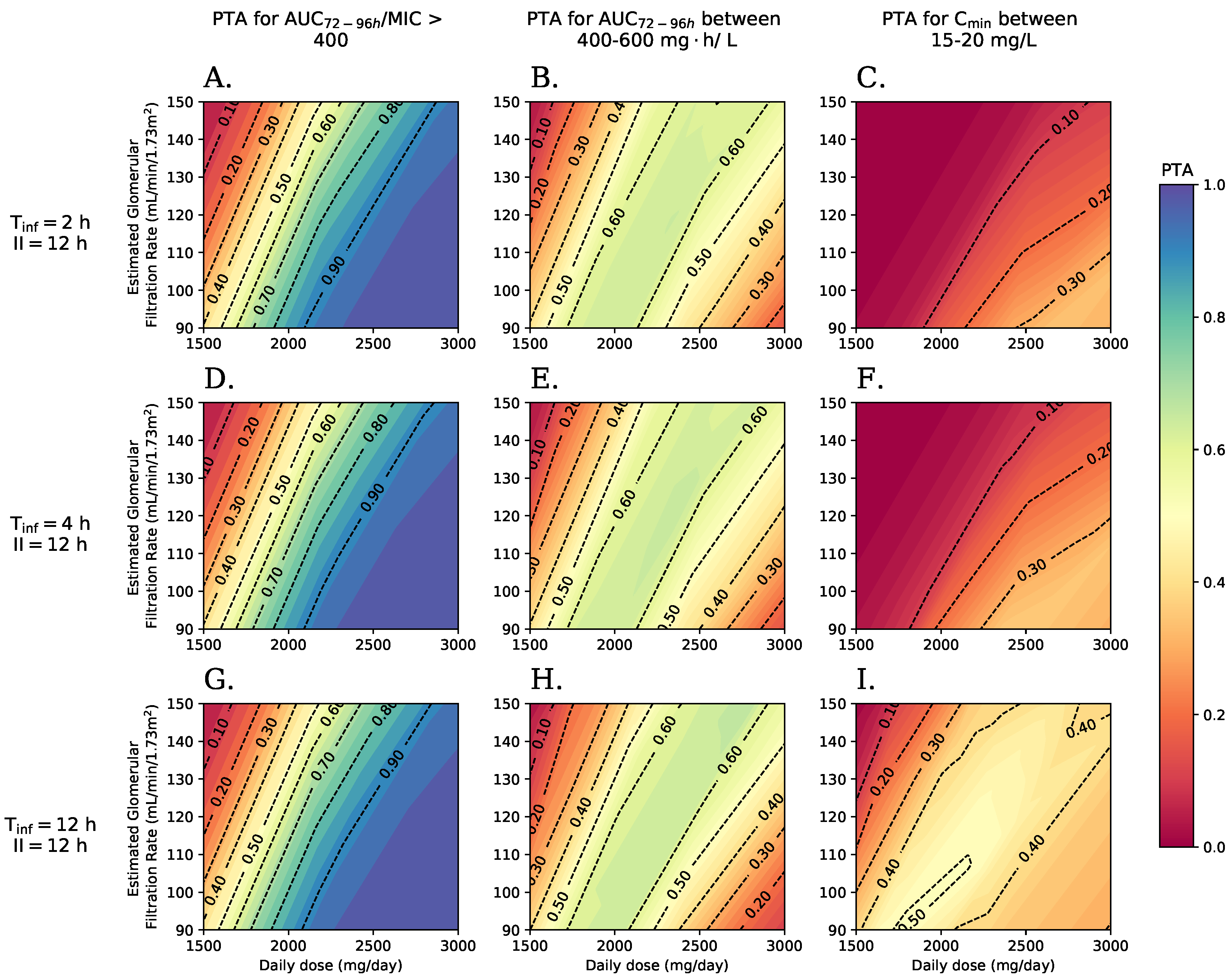
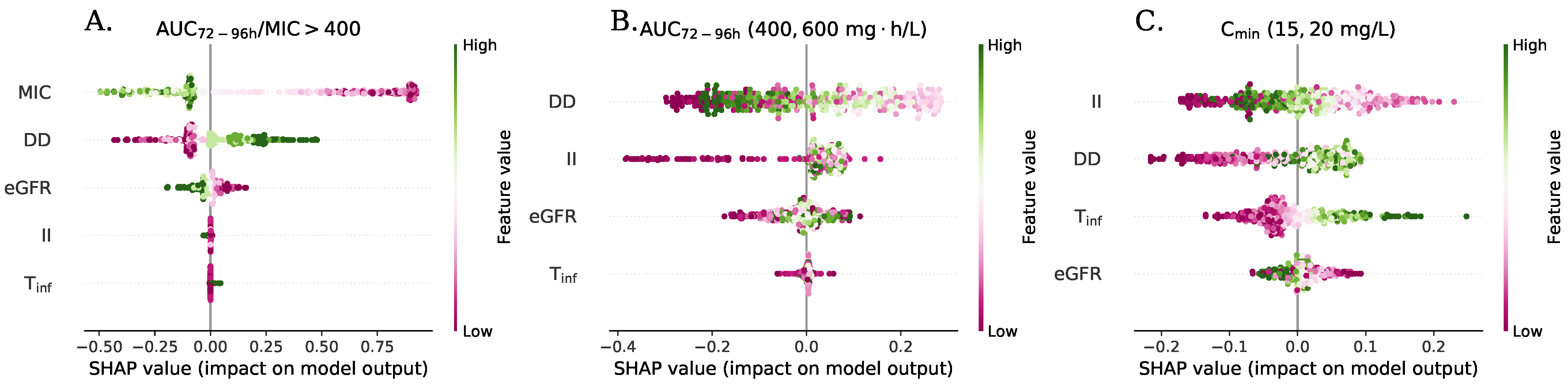
2.4. Pharmacodynamics Assessment/Simulations
- Preprocessing layer with normalization of input variables.
- Input layer with 4 to 5 nodes according to the type of index with rectified linear units (ReLUs).
- Hidden layer 1 with 5 (network A) or 10 nodes (networks B, C, and D) with ReLUs.
- Hidden layer 2 with 10 nodes (C and D networks), with ReLUs.
- Hidden layer 3 with 10 nodes (network D only), with ReLUs.
- Output layer with a sigmoid activation function (PTA only defined between 0 and 1). The ANN for prediction of the -based index contain only one node in this layer, while the ANN for secondaryindices was considered as a multi-output model with two nodes in the output layer.
3. Results
3.1. Patient Characteristics
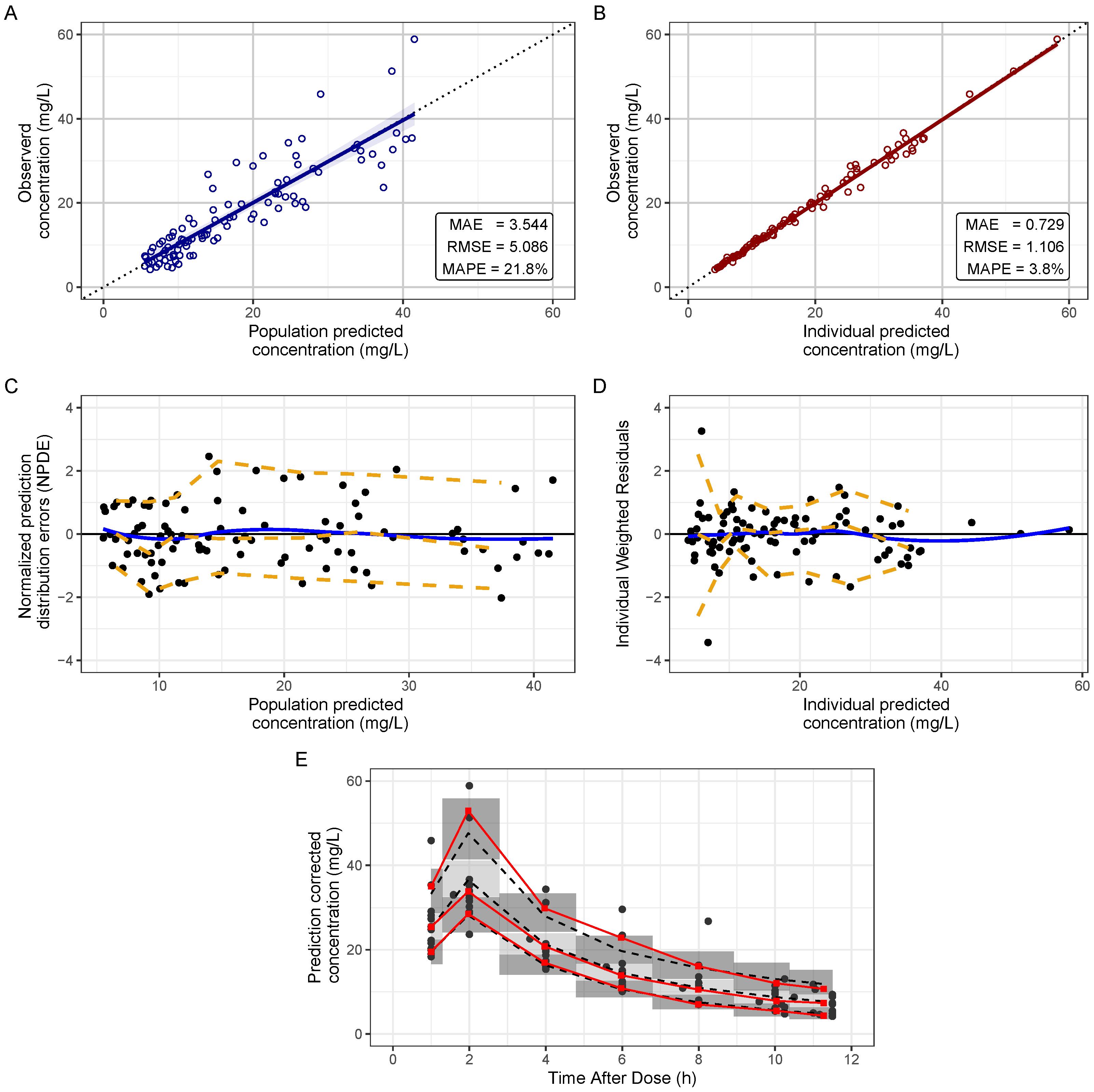
3.2. Pharmacokinetic Analysis
3.3. Pharmacodynamic Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANC | Absolute neutrophil count |
| ANN | Artificial Neural Network |
| AKI | Acute Kidney Injury |
| Area under the curve | |
| Ratio of AUC over MIC | |
| Minimal concentration | |
| Predicted concentration | |
| Clearance | |
| Creatinine clearance | |
| Daily dose | |
| GAM | Generalized additive models |
| Estimated glomerular filtration rate | |
| Interval between doses | |
| IQR | Interquartile range |
| MAE | Mean absolute error |
| MAPE | Mean absolute error percentage |
| MIC | Minimal Inhibitory Concentration |
| pcVPC | Prediction-corrected visual predictive check |
| Probability of Target Attainment | |
| Pharmacokinetic and Pharmacodynamic | |
| Serum creatinine | |
| Infusion time | |
| Typical value of clearance | |
| ReLUs | Rectified linear units |
| RMSE | Root mean quadratic error |
| Volume of distribution | |
| Vancomycin |
References
- Wisplinghoff, H.; Seifert, H.; Wenzel, R.; Edmond, M. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis. 2003, 36, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, B.; Wendelbo, Ø.; Bruserud, Ø.; Hemsing, A.; Mosevoll, K.; Reikvam, H. Febrile neutropenia in acute leukemia. Epidemiology, etiology, pathophysiology and treatment. Mediterr. J. Hematol. Infect. Dis. 2020, 11, e2020009. [Google Scholar]
