Imidazothiazole Derivatives Exhibited Potent Effects against Brain-Eating Amoebae
Abstract
:1. Introduction
2. Results
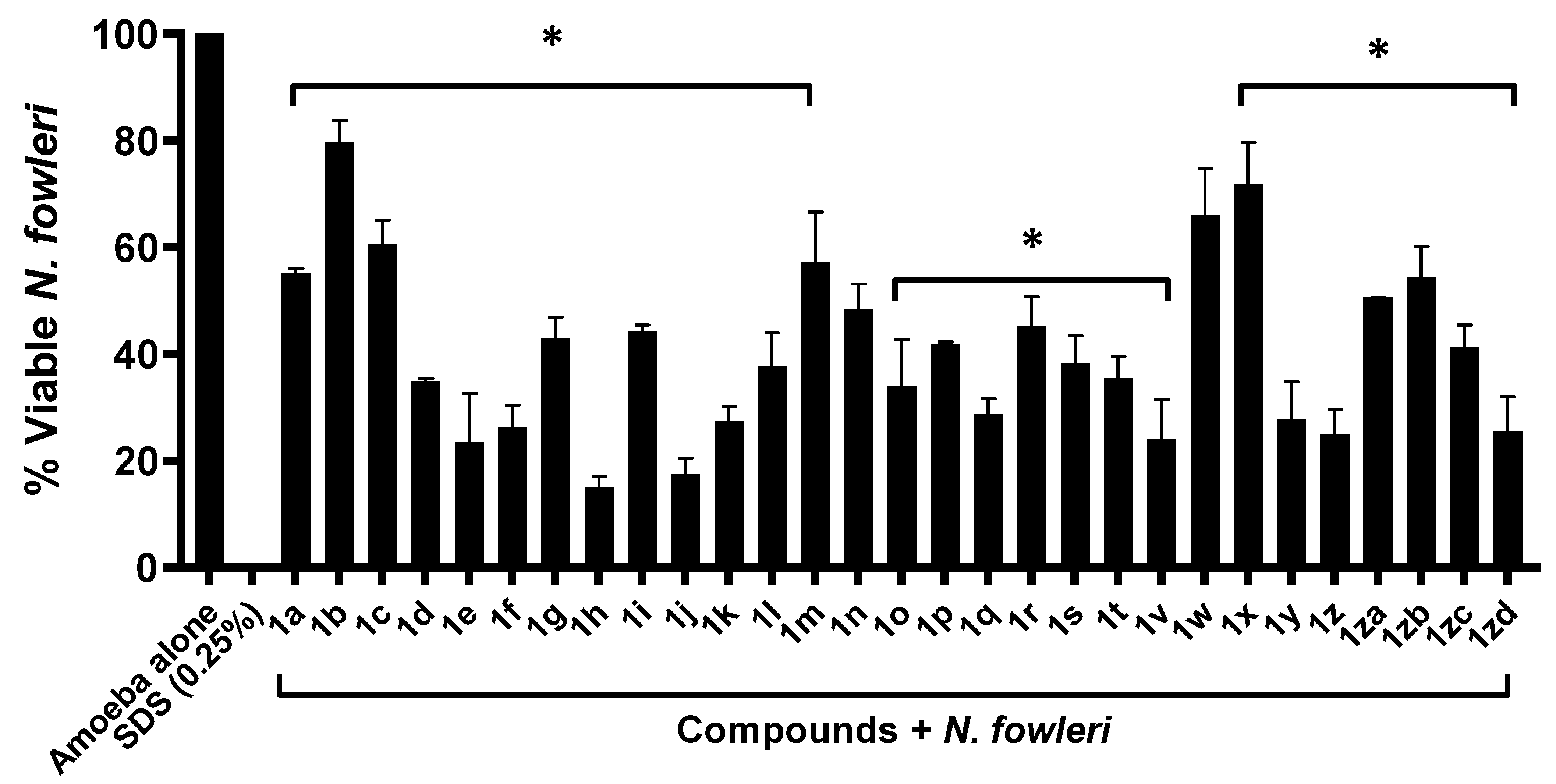
2.1. Majority of Compounds Exhibited Significant Amoebicidal Properties against Naegleria fowleri
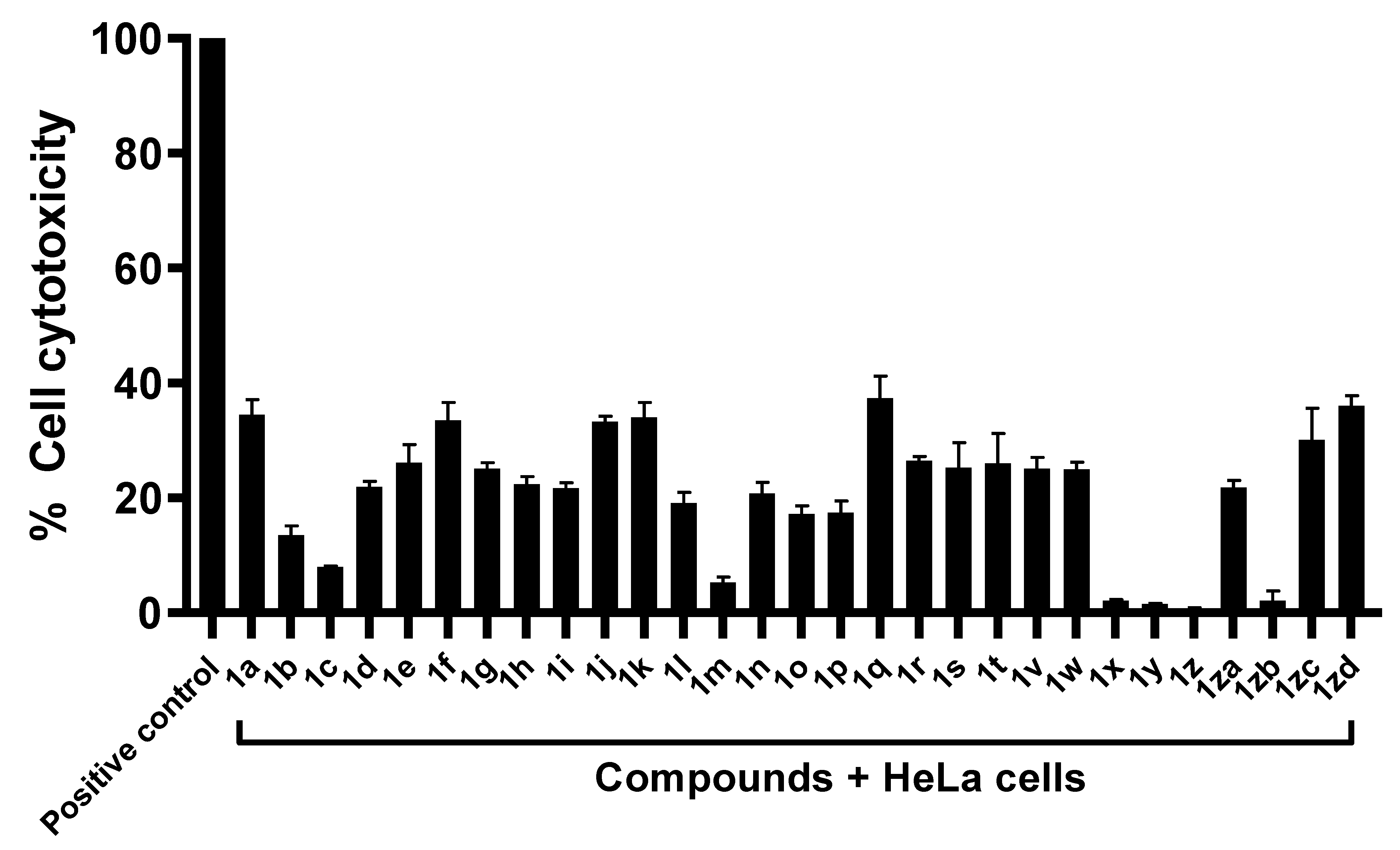
2.2. Compounds Exhibited Limited Cytotoxicity against Human Cell Lines
2.3. Majority of Compounds Resulted in Decreased in Amoeba-Mediated Cytotoxic Activity against Human Cells
3. Discussion
4. Materials and Methods
4.1. The Tested Compounds
4.2. Henrietta Lacks (HeLa) Cervical Cancer Cells
4.3. Naegleria Fowleri Culture
4.4. Amoebicidal Assay
4.5. Cytotoxicity Assay
4.6. Cytopathogenicity Assay
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pugh, J.J.; Levy, R.A. Naegleria fowleri: Diagnosis, pathophysiology of brain inflammation, and antimicrobial treatments. ACS Chem. Neurosci. 2016, 7, 1178–1179. [Google Scholar] [CrossRef] [Green Version]
- Maciver, S.K.; Piñero, J.E.; Lorenzo-Morales, J. Is Naegleria fowleri an emerging parasite? Trends Parasitol. 2020, 36, 19–28. [Google Scholar] [CrossRef] [PubMed]
- De Jonckheere, J.F. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect. Genet. Evol. 2011, 11, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, R.; Bliton, J.; Goodman, A.; Ali, I.K.M.; Yoder, J.; Cope, J.R. Epidemiology and clinical characteristics of primary amebic meningoencephalitis caused by Naegleria fowleri: A global review. Clin. Infect. Dis. 2021, 73, e19–e27. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; MacLean, R.; Mensah, A.; LaPat-Polasko, L. Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl. Environ. Microbiol. 2003, 69, 5864–5869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciano-Cabral, F.; Cabral, G.A. The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol. Med. Microbiol. 2007, 51, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, R.; Khamis, M.; Ibrahim, T.; Khan, N.A. Brain-Eating Amoebae in the United Arab Emirates? ACS Pharmacol. Transl. Sci. 2021, 4, 1014–1015. [Google Scholar] [CrossRef]
- Matin, A.; Siddiqui, R.; Jayasekera, S.; Khan, N.A. Increasing importance of Balamuthia mandrillaris. Clin. Microbiol. Rev. 2008, 21, 435–448. [Google Scholar] [CrossRef] [Green Version]
- da Rocha-Azevedo, B.; Tanowitz, H.B.; Marciano-Cabral, F. Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 251406. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, R.; Ali, I.K.M.; Cope, J.R.; Khan, N.A. Biology and pathogenesis of Naegleria fowleri. Acta Trop. 2016, 164, 375–394. [Google Scholar] [CrossRef]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Siddiqui, R. Brain-Eating Amoebae: Silver Nanoparticle Conjugation Enhanced Efficacy of Anti-Amoebic Drugs against Naegleria fowleri. ACS Chem. Neurosci. 2017, 8, 2626–2630. [Google Scholar] [CrossRef] [PubMed]
- Mungroo, M.R.; Anwar, A.; Khan, N.A.; Siddiqui, R. Gold-Conjugated Curcumin as a Novel Therapeutic Agent against Brain-Eating Amoebae. ACS Omega 2020, 5, 12467–12475. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Mungroo, M.R.; Khan, S.; Fatima, I.; Rafique, R.; Kanwal; Khan, K.M.; Siddiqui, R.; Khan, N.A. Novel Azoles as Antiparasitic Remedies against Brain-Eating Amoebae. Antibiotics 2020, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Muhammad, J.S.; Siddiqui, R. Brain-eating amoebae: Is killing the parasite our only option to prevent death? Expert Rev. Anti-Infect. Ther. 2021, 20, 1–2. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Contemporary approaches to treat Naegleria fowleri: A patent overview. Pharm. Pat. Anal. 2020, 10, 99–101. [Google Scholar] [CrossRef]
- Baig, A.M. Global warming favors pathogenicity of the brain-eating amoebae. Anti-Infect. Agents 2019, 17, 2–3. [Google Scholar] [CrossRef]