- Kern, W.; Roth, J.; Bertz, H.; Götting, T.; Dettenkofer, M.; Widmer, A.; Theilacker, C. Contribution of specific pathogens to bloodstream infection mortality in neutropenic patients with hematologic malignancies: Results from a multicentric surveillance cohort study. Transpl. Infect. Dis. 2019, 21, e13186. [Google Scholar] [CrossRef] [PubMed]
- Kara Ali, R.; Surme, S.; Balkan, I.; Salihoglu, A.; Sahin Ozdemir, M.; Ozdemir, Y.; Mete, B.; Can, G.; Ar, M.; Tabak, F.; et al. An eleven-year cohort of bloodstream infections in 552 febrile neutropenic patients: Resistance profiles of Gram-negative bacteria as a predictor of mortality. Ann. Hematol. 2020, 99, 1925–1932. [Google Scholar]
- Daniels, L.; Durani, U.; Barreto, J.; O’Horo, J.; Siddiqui, M.; Park, J.; Tosh, P. Impact of time to antibiotic on hospital stay, intensive care unit admission, and mortality in febrile neutropenia. Support. Care Cancer 2019, 27, 4171–4177. [Google Scholar] [CrossRef]
- Rybak, M.; Le, J.; Lodise, T.; Levine, D.; Bradley, J.; Liu, C.; Mueller, B.; Pai, M.; Wong-Beringer, A.; Rotschafer, J.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatr. Am. J.-Health Pharm. 2020, 77, 835–863. [Google Scholar] [CrossRef] [Green Version]
- Freifeld, A.; Bow, E.; Sepkowitz, K.; Boeckh, M.; Ito, J.; Mullen, C.; Raad, I.; Rolston, K.; Young, J.; Wingard, J. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Men, P.; Li, H.; Zhai, S.; Zhao, R. Association between the AUC0-24 /MIC Ratio of Vancomycin and Its Clinical Effectiveness: A Systematic review and meta-analysis. PLoS ONE 2016, 11, e0146224. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Song, K.; Cho, J.; Kim, H.; Kim, N.; Kim, T.; Choe, P.; Chung, J.; Park, W.; Bang, J.; et al. Area under the concentration-time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant Staphylococcus aureus bacteraemia. Int. J. Antimicrob. Agents 2014, 43, 179–183. [Google Scholar] [CrossRef]