- Trabelsi, H.; Dendana, F.; Sellami, A.; Sellami, H.; Cheikhrouhou, F.; Neji, S.; Makni, F.; Ayadi, A. Pathogenic free-living amoebae: Epidemiology and clinical review. Pathol. Biol. 2012, 60, 399–405. [Google Scholar] [CrossRef]
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015, 59, 6677–6681. [Google Scholar] [CrossRef] [Green Version]
- Rice, C.A.; Colon, B.L.; Chen, E.; Hull, M.V.; Kyle, D.E. Discovery of repurposing drug candidates for the treatment of diseases caused by pathogenic free-living amoebae. PLoS Negl. Trop. Dis. 2020, 14, e0008353. [Google Scholar] [CrossRef]
- Park, J.-H.; Oh, C.-H. Synthesis of new 6-(4-fluorophenyl)-5-(2-substituted pyrimidin-4-yl)imidazo[2,1-b]thiazole derivatives and their antiproliferative activity against melanoma cell line. Bull. Korean Chem. Soc. 2010, 31, 2854–2860. [Google Scholar] [CrossRef]
- Park, J.-H.; El-Gamal, M.I.; Lee, Y.S.; Oh, C.-H. New imidazo[2,1-b]thiazole derivatives: Synthesis, in vitro anticancer evaluation, and in silico studies. Eur. J. Med. Chem. 2011, 46, 5769–5777. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maksoud, M.S.; El-Gamal, M.I.; Gamal El-Din, M.M.; Kwak, S.-S.; Kim, H.-I.; Oh, C.-H. Broad-spectrum antiproliferative activity of a series of 6-(4-fluorophenyl)-5-(2-substituted pyrimidin-4-yl)imidazo[2,1-b]thiazole derivatives. Med. Chem. Res. 2016, 25, 824–833. [Google Scholar] [CrossRef]
- Anbar, H.S.; El-Gamal, M.I.; Tarazi, H.; Lee, B.S.; Jeon, H.R.; Kwon, D.; Oh, C.-H. Imidazothiazole-based potent inhibitors of V600E-B-RAF kinase with promising anti-melanoma activity: Biological and computational studies. J. Enz. Inhibit. Med. Chem. 2020, 35, 1712–1726. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.S.; El-Gamal, M.I.; Lee, B.S.; Gamal El-Din, M.M.; Jeon, H.R.; Kwon, D.; Ammar, U.M.; Mersal, K.I.; Ali, E.M.H.; Lee, K.-T.; et al. Discovery of New Imidazo[2,1-b]thiazole Derivatives as Potent Pan-RAF Inhibitors with Promising In vitro and In vivo Anti-melanoma Activity. J. Med. Chem. 2021, 64, 6877–6901. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; El-Gamal, M.I.; Saeed, B.Q.; Oh, C.-H.; Abdel-Maksoud, M.S.; Khan, N.A.; Alharbi, A.M.; Alfahemi, H.; Siddiqui, R. Antiamoebic activity of imidazothiazole derivatives against opportunistic pathogen Acanthamoeba castellanii. Antibiotic 2022, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Khan, N.A. Antibacterial activities of selected pure compounds isolated from gut bacteria of animals living in polluted environments. Antibiotics 2020, 9, 190. [Google Scholar] [CrossRef]
- Król-Turmińska, K.; Olender, A. Human infections caused by free-living amoebae. Ann. Agric. Environ. Med. 2017, 24, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.Y.Y.; Khan, N.A.; Siddiqui, R. Brain-eating amoebae: Predilection sites in the brain and disease outcome. J. Clin. Microbiol. 2017, 55, 1989–1997. [Google Scholar] [CrossRef] [Green Version]
- Matanock, A.; Mehal, J.M.; Liu, L.; Blau, D.M.; Cope, J.R. Estimation of undiagnosed Naegleria fowleri primary amebic meningoencephalitis, United States. Emerg. Infect. Dis. 2018, 24, 162. [Google Scholar] [CrossRef] [Green Version]
- Taravaud, A.; Fechtali-Moute, Z.; Loiseau, P.M.; Pomel, S. Drugs used for the treatment of cerebral and disseminated infections caused by free-living amoebae. Clin. Transl. Sci. 2021, 14, 791–805. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Shah, M.R.; Siddiqui, R. trans-Cinnamic acid conjugated gold nanoparticles as potent therapeutics against brain-eating amoeba Naegleria fowleri. ACS Chem. Neurosci. 