- Casapao, A.; Lodise, T.; Davis, S.; Claeys, K.; Kullar, R.; Levine, D.; Rybak, M. Association between Vancomycin Day 1 Exposure Profile and Outcomes among Patients with Methicillin-Resistant Staphylococcus aureus Infective Endocarditis. Antimicrob. Agents Chemother. 2015, 59, 2978–2985. [Google Scholar] [CrossRef] [Green Version]
- Gawronski, K.; Goff, D.; Brown, J.; Khadem, T.; Bauer, K. A stewardship program’s retrospective evaluation of vancomycin auc24/mic and time to microbiological clearance in patients with methicillin-resistant staphylococcus aureus bacteremia and osteomyelitis. Clin. Ther. 2013, 35, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Davis, S.; Levine, D.; Rybak, M. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant staphylococcus aureus bacteremia: Support for consensus guidelines suggested targets. Clin. Infect. Dis. 2011, 52, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Aljefri, D.; Avedissian, S.; Rhodes, N.; Postelnick, M.; Nguyen, K.; Scheetz, M. Vancomycin Area under the Curve and Acute Kidney Injury: A Meta-analysis. Clin. Infect. Dis. 2019, 69, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.; Moellering, R.; Craig, W.; Billeter, M.; Dalovisio, J.; Levine, D.; Reilly, C. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J.-Health-Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.; Kato, L.; Youn, G.; Kraler, L.; Bayard, D.; Van Guilder, M.; Schumitzky, A.; Yamada, W.; Jones, B.; Minejima, E. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob. Agents Chemother. 2018, 62, e02042-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch, N.; Zasowski, E.; Murray, K.; Mynatt, R.; Zhao, J.; Yost, R.; Pogue, J.; Rybak, M. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob. Agents Chemother. 2017, 61, e01293-17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Cheng, H.; Pan, Z.; Mi, J.; Dong, Y.; Zhang, Y.; Sun, D.; Du, Q.; Cheng, X.; Dong, Y. Desired vancomycin trough concentration to achieve an AUC0-24/MIC >= 400 in Chinese children with complicated infectious diseases. Basic Clin. Pharmacol. Toxicol. 2020, 126, 75–85. [Google Scholar] [CrossRef]
- Clark, L.; Skrupky, L.; Servais, R.; Brummitt, C.; Dilworth, T. Examining the Relationship between Vancomycin Area under the Concentration Time Curve and Serum Trough Levels in Adults with Presumed or Documented Staphylococcal Infections. Ther. Drug Monit. 2019, 41, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Gregory, E.; Burgess, D.; Cotner, S.; VanHoose, J.; Flannery, A.; Gardner, B.; Autry, E.; Forster, D.; Burgess, D.; Wallace, K. Vancomycin Area Under the Curve Dosing and Monitoring at an Academic Medical Center: Transition Strategies and Lessons Learned. J. Pharm. Pract. 2020, 33, 774–778. [Google Scholar] [CrossRef]
- Hirai, K.; Ishii, H.; Shimoshikiryo, T.; Shimomura, T.; Tsuji, D.; Inoue, K.; Kadoiri, T.; Itoh, K. Augmented renal clearance in patients with febrile neutropenia is associated with increased risk for subtherapeutic concentrations of vancomycin. Ther. Drug Monit. 2016, 38, 706–710. [Google Scholar] [CrossRef]
- Haeseker, M.; Croes, S.; Neef, C.; Bruggeman, C.; Stolk, L.; Verbon, A. Vancomycin dosing in neutropenic patients. PLoS ONE 2014, 9, e112008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarkowski, A., III; Forrest, A.; Sweeney, R.; Tan, W.; Segal, B.; Almyroudis, N.; Wang, E.; Wetzler, M. Characterization of vancomycin pharmacokinetics in the adult acute myeloid leukemia population. J. Oncol. Pharm. Pract. 2012, 18, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Bury, D.; ter Heine, R.; van de Garde, E.M.W.; Nijziel, M.R.; Grouls, R.J.; Deenen, M.J. The effect of neutropenia on the clinical pharmacokinetics of vancomycin in adults. Eur. J. Clin. Pharmacol. 2019, 75, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buelga, D.S.; del Mar Fernandez de Gatta, M.; Herrera, E.V.; Dominguez-Gil, A.; García, M.J. Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies. Antimicrob. Agents Chemother. 2005, 49, 4934–4941. [Google Scholar] [CrossRef] [Green Version]