2019, 10, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Abagyan, R.; Podust, L.M.; Roy, S.; Ali, I.K.M.; Debnath, A. HMG-CoA reductase inhibitors as drug leads against Naegleria fowleri. ACS Chem. Neurosci. 2020, 11, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Smego, R.A.; Durack, D.T. An experimantal model for Naegleria fowleri-induced primary amebic meningoencephalitis in rabbits. J. Parasitol. 1984, 70, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Ramirez, D.A.; Carrasco-Yepez, M.M.; Rodriguez-Mera, I.B.; Resendiz-Albor, A.A.; Rosales-Cruz, E.; Rojas-Hernandez, S. A 250-kDa glycoprotein of Naegleria fowleri induces protection and modifies the expression of α4β1 and LFA-1 on T and B lymphocytes in mouse meningitis model. Parasite Immunol. 2021, 43, e12882. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Anwar, A.; Shah, M.R.; Siddiqui, R. Cytotoxic effects of Benzodioxane, Naphthalene diimide, Porphyrin and Acetamol derivatives on HeLa cells. SAGE Open Med. 2018, 6, 2050312118781962. [Google Scholar] [CrossRef]

| Naegleria fowleri | ||||
|---|---|---|---|---|
| 25 µM | 50 µM | 75 µM | MIC50 | |
| Amoeba Alone | 100 | |||
| 1e | 93 ± 9.7 | 52.16 ± 1.7 | 19 ± 1.7 | 50.96 |
| 1f | 99 ± 7.3 | 44 ± 3.9 | 17 ± 4.4 | 48.45 |
| 1h | 96 ± 8.5 | 73 ± 2.4 | 26 ± 0.8 | 60.87 |
| Compound No. | Structure |
|---|---|
| 1a | 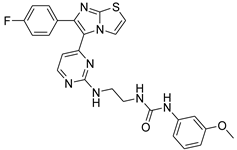 |
| 1b | 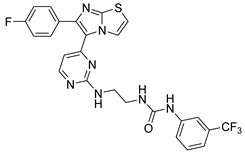 |
| 1c | 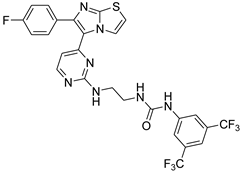 |
| 1d | 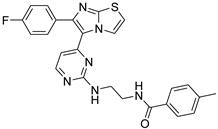 |
| 1e |  |
| 1f | 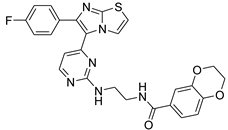 |
| 1g |  |
| 1h |  |
| 1i |  |
| 1j |  |
| 1k |  |
| 1l |  |
| 1m | 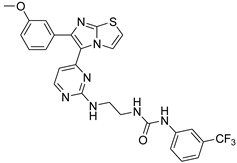 |
| 1n | 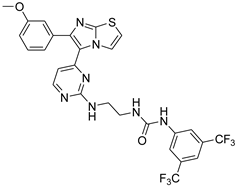 |
| 1o | 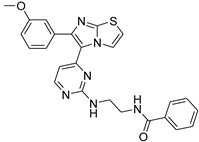 |
| 1p | 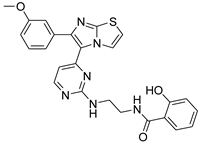 |
| 1q | 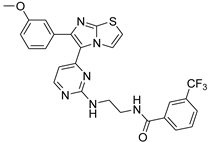 |
| 1r | 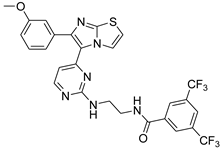 |