- Tapliz, R.; Kennedy, E.; Bow, E.; Crew, J.; Gleason, C.; Hawley, D.; Langston, A.; Nastoupil, L.; Rajotte, M.; Rolston, K.; et al. Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1443–1453. [Google Scholar] [CrossRef] [Green Version]
- Akiwg, K. Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 138–141. [Google Scholar]
- Levey, A.; Stevens, L.; Schmid, C.; Zhang, Y.; Castro, A.; Feldman, H.; Kusek, J.; Eggers, P.; Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Peng, Y.; Qi, X.; Guo, X. Child-pugh versus MELD score for the assessment of prognosis in liver cirrhosis a systematic review and meta-analysis of observational studies. Medicine 2016, 95, e2877. [Google Scholar] [CrossRef]
- Schouwers, S.; Verstraete, A.; Stove, V. Analytical evaluation of phenytoin, phenobarbital, valproic acid and vancomycin assays on the Abbott Architect (TM). Acta Clinica Belgica 2010, 65, 211. [Google Scholar]
- Lixoft SAS Monolix Version 2019R1. 2019. Available online: http://lixoft.com/products/monolix/ (accessed on 12 June 2021).
- Delattre, M.; Lavielle, M.; Poursat, M. A note on BIC in mixed-effects models. Electron. J. Stat. 2014, 8, 456–475. [Google Scholar] [CrossRef]
- Aljutayli, A.; Marsot, A.; Nekka, F. An Update on Population Pharmacokinetic Analyses of Vancomycin, Part I: In Adults. Clin. Pharmacokinet. 2020, 59, 671–698. [Google Scholar] [CrossRef] [PubMed]
- Ayral, G.; Si Abdallah, J.F.; Magnard, C.; Chauvin, J. A novel method based on unbiased correlations tests for covariate selection in nonlinear mixed effects models: The COSSAC approach. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Mouksassi, M.; Holford, N.; Al-Huniti, N.; Freedman, I.; Hooker, A.; John, J.; Karlsson, M.; Mould, D.; Perez Ruixo, J.; et al. Model evaluation of continuous data pharmacometric models: Metrics and graphics. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Lavielle, M. mlxR: Simulation of Longitudinal Data. R Package Version 4.2.0. 2021. Available online: https://CRAN.R-project.org/package=mlxR (accessed on 12 June 2021).
- Sime, F.B.; Hahn, U.; Warner, M.S.; Tiong, I.S.; Roberts, M.S.; Lipman, J.; Peake, S.L.; Roberts, J.A. Using population pharmacokinetic modelling and Monte Carlo simulations to determine whether standard doses of piperacillin in piperacillin/tazobactam regimens adequate for the management of febrile neutropenia? Antimicrob. Agents Chemother 2017, 61, e00311-17. [Google Scholar] [CrossRef] [PubMed]
- Derbalah, A.; Al-Sallami, H.; Duffull, S. Reduction of quantitative systems pharmacology models using artificial neural networks. J. Pharmacokinet. Pharmacodyn. 2021, 48, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.; Davis, A.; Dean, J.; Devin, M.; et al. TensorFlow: Large-Scale Machine Learning on Heterogeneous Distributed Systems. 2015. Available online: https://www.tensorflow.org/ (accessed on 12 June 2021).
- Kingma, D.; Ba, J. Adam: A method for stochastic optimization. In Proceedings of the 3rd International Conference On Learning Representations, ICLR 2015—Conference Track Proceedings, San Diego, CA, USA, 7–9 May 2015; pp. 1–15. [Google Scholar]
- Lundberg, S.; Lee, S. A Unified Approach to Interpreting Model Predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 1–10. Available online: https://proceedings.neurips.cc/paper/2017/file/8a20a8621978632d76c43dfd28b67767-Paper.pdf (accessed on 14 May 2021).
- Bonate, P. Pharmacokinetic-Pharmacodynamic Modeling and Simulation; Springer International Publishing: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Chung, J.; Jin, S.; Yoon, J.; Song, Y. Serum cystatin C Is a major predictor of vancomycin clearance in a population pharmacokinetic analysis of patients with normal serum creatinine concentrations. J. Korean Med Sci. 2013, 28, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Pang, H.; Jing, L.; Wei, W.; Qin, X.; Guo, Q.; Lu, H.; Cheng, D.; Jiang, W. A population pharmacokinetic model of vancomycin for dose individualization based on serum cystatin C as a marker of renal function. J. Pharm. Pharmacol. 2019, 71, 945–955. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.; Daum, R.; Fridkin, S.; Gorwitz, R.; Kaplan, S.; Karchmer, A.; Levine, D.; Murray, B.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [Green Version]
- Al-Kofide, H.; Zaghloul, I.; Al-Naim, L. Pharmacokinetics of vancomycin in adult cancer patients. J. Oncol. Pharm. Pract. 2010, 16, 245–250. [Google Scholar] [CrossRef]
- Gatta, D.; Buelga, D.; Sa, A.; Dominguez-gil, A.; Ferna, M.; Garcı, M. Vancomycin Dosage Optimization in Patients with Malignant Haematological Disease by Pharmacokinetic/Pharmacodynamic Analysis. Clin. Pharmacokinet. 2009, 48, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Snowden, T.; Graaf, P.; Tindall, M. Model reduction in mathematical pharmacology. J. Pharmacokinet. Pharmacodyn. 2018, 48, 537–555. [Google Scholar] [CrossRef] [PubMed]
| Variables | Value |
|---|---|
| Age (years) [Mean (95% CI)] | 44 (33.6, 54.4) |
| Sex (Male/Female) | 8 (57.1%)/6(42.9%) |
| Weight (kg) [Median (IQR)] | 60.0 (55.0, 67.0) |