| 1s | 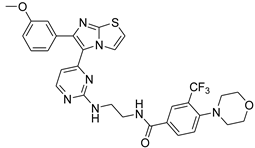 |
| 1t | 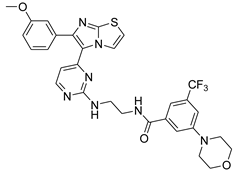 |
| 1u | 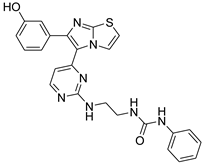 |
| 1v | 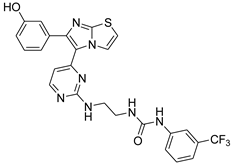 |
| 1w | 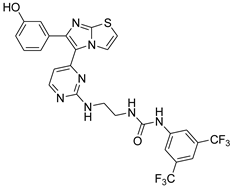 |
| 1x | 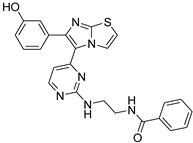 |
| 1y | 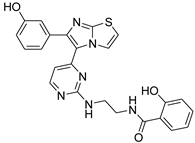 |
| 1z | 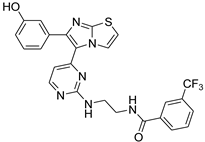 |
| 1za |  |
| 1zb | 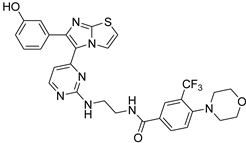 |
| 1zc | 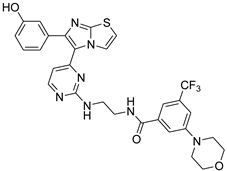 |
| 1zd | 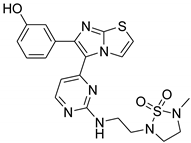 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, R.; El-Gamal, M.I.; Boghossian, A.; Saeed, B.Q.; Oh, C.-H.; Abdel-Maksoud, M.S.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. Imidazothiazole Derivatives Exhibited Potent Effects against Brain-Eating Amoebae. Antibiotics 2022, 11, 1515. https://doi.org/10.3390/antibiotics11111515
Siddiqui R, El-Gamal MI, Boghossian A, Saeed BQ, Oh C-H, Abdel-Maksoud MS, Alharbi AM, Alfahemi H, Khan NA. Imidazothiazole Derivatives Exhibited Potent Effects against Brain-Eating Amoebae. Antibiotics. 2022; 11(11):1515. https://doi.org/10.3390/antibiotics11111515
Chicago/Turabian StyleSiddiqui, Ruqaiyyah, Mohammed I. El-Gamal, Anania Boghossian, Balsam Qubais Saeed, Chang-Hyun Oh, Mohammed S. Abdel-Maksoud, Ahmad M. Alharbi, Hasan Alfahemi, and Naveed Ahmed Khan. 2022. "Imidazothiazole Derivatives Exhibited Potent Effects against Brain-Eating Amoebae" Antibiotics 11, no. 11: 1515. https://doi.org/10.3390/antibiotics11111515
APA StyleSiddiqui, R., El-Gamal, M. I., Boghossian, A., Saeed, B. Q., Oh, C.-H., Abdel-Maksoud, M. S., Alharbi, A. M., Alfahemi, H., & Khan, N. A. (2022). Imidazothiazole Derivatives Exhibited Potent Effects against Brain-Eating Amoebae. Antibiotics, 11(11), 1515. https://doi.org/10.3390/antibiotics11111515