| Height (cm) [Mean (95% CI)] | 163.9 (159.3, 168.4) |
| Body Surface Area () [Median (IQR)] | 1.64 (1.55, 1.72) |
| () [Mean (95% CI)] | 0.582 (0.501, 0.663) |
| () [Mean (95% CI)] | 120.3 (109.9, 130.7) |
| Protein (g/dL) [Mean (95% CI)] | 5.59 (5.07, 6.12) |
| Albumin (g/dL) [Mean (95% CI)] | 3.12 (2.84, 3.41) |
| RAL (/) [Median (IQR)] | 960 (650, 1260) |
| RAN (/) [Mean (95% CI)] | 264 (187, 341) |
| Parameter | Final Model (RSE%) | Median Bootstrap Value [95% CI] | Shrinkage (%) |
|---|---|---|---|
| Population Parameters | |||
| : | 5.2 (5.94) | 5.19 [2.86, 5.85] | |
| : | 0.62 (35.3) | 0.58 [0.04, 1.35] | |
| 4.99 (21.6) | 5.24 [3.50, 27.17] | ||
| 21.22 (7.28) | 20.62 [8.03, 25.48] | ||
| 28.28 (13.5) | 28.61 [19.28, 312.98] | ||
| Between-subject variability | |||
| 22.3 (19.4) | 21.2 [9.0, 40.5] | 3.11 | |
| 76.7 (25.9) | 68.5 [9.0, 129.0] | −1.03 | |
| 22.3 (24.8) | 26.4 [8.0, 99.6] | 13.4 | |
| 0.62 (32.2) | 0.61 [−0.24, 0.95] | ||
| Residual variability | |||
| b | 0.025 (9.5) | 0.03 [0.02, 0.04] | 19.5 |
| Model | MAE | RMSE | ||
|---|---|---|---|---|
| Training | Test | Training | Test | |
| Model 1: ANN with one output PTA of | 0.00189 | 0.00199 | 0.00665 | 0.00697 |
| Model 2: ANN with two outputs: PTA of between 15–20 and PTA of between 400 and 600 | 0.0327 | 0.0313 | 0.0572 | 0.0542 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra González, D.; Pérez Mesa, J.A.; Cuervo Maldonado, S.I.; Díaz Rojas, J.A.; Cortés, J.A.; Silva Gómez, E.; Saavedra Trujillo, C.H.; Gómez, J. Pharmacokinetics of Vancomycin among Patients with Chemotherapy-Associated Febrile Neutropenia: Which Would Be the Best Dosing to Obtain Appropriate Exposure? Antibiotics 2022, 11, 1523. https://doi.org/10.3390/antibiotics11111523
Parra González D, Pérez Mesa JA, Cuervo Maldonado SI, Díaz Rojas JA, Cortés JA, Silva Gómez E, Saavedra Trujillo CH, Gómez J. Pharmacokinetics of Vancomycin among Patients with Chemotherapy-Associated Febrile Neutropenia: Which Would Be the Best Dosing to Obtain Appropriate Exposure? Antibiotics. 2022; 11(11):1523. https://doi.org/10.3390/antibiotics11111523
Chicago/Turabian StyleParra González, Daniel, Jefferson Alejandro Pérez Mesa, Sonia Isabel Cuervo Maldonado, Jorge Augusto Díaz Rojas, Jorge Alberto Cortés, Edelberto Silva Gómez, Carlos Humberto Saavedra Trujillo, and Julio Gómez. 2022. "Pharmacokinetics of Vancomycin among Patients with Chemotherapy-Associated Febrile Neutropenia: Which Would Be the Best Dosing to Obtain Appropriate Exposure?" Antibiotics 11, no. 11: 1523. https://doi.org/10.3390/antibiotics11111523
APA StyleParra González, D., Pérez Mesa, J. A., Cuervo Maldonado, S. I., Díaz Rojas, J. A., Cortés, J. A., Silva Gómez, E., Saavedra Trujillo, C. H., & Gómez, J. (2022). Pharmacokinetics of Vancomycin among Patients with Chemotherapy-Associated Febrile Neutropenia: Which Would Be the Best Dosing to Obtain Appropriate Exposure? Antibiotics, 11(11), 1523. https://doi.org/10.3390/antibiotics11111523